Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.436
Peer-review started: January 30, 2019
First decision: March 14, 2019
Revised: March 19, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: May 15, 2019
Processing time: 106 Days and 3.3 Hours
Hepatic neuroendocrine neoplasm (hNEN) is a highly heterogeneous tumor. The exact identification of the source and malignant degree of hNEN is important. However, there is a lack of information regarding diagnosis of hNEN with imaging. In addition, no studies have compared the imaging between hNEN and hepatocellular carcinoma (HCC) and among different sources and malignant degrees of hNEN.
To compare the ultrasound characteristics between hNEN and HCC and among different sources and malignant degrees of hNEN.
A total of 55 patients with hNEN were recruited and defined as the hNEN group. Among them, 35 cases of hNET were defined as the hNET group. Twenty cases of hepatic neuroendocrine carcinoma (hNEC) were defined as the hNEC group. Among the 55 lesions, 29 were transferred from the pancreas, 20 were from the gastrointestinal tract, and six were from other sites. In total, 55 patients with HCC were recruited and defined as the HCC group. The characteristic differences of B-mode ultrasound and contrast-enhanced ultrasound (CEUS) between hNEN and HCC and among different sources and malignant degrees of hNEN were compared.
In the hNEN group, the proportions of multiple liver lesions, unclear borders, and high echo lesions were higher than those in the HCC group. The proportions of non-uniform echo and peripheral acoustic halo were lower than those in the HCC group (P < 0.05). The washout to iso-enhancement time and washout to hypo-enhancement time were lower than those in the HCC group (P < 0.05). The characteristics of B-ultrasound and CEUS among different sources of hNEN were similar, and the differences were not statistically significant (P > 0.05). B-mode ultrasound characteristics of hNET and hNEC were similar. The proportions of low enhancement at portal venous phase, non-uniform enhancement forms, and combined tumor vasculature in the hNEC group were larger than those in the hNEN group (P < 0.05).
Compared with HCC, hNEN showed multiple intrahepatic lesions, uniform high echo, uniform high enhancement at arterial phase, and rapid washout. Low enhancement at portal venous phase, overall non-uniform enhancement form, and the proportion of combined tumor vasculature in hNEC were larger than those in hNET.
Core tip: Clinically, hepatic neuroendocrine neoplasm (hNEN) is rare, and few reports are currently available on the imaging diagnosis of hNENs. In this study, by comparing hNEN and hepatocellular carcinoma, hNEN from different sources, and differentiation, it was found that the ultrasound characteristics of hNEN are mostly multiple, uniform hyperechoic masses. The enhancement at the arterial phase was mostly uniform and high, and the washout was rapid compared with hepatocellular carcinoma. Compared with hepatic neuroendocrine tumor, the enhancement at the portal venous phase of hepatic neuroendocrine carcinoma was low, and the enhancement form was non-uniform.
- Citation: Kang XN, Zhang XY, Bai J, Wang ZY, Yin WJ, Li L. Analysis of B-ultrasound and contrast-enhanced ultrasound characteristics of different hepatic neuroendocrine neoplasm. World J Gastrointest Oncol 2019; 11(5): 436-448
- URL: https://www.wjgnet.com/1948-5204/full/v11/i5/436.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i5.436
Neuroendocrine neoplasm (NEN) is a highly heterogeneous tumor. The liver is the most important metastatic part of NEN, which is mostly transferred from other organs, such as the gastrointestinal tract. Therefore, hepatic NEN (hNEN) is more common than primary hNEN[1-3]. The manifestations of hNEN patients are complex and mostly non-specific. Patients with hNEN often present with liver discomfort and bloating. It is necessary to identify hNEN and hepatocellular carcinoma (HCC). In addition, all NENs have malignant potential. NEN from different sources and malignant degrees differ greatly in outcome and treatment. Hence, it is important to identify accurately the source and malignant degree of hNEN.
Currently, the diagnosis of hNEN mainly depends on the results of pathological examination and immunohistochemistry[4-6]. Although pathological examination and immunohistochemistry are the gold standard for diagnosis, they are invasive ex-aminations. They can only be used as a means of verification and cannot be used as a screening tool for diseases. Clinically, initial screening is required through non-invasive examinations (e.g., imaging examinations, laboratory examinations, etc), and pathological diagnosis is performed on highly suspected patients. However, due to the rareness of hNEN, there is a lack of current information regarding imaging examinations, and there is little experience in identifying hNEN and HCC, hNEN from different sources, and malignant degrees.
Beard et al[7] reported that hNEN and HCC had similarities in ultrasound performance, which may cause misdiagnosis due to insufficient understanding. Some studies compared the hNEN characteristics of different sources and malignant degrees and found that the B-ultrasound and contrast-enhanced ultrasound (CEUS) performance of hNEN from different sources and malignant degrees were diffe-rent[8-10]. These findings suggest that we can identify hNEN by ultrasound and CEUS, but its clinical application value has not been confirmed. Therefore, the present study compared the ultrasound performance between hNEN and HCC. In addition, the characteristics of B-mode ultrasound and CEUS from different sources and malignant degrees of hNEN were analyzed in order to provide a reference for the diagnosis and treatment of hNEN.
A total of 55 patients with hNEN admitted to Cangzhou Central Hospital from Jan-uary 2014 to May 2018 were recruited. All patients obtained a complete B-mode ultrasound and CEUS data. They were defined as the hNEN group. Among them, 27 were males and 28 were females with an age range of 36-68 years old and an average of 55.23 ± 14.52 years old. Three patients in the hNEN group had hepatitis. The inclusion criteria of hNEN were: Surgical resection or biopsy was confirmed as hNEN, and immunohistochemistry confirmed that ChrA or Syno was positive. The exclusion criteria were: HCC, mixed liver cancer, hilar cholangiocarcinoma, and extrahepatic cholangiocarcinoma. According to the World Health Organization classification of the digestive system tumor (2010) neuroendocrine tumor (NET) grading standard[11], 35 cases of hepatic NET (hNET) (G1 and G2) were defined as the hNET group, and 20 cases of hNEC (G3) were defined as the hNEC group. Among the 55 hNEN lesions, 29 were transferred from the pancreas, 20 were from the gastrointestinal tract, and six were from other sites (two cases from the gallbladder, two cases from the abdomen, and two cases from the lung). During the study period, 55 patients with HCC were recruited as the HCC group. There were 38 males and 17 females with an age range of 35-71 years old and an average age of 54.29 ± 17.27 years old. There were 51 HCC patients associated with hepatitis, and the hepatitis infection rate was significantly higher than that of hNEN patients. The difference was statistically significant (χ2 = 86.443, P = 0.000). All patients signed informed consent, and this study was reviewed by the Ethics Committee of Cangzhou Central Hospital.
Ultrasound examination: Ultrasound examination was performed using a Philips ultrasound affinity 70 diagnostic instruments equipped with CEUS imaging software. B-mode ultrasound and CEUS examinations were performed in each patient. The patient was placed in a supine position. The depth, focus, gain, and grayscale and color Doppler (CDFI) range were adjusted before examination. B-mode ultrasound examinations, including CDFI scans, were performed first. Lesion diameter (unit: cm), number (single/multiple), lesion property (solid/cyst), echo uniformity (uniform/non-uniform), echo level (high/low/mixed/equal), boundary (clear/unclear), accompanying signs (peripheral acoustic halo, posterior echo attenuation) of the liver lesions, and CDFI images were recorded.
Subsequently, a 2.4 mL contrast agent of SonoVue (Bracco) was used for CEUS. After the bolus injection into the left median cubital vein, 5 mL of saline was injected. The timing was started when the injection began. The whole examination process was about 3-5 min, and the image data were recorded. The time phase of hepatic CEUS was: 10-30 s after the injection of the contrast agent was the arterial phase, 31-120 s was the portal venous phase, and 121-360 s was the late phase. All examinations were performed by physicians with more than 10 years of ultrasound experience in our hospital.
Data collection and image analysis: B-mode ultrasound lesion diameter, number, boundary, lesion property, echo level, echo uniformity, and the number and proportion of accompanying signs (peripheral acoustic halo, posterior echo attenuation) of the liver lesions were observed and recorded. The characteristic differences of B-mode ultrasound between hNEN and HCC groups, transferred from different hNEN sources, and between hNEC and hNET groups were compared.
CEUS: The initial enhancement time (unit:s) of liver parenchyma and lesions was recorded. The washout to iso-enhancement time (unit:s) and washout to hypo-enhancement time (unit:s) of liver lesions were recorded as well. Then, the number and proportion of different enhancement levels at arterial phase (reference to the enhancement level of adjacent liver tissue, divided into high/equal/low enhan-cement), enhancement levels at portal venous phase and late phase (equal/low enhancement), enhancement forms (uniform or non-uniform enhancement), enhancement-washout modes (fast enhancement and washout/equal enhancement and fast washout/low enhancement and fast washout), and special signs (adjacent and internal tumor vasculature, tumor necrosis no-enhancement zone, capsule enhancement in the late phase) of liver lesions were recorded. The characteristic differences of CEUS between hNEN and HCC groups, transferred from different hNEN sources, and between hNEC and hNET groups were compared.
All statistical analyses were performed with SPSS version 19.0 (IBM, Armonk, NY, United States) software. The numerical data were expressed as mean ± SD and the categorical variables as number and percentage. The t test was used to compare the two groups of numerical data, and the three groups of numerical data were compared using one-way analysis of variance. The comparisons between the categorical variables were performed by chi-square test. If the minimum theoretical frequency was less than one, the Fisher’s exact test was used. P < 0.05 was considered a statis-tically significant difference.
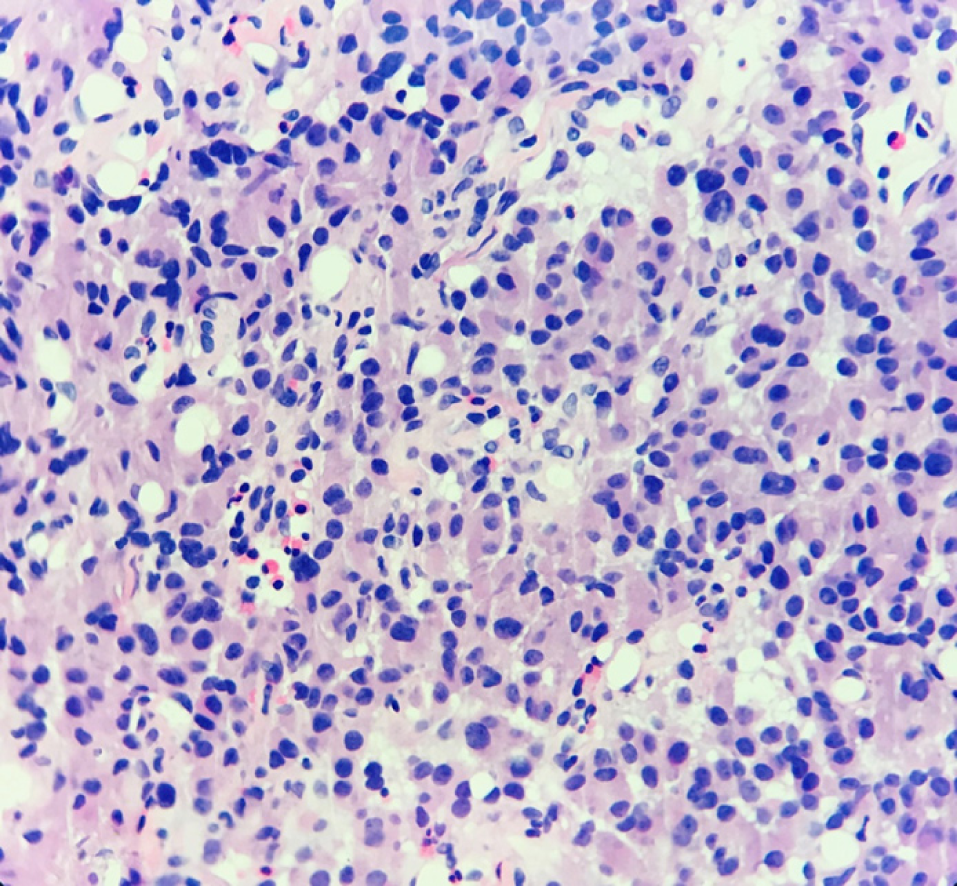
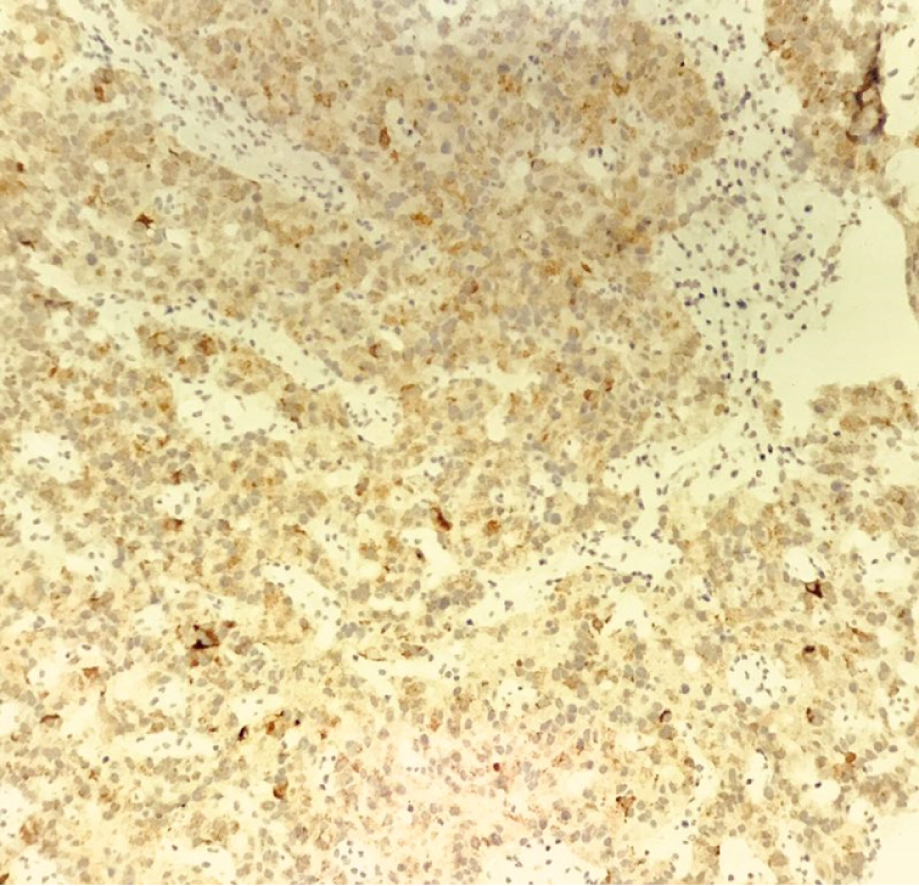
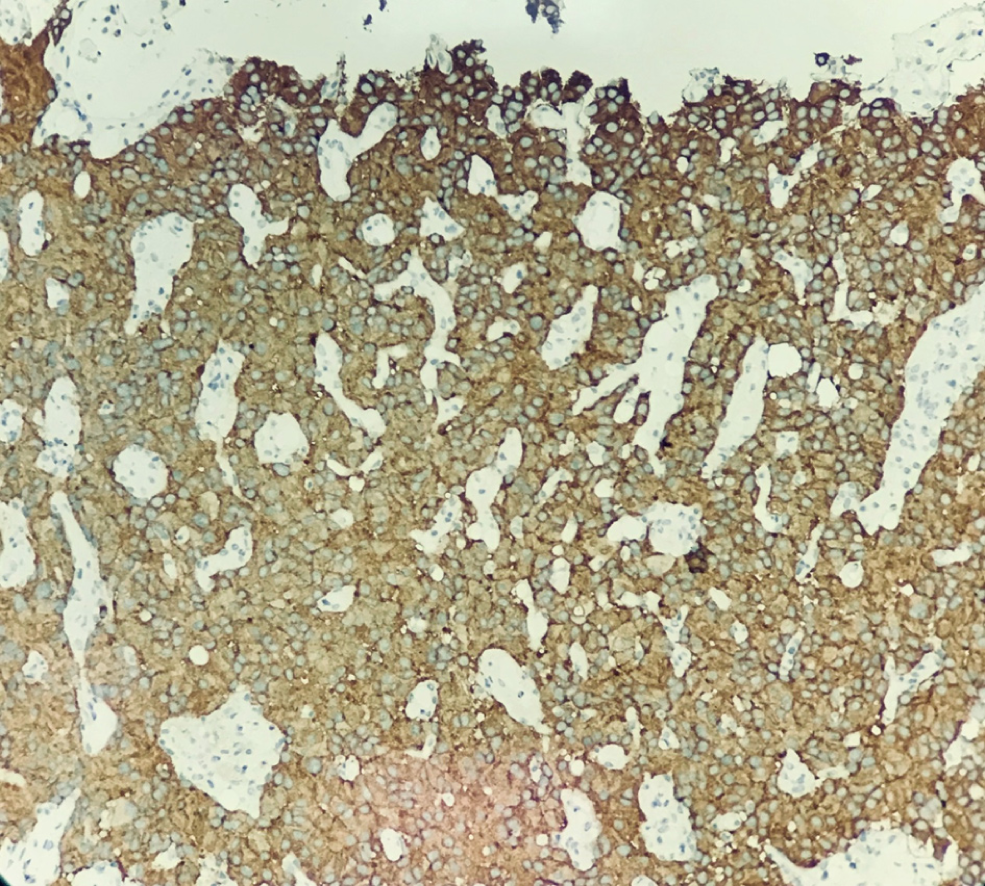
Hematoxylin-eosin staining showed that the tumors were arranged by uniform circular or oval cells, which were nested or glandularly distributed. The cells were well-differentiated. There were fewer mitotic figures, and the atypia was not obvious (Figure 1). Immunohistochemical staining showed that 42 patients with hNEN were positive for ChrA (Figure 2), and 45 patients were positive for Syno (Figure 3).
Among all the B-mode ultrasound features, lesion diameter and the proportions of different lesion property and posterior echo attenuation were similar between the hNEN and HCC groups, and the differences were not statistically significant (P > 0.05). The proportions of multiple liver lesions, unclear boundary, and high echo lesion in the hNEN group were higher than those in the HCC group, and the diffe-rences were statistically significant (P < 0.05). The proportions of non-uniform echo and peripheral acoustic halo in the hNEN group were lower than those in the HCC group, and the differences were statistically significant (P < 0.05; Table 1).
| B-mode Ultrasound characteristics | hNEN group, n = 55 | HCC group, n = 55 | t/χ2 | P value | |
| Diameter in cm | 4.32 ± 1.38 | 3.91 ± 1.27 | 1.621 | 0.108 | |
| Number of liver lesions | Single | 19 (34.5) | 47 (85.5) | 29.697 | 0.000 |
| Multiple | 36 (65.5) | 8 (14.5) | |||
| Lesion property | Solid | 50 (90.9) | 47 (85.5) | 0.785 | 0.376 |
| Cyst | 5 (9.1) | 8 (14.5) | |||
| Boundary | Clear | 29 (52.7) | 40 (72.7) | 4.705 | 0.030 |
| Unclear | 26 (47.3) | 15 (27.3) | |||
| Echo level | High | 28 (50.9) | 13 (23.6) | 9.498 | 0.023 |
| Low | 17 (30.9) | 24 (43.6) | |||
| Mixed | 10 (18.2) | 17 (30.9) | |||
| Equal | 0 (0.0) | 1 (1.8) | |||
| Echo uniformity | Uniform | 39 (70.9) | 26 (47.3) | 6.356 | 0.012 |
| Non-uniform | 16 (29.1) | 29 (52.7) | |||
| Posterior echo attenuation | Yes | 6 (10.9) | 2 (3.6) | - | 0.2711 |
| No | 49 (89.1) | 53 (96.4) | |||
| Peripheral acoustic halo | Yes | 13 (23.6) | 27 (49.1) | 7.700 | 0.006 |
| No | 42 (76.4) | 28 (50.9) | |||
The initial enhancement time was similar in the hNEN and HCC groups, and the difference was not statistically significant (P > 0.05). The washout to iso-enhancement time and washout to hypo-enhancement time in the hNEN group were lower than those in the HCC group. The differences were statistically significant (P < 0.05).
The proportions of different CEUS enhancement characteristics, including enhancement at arterial phase, portal venous phase, and late phase, enhancement-washout mode, enhancement form, tumor vasculature, tumor necrosis, and capsule enhancement were similar in the two groups, and the differences were not statistically significant (P > 0.05; Table 2).
| Contrast-enhanced Ultrasound characteristics | hNEN group, n = 55 | HCC group, n = 55 | t/χ2 | P value | |
| Initial enhancement time in s | 16.23 ± 5.29 | 16.52 ± 5.17 | 0.291 | 0.772 | |
| Washout to iso-enhancement time in s | 26.91 ± 15.39 | 47.26 ± 16.84 | 6.615 | 0.000 | |
| Washout to hypo-enhancement time in s | 59.84 ± 37.91 | 99.63 ± 61.82 | 5.092 | 0.000 | |
| Enhancement level at arterial phase | High | 53 (96.4) | 55 (100) | 2.037 | 0.154 |
| Equal | 2 (3.6) | 0 (0) | |||
| Low | 0 (0) | 0 (0) | |||
| Enhancement level at portal venous phase | Equal | 7 (12.7) | 11 (20.0) | 1.063 | 0.303 |
| Low | 48 (87.3) | 44 (80.0) | |||
| Enhancement level at late phase | Equal | 2 (3.6) | 1 (1.8) | 0.343 | 0.558 |
| Low | 53 (96.4) | 54 (98.2) | |||
| Enhancement forms | Fast forward and fast out | 53 (96.4) | 51 (92.8) | 0.705 | 0.401 |
| Equal/slow forward and fast out | 2 (3.6) | 4 (7.2) | |||
| Enhancement forms | Uniform | 33 (60) | 39 (70.9) | 1.447 | 0.229 |
| Non-uniform | 22 (40) | 16 (29.1) | |||
| Tumor vasculature | Yes | 34 (61.8) | 38 (69.1) | 0.643 | 0.423 |
| No | 21 (38.2) | 17 (30.9) | |||
| Tumor necrosis | Yes | 16 (29.1) | 19 (34.5) | 0.377 | 0.539 |
| No | 39 (70.9) | 36 (65.5) | |||
| Capsule enhancement | Yes | 7 (12.7) | 2 (3.6) | 3.025 | 0.082 |
| No | 48 (87.3) | 53 (96.4) | |||
The lesion diameter in hNEN lesions transferred from the gastrointestinal tract, pancreas, and other sites was similar, and there was no statistical significance (P > 0.05). In addition, the proportions of hNEN B-mode ultrasound characteristics, including number of liver lesions, lesion property, boundary, echo level, echo uniformity, posterior echo attenuation, and peripheral acoustic halo, transferred from different sources were similar, and the differences were not statistically significant (P > 0.05; Table 3).
| B-mode Ultrasound characteristics | gastrointestinal tract, n = 20 | Pancreas, n = 29 | Other sites, n = 6 | F/χ2 | P value | |
| Diameter in cm | 3.24 ± 1.96 | 2.98 ± 1.95 | 3.41 ± 2.06 | 1.772 | 0.163 | |
| Number of liver lesions | Single | 7 (35) | 10 (34.5) | 2 (33.3) | - | 1.0001 |
| Multiple | 13 (65) | 19 (65.5) | 4 (66.7) | |||
| Lesion property | Solid | 19 (95.0) | 25 (86.0) | 6 (100.0) | 1.781 | 0.410 |
| Cyst | 1 (5.0) | 4 (13.8) | 0 (0) | |||
| Boundary | Clear | 10 (50.0) | 15 (51.7) | 4 (66.47) | - | 0.8561 |
| Unclear | 10 (50.0) | 14(48.3) | 2 (16.7) | |||
| Echo level | High | 6 (30.0) | 17 (58.6) | 4 (66.7) | - | 0.2281 |
| Low | 10 (50.0) | 7 (24.1) | 1 (33.3) | |||
| Mixed | 4 (20.0) | 5 (17.2) | 1 (33.3) | |||
| Equal | 0 (0) | 0 (0) | 0 (0) | |||
| Echo uniformity | Uniform | 15 (75.0) | 20 (69.0) | 4 (66.7) | - | 0.9161 |
| Non-uniform | 5 (25.0) | 9 (31.0) | 2 (33.3) | |||
| Posterior echo attenuation | Yes | 2 (10.0) | 4 (13.8) | 0 (0) | - | 1.0001 |
| No | 18 (90.0) | 25 (86.2) | 6 (100.0) | |||
| Peripheral acoustic halo | Yes | 16 (80.0) | 21 (72.4) | 5 (83.3) | - | 0.9001 |
| No | 4 (20.0) | 8 (27.6) | 1 (16.7) | |||
The initial enhancement time, washout to iso-enhancement time, and washout to hypo-enhancement time of hNEN transferred from the gastrointestinal tract, pancreas, and other sites were similar. The differences were not statistically significant (P > 0.05). The proportions of CEUS enhancement characteristics transferred from different sources of hNEN, including enhancement at arterial phase, portal venous phase and late phase, enhancement-washout mode, enhancement form, tumor vasculature, tumor necrosis, and capsule enhancement, were similar, and the differences were not statistically significant (P > 0.05; Table 4).
| Contrast-enhanced ultrasound characteristics | Gastrointestinal tract, n = 20 | Pancreas, n = 29 | Other sites, n = 6 | t/χ2 | P value | |
| Initial enhancement time in s | 16.28 ± 5.82 | 16.83 ± 6.16 | 15.22 ± 4.92 | 1.305 | 0.372 | |
| Washout to iso-enhancement time in s | 28.82 ± 12.38 | 27.29 ± 14.92 | 2 1.83 ± 11.23 | 0.924 | 0.477 | |
| Washout to hypo-enhancement time in s | 64.93 ± 36.29 | 55.28 ± 31.83 | 58.21 ± 29.65 | 0.874 | 0.592 | |
| Enhancement level at arterial phase | High | 19 (95.0) | 28 (96.6) | 6 (100.0) | 0.335 | 0.846 |
| Equal | 1 (5.0) | 1 (3.4) | 0 (0) | |||
| Low | 0 (0) | 0 (0) | 0 (0) | |||
| Enhancement level at portal venous phase | Equal | 2 (10.0) | 5 (17.2) | 0 (0) | - | 0.6101 |
| Low | 18 (90.0) | 24 (82.8) | 6 (100.0) | |||
| Enhancement level at late phase | Equal | 1(5.0) | 1(3.4) | 0 (0) | - | 1.0001 |
| Low | 19 (95.0) | 28 (96.6) | 6 (100.0) | |||
| Enhancement forms | Fast forward and fast out | 19 (95.0) | 28 (96.6) | 6 (100.0) | - | 1.0001 |
| Equal/slow forward and fast out | 1 (5.0) | 1 (3.4) | 0 (0) | |||
| Enhancement forms | Uniform | 13 (65.0) | 16 (55.2) | 4 (66.7) | - | 0.7291 |
| Non-uniform | 7 (35.0) | 13 (44.8) | 2 (33.3) | |||
| Tumor vasculature | Yes | 14 (70.0) | 17 (58.6) | 3 (50.0) | - | 0.6671 |
| No | 6 (30.0) | 12 (41.4) | 3 (50.0) | |||
| Tumor necrosis | Yes | 4 (20.0) | 10 (34.5) | 2 (33.3) | - | 0.5691 |
| No | 16 (80.0) | 19 (65.5) | 4 (66.7) | |||
| Capsule enhancement | Yes | 2 (10.0) | 4 (13.8) | 1 (16.7) | - | 1.0001 |
| No | 18 (90.0) | 25 (86.2) | 5 (83.3) | |||
The difference in lesion diameter between hNET and hNEC groups was not statistically significant (P > 0.05). The proportions of B-mode ultrasound features, including number of liver lesions, lesion property, boundary, echo level, echo uniformity, posterior echo attenuation, and peripheral acoustic halo, between hNET and hNEC groups were similar, and the differences were not statistically significant (P > 0.05; Table 5).
| B-mode Ultrasound characteristics | hNET group, n = 35 | hNEC group, n = 20 | t/χ2 | P value | |
| Diameter in cm | 4.58 ± 2.91 | 5.08 ± 3.87 | 0.543 | 0.590 | |
| Number of liver lesions | Single | 12 (34.3) | 7 (35.0) | 0.003 | 0.957 |
| Multiple | 23 (65.7) | 13 (65.0) | |||
| Lesion property | Solid | 31 (88.6) | 19 (95.0) | 0.636 | 0.425 |
| Cyst | 4 (11.4) | 1 (5.0) | |||
| Boundary | Clear | 18 (51.4) | 11 (55.0) | 0.065 | 0.799 |
| Unclear | 17 (48.6) | 9 (45.0) | |||
| Echo level | High | 19 (54.3) | 9 (45.0) | 0.443 | 0.801 |
| Low | 10 (28.6) | 7 (35.0) | |||
| Mixed | 6 (17.1) | 4 (20.0) | |||
| Equal | 0 (0) | 0 (0) | |||
| Echo uniformity | Uniform | 25 (71.4) | 14 (70.0) | 0.013 | 0.911 |
| Non-uniform | 10 (28.6) | 6 (30.0) | |||
| Posterior echo attenuation | Yes | 5 (14.3) | 1 (5.0) | - | 0.3991 |
| No | 30 (85.7) | 19 (95.0) | |||
| Peripheral acoustic halo | Yes | 9 (25.7) | 4 (20.0) | - | 0.7491 |
| No | 26 (74.3) | 16 (80.0) | |||
There was no significant difference between hNEN and hNEC groups in terms of initial enhancement time, washout to iso-enhancement time, and washout to hypo-enhancement time (P > 0.05). Among the CEUS enhancement characteristics, the proportions of low enhancement at portal venous phase, non-uniform enhancement forms, and no tumor vasculature in the hNEC group were greater than those in the hNEN group (P < 0.05). The remaining CEUS enhancement characteristics, including the proportions of enhancement at arterial phase, enhancement at late phase, tumor necrosis, and capsule enhancement, were similar between the two groups. The differences were not statistically significant (P > 0.05; Table 6).
| Contrast-enhanced Ultrasound characteristics | hNET group, n = 35 | hNEC group, n = 20 | t/χ2 | P value | |
| Initial enhancement time in s | 16.83 ± 5.08 | 16.28 ± 4.93 | 0.834 | 0.854 | |
| Washout to iso-enhancement time in s | 30.84 ± 10.38 | 27.68 ± 9.74 | 1.856 | 0.804 | |
| Washout to hypo-enhancement time in s | 65.28 ± 37.84 | 51.72 ± 31.85 | 1.152 | 0.833 | |
| Enhancement level at arterial phase | High | 33 (94.3) | 20 (100.0) | 1.186 | 0.276 |
| Equal | 2 (5.7) | 0 (0) | |||
| Low | 0 (0) | 0 (0) | |||
| Enhancement level at portal venous phase | Equal | 7 (20.0) | 0 (0) | 4.583 | 0.032 |
| Low | 28 (80.0) | 20 (100.0) | |||
| Enhancement level at late phase | Equal | 1 (2.9) | 1 (5.0) | - | 1.0001 |
| Low | 34 (97.1) | 19 (95.0) | |||
| Enhancement forms | Fast forward and fast out | 34 (97.1) | 19 (95.0) | - | 1.0001 |
| Equal/slow forward and fast out | 1 (2.9) | 1 (5.0) | |||
| Enhancement forms | Uniform | 28 (80.0) | 5 (25.0) | 16.042 | 0.000 |
| Non-uniform | 7 (20.0) | 15 (75.0) | |||
| Tumor vasculature | Yes | 15 (42.9) | 19 (95.0) | 14.661 | 0.000 |
| No | 20 (57.1) | 1 (5.0) | |||
| Tumor necrosis | Yes | 10 (28.6) | 6 (30.0) | 0.013 | 0.911 |
| No | 25 (71.4) | 14 (70.0) | |||
| Capsule enhancement | Yes | 6 (17.1) | 1 (5.0) | - | 0.4021 |
| No | 29 (82.9) | 19 (95.0) | |||
Imaging examination plays an important role in tumor discovery, auxiliary diagnosis, treatment, and follow-up. B-mode ultrasound and CEUS are widely used in clinical practice as non-invasive and simple imaging methods. However, due to the rareness of hNEN, there is currently little experience in imaging diagnosis of hNEN, which may result in clinicians not being able to obtain correct imaging results for hNEN, thus affecting the diagnosis and treatment of hNEN. Therefore, the present study first compared the B-mode ultrasound and CEUS performance between hNEN and HCC. Then, we compared the B-mode ultrasound and CEUS characteristics of different sources of hNEN and different malignant degrees of hNEN in order to report clinical diagnostic experience for hNEN.
Recent studies have reported that the characteristics of hNEN B-mode ultrasound are uniform hyperechoic or hypoechoic masses with clear boundaries[12,13]. Most of hNEN CEUS characteristics are "fast forward and fast out"[14]. Centripetal enhancement at the arterial phase appears first, and then uniform high enhancement appears[15]. The characteristics of HCC B-mode ultrasound are hypoechoic or mixed echo masses with clear boundaries[16]. The CEUS characteristics are “fast forward and fast out” as well. But most of the CEUS characteristics of HCC showed uniform high enhancement at the arterial phase[17-19]. In this study, there was no significant difference in lesion size and the proportions of different lesion property and posterior echo attenuation in the comparison of B-mode ultrasound and CEUS results between hNEN and HCC groups. The possible reason is that both hNEN and HCC are solid and blood-rich tumors[20]. They have similar characteristics in B-ultrasound signs and enhancement features. However, the proportions of multiple liver lesion, unclear border, and high echo lesion in the hNEN group were higher than those in the HCC group. The proportions of non-uniform echo and peripheral acoustic halo in the hNEN group were lower than those in the HCC group. It has been suggested that if the liver lesions found in the ultrasound examination are multiple, uniform high echo, and without peripheral acoustic halo, it may be hNEN. Further examination should be performed to determine if there are extrahepatic lesions.
In the comparison of CEUS results, the initial enhancement time was similar between the hNEN and HCC groups, but the washout to iso-enhancement time and washout to hypo-enhancement time in the hNEN group were lower than those in the HCC group. These findings indicated that the washout time in hNEN was earlier than that in HCC. The possible reason is that hNENs are transferred from different sources. The blood flow supply composition is different, which results in a different washout time than HCC[21,22]. In addition, the proportions of CEUS characteristics, including enhancement of arterial phase, portal venous phase, and enhancement of late phase, enhancement forms, tumor vasculature, tumor necrosis, and capsule enhancement, were similar in the hNEN and HCC groups. Because the CEUS enhancement features of hNEN and HCC are similar, it is difficult to distinguish clinically. It is necessary to pay special attention to the difference of contrast agent washout time between hNEN and HCC.
Therefore, this study suggests two points in the ultrasound examination: (1) Intra-hepatic lesions are multiple, uniform, and high echo and without peripheral acoustic halo; and (2) In the CEUS performance of intrahepatic lesions, the uniform high enhancement at arterial phase was found, and the washout is rapid. The diagnosis of hNEN needs to be considered.
hNEN can be transferred from multiple sites, including the pancreas, gastrointestinal tract, liver, lungs, adrenal glands, etc. Gastroenteropancreatic NENs are the main source of hNEN[23]. Previous studies have revealed that although the treatment of hNEN is surgery, the efficacy and 5-year survival of different sources of hNEN are different[24-26]. The survival time of hNEN patients from the gastrointestinal tract is significantly longer than that of hNEN patients from the pancreas[27]. Ablation, embolism, and liver transplantation have different effects on hNEN from different sources[28,29]. In addition, some patients with hNEN need to undergo surgery again to remove the primary lesion because they have misjudged the source of hNEN before surgery[30-33]. Therefore, predicting the possible primary site of hNEN is important in guiding the patient's examination, such as finding the extrahepatic primary tumor and the treatment plan. This study analyzed B-mode ultrasound and CEUS results of hNENs from the gastrointestinal tract, pancreas, and other sites. We found there was no significant difference in B-mode ultrasound and CEUS characteristics of hNEN from different sources. All of them were mainly multiple hyperechoic lesions, and the CEUS showed uniformly high enhancement at arterial phase and rapid washout. This indicated that it is difficult to identify hNEN from different sources only by ultrasound. Therefore, this study suggests that when hNEN is suspected to be a metastatic tumor, the pathological examination should be performed to clarify the primary lesion to prevent missed diagnosis.
All hNENs have malignant potential[34-37], and hNEN can be divided into poorly differentiated hNEC (G3 grade) and highly differentiated hNET (G1 and G2 grade) according to its degree of differentiation[38-40]. Most hNET patients require local therapy, and most hNEC patients require systemic therapy[41-43]. Therefore, accurate identification of hNEC and hNET has great significance for clinical treatment of patients. In this study, the characteristics of B-mode ultrasound of hNEC and hNET groups were compared. It was found that both hNEC and hNET groups showed multiple solid lesions, uniform and high echo, no posterior echo attenuation, and peripheral acoustic halo. The difference was not obvious. When comparing CEUS features, it was found that the initial enhancement time, washout to iso-enhancement time, and washout to hypo-enhancement time were similar between the two groups.
The proportions of enhancement at arterial phase, enhancement at late phase, tumor necrosis, and capsule enhancement were similar as well. However, there were differences in the enhancement level at the portal vein phase. It was low enhancement in the hNEC group, while some of the hNETs showed partial equal enhancement. In addition, the proportion of combined tumor vasculature in the hNEC group was larger than that in the hNET group. The possible reason is that hNEC is mainly supplied by arteries, and washout is fast at the portal venous phase. Compared with hNEC, hNET has more portal blood supply, which leads to equal enhancement at the portal venous phase. This is consistent with the biological behavior and malignancy of the tumor[44]. It also explains to some extent why hNEC has a low enhancement level in the portal venous phase and a large proportion of tumor blood vessels[45,46]. In addition, compared with hNET, hNEC has more non-uniform enhancement form at the portal venous phase, probably because hNEC is more prone to cystic lesions, resulting in non-uniform enhancement in CEUS[15,47,48]. Therefore, when the CEUS result of hNEN is equal enhancement at the portal venous phase and uniform enhancement form, hNET can be considered. If there is low enhancement at the portal venous phase, non-uniform enhancement form, and combined tumor vasculature, hNEC should be highly suspected. Further medical treatment measures should be taken.
Because patients with hNEN are rare, there are currently few targeted studies about hNEN. The number of patients recruited in this study was limited. Patients with primary hNEN were not included in this study. There are further research plans to conduct a multi-center study to collect detailed data from hNEN patients to make the results more comprehensive.
In summary, this study compared the ultrasound characteristics between hNEN and HCC and among hNENs from different sources and malignant degrees. We found that compared with HCC, hNEN showed multiple intrahepatic lesions, uniform high echo, uniform high enhancement at the arterial phase, and rapid washout. The ultrasound characteristics of hNENs from different sources were similar. The low enhancement at portal venous phase, overall non-uniform enhancement form, and the proportion of combined tumor vasculature in hNEC were larger than those of hNET, indicating that hNEC and hNET can be initially identified based on CEUS results.
Hepatic neuroendocrine neoplasm (hNEN) is a rare tumor clinically. It is important to identify the source and malignant degree of hNEN and distinguish it from hepatocellular carcinoma (HCC). Imaging examination is required for the initial screening of hNEN. However, there is a lack of data regarding imaging diagnosis of hNEN.
Because of the lack of imaging examination experience, the screening and identification of hNEN is difficult. Research has revealed that there are some differences among hNEN with different sources and malignant degrees screened by ultrasound and contrast-enhanced ultrasound (CEUS). By analyzing the characteristics of ultrasound and CEUS, our study hopes to provide more helpful information in the diagnosis of hNEN.
In this study, the ultrasound performance between hNEN and HCC and data of hNEN with different sources and malignant degrees were compared. The purpose of this study was to improve the accuracy of the identification of hNEN and provide useful information for its clinical diagnosis.
A total of 55 patients with hNEN were recruited, the hNEN group. There were 35 cases in the hepatic neuroendocrine tumor (hNET) group, and 20 cases in the neuroendocrine carcinoma (hNEC) group. About 55 patients with HCC were recruited as the HCC group. The characteristic differences of B-mode ultrasound and CEUS between hNEN and HCC, hNEN from different sources, and between hNEC and hNET were compared and analyzed.
Compared with the HCC group, the proportions of multiple liver lesions, unclear borders, and high echo lesions were higher and the proportions of non-uniform echo and peripheral acoustic halo were lower in the hNEN group. In the NEN group, the washout to iso-enhancement time and washout to hypo-enhancement time were lower than those of the HCC group. The proportion of low enhancement of portal venous phase, non-uniform enhancement forms, and combined tumor vasculature in the hNEC group was greater than that in the hNEN group.
Compared with HCC, the ultrasound performance of hNEN showed more intrahepatic lesions, uniform high echo, uniform high enhancement at arterial phase, and rapid washout. Compared with hNET, the CEUS characteristics of hNEC are low enhancement of portal venous phase, non-uniform enhancement forms, and combined tumor vasculature.
To expand this research, future studies should include more hospitals in order to collect detailed data from more hNEN patients. The ultrasound results of primary hNEN also need to be analyzed further to provide stronger evidence for clinical diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Snyder J, Tatsuya O, Yukihiko T S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with review of the literature. World J Gastroenterol. 2015;21:3132-3138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tamburrino D, Spoletini G, Partelli S, Muffatti F, Adamenko O, Crippa S, Falconi M. Surgical management of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2016;30:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ortiz J, Balasubramanian M, Brown T, Cetrulo L. Liver transplant for neuroendocrine tumor metastatic to the liver: literature review and report of extirpation at 16-year recurrence. Exp Clin Transplant. 2015;13:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Haug AR, Cindea-Drimus R, Auernhammer CJ, Reincke M, Beuschlein F, Wängler B, Uebleis C, Schmidt GP, Spitzweg C, Bartenstein P, Hacker M. Neuroendocrine tumor recurrence: diagnosis with 68Ga-DOTATATE PET/CT. Radiology. 2014;270:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Wang LM, An SL, Wu JX. Diagnosis and therapy of primary hepatic neuroendocrine carcinoma: clinical analysis of 10 cases. Asian Pac J Cancer Prev. 2014;15:2541-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Zhao ZM, Wang J, Ugwuowo UC, Wang L, Townsend JP. Primary hepatic neuroendocrine carcinoma: report of two cases and literature review. BMC Clin Pathol. 2018;18:3. [PubMed] |
| 7. | Beard RE, Finkelstein SD, Borhani AA, Minervini MI, Marsh JW. A massive hepatic tumor demonstrating hepatocellular, cholangiocarcinoma and neuroendocrine lineages: A case report and review of the literature. Int J Surg Case Rep. 2017;37:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Manta R, Nardi E, Pagano N, Ricci C, Sica M, Castellani D, Bertani H, Piccoli M, Mullineris B, Tringali A, Marini F, Germani U, Villanacci V, Casadei R, Mutignani M, Conigliaro R, Bassotti G, Zullo A. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J Gastrointestin Liver Dis. 2016;25:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Hoeffel C, Job L, Ladam-Marcus V, Vitry F, Cadiot G, Marcus C. Detection of hepatic metastases from carcinoid tumor: prospective evaluation of contrast-enhanced ultrasonography. Dig Dis Sci. 2009;54:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20:3590-3596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Bosman FT. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer 2010; . |
| 12. | Nomura Y, Nakashima O, Akiba J, Ogasawara S, Fukutomi S, Yamaguchi R, Kusano H, Kage M, Okuda K, Yano H. Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver. J Clin Pathol. 2017;70:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Sahu S, Schernthaner R, Ardon R, Chapiro J, Zhao Y, Sohn JH, Fleckenstein F, Lin M, Geschwind JF, Duran R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology. 2017;283:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma KS, Guo DY, Ding SY. Primary hepatic neuroendocrine tumors: clinical characteristics and imaging features on contrast-enhanced ultrasound and computed tomography. Abdom Radiol (NY). 2016;41:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Li W, Zhuang BW, Wang Z, Liao B, Hong LY, Xu M, Lin XN, Xie XY, Lu MD, Chen LD, Wang W. Case Report of Contrast-Enhanced Ultrasound Features of Primary Hepatic Neuroendocrine Tumor: A CARE-Compliant Article. Medicine (Baltimore). 2016;95:e3450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Lekht I, Nayyar M, Luu B, Guichet PL, Ho J, Ter-Oganesyan R, Katz M, Gulati M. Intra-arterial contrast-enhanced ultrasound (IA CEUS) for localization of hepatocellular carcinoma (HCC) supply during transarterial chemoembolization (TACE): a case series. Abdom Radiol (NY). 2017;42:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord. 2014;14:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist. 2016;21:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Valadares LJ, Costa Junior W, Ribeiro HS, Diniz AL, Coimbra FJ, Herman P. Resection of liver metastasis from neuroendocrine tumors: evaluation of results and prognostic factors. Rev Col Bras Cir. 2015;42:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Watson GA, Ahmed Y, Picardo S, Chew S, Cobbe S, Mahony C, Crotty J, Wallis F, Shelly MJ, Kiely P, Ipadeola OB, Healy V, Osman N, Gupta RK. Unusual Sites of High-Grade Neuroendocrine Carcinomas: A Case Series and Review of the Literature. Am J Case Rep. 2018;19:710-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Fiore F, Del Prete M, Franco R, Marotta V, Ramundo V, Marciello F, Di Sarno A, Carratù AC, de Luca di Roseto C, Colao A, Faggiano A. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Memon K, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, Gates VL, Atassi B, Newman S, Omary RA, Benson AB, Salem R. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | de Herder WW, Mazzaferro V, Tavecchio L, Wiedenmann B. Multidisciplinary approach for the treatment of neuroendocrine tumors. Tumori. 2010;96:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Shin Y, Ha SY, Hyeon J, Lee B, Lee J, Jang KT, Kim KM, Park YS, Park CK. Gastroenteropancreatic Neuroendocrine Tumors with Liver Metastases in Korea: A Clinicopathological Analysis of 72 Cases in a Single Institute. Cancer Res Treat. 2015;47:738-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Iwasaki M, Tsuchida K, Jinnai H, Komatsubara T, Arisaka T, Tsunemi M, Nakano M, Iijima M, Hiraishi H. Multimodal Treatment of Vasoactive Intestinal Polypeptide-producing Pancreatic Neuroendocrine Tumors with Liver Metastases. Intern Med. 2017;56:517-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Pusceddu S, Femia D, Lo Russo G, Ortolani S, Milione M, Maccauro M, Vernieri C, Prinzi N, Concas L, Leuzzi L, De Braud F, Buzzoni R. Update on medical treatment of small intestinal neuroendocrine tumors. Expert Rev Anticancer Ther. 2016;16:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | de Baere T, Deschamps F, Tselikas L, Ducreux M, Planchard D, Pearson E, Berdelou A, Leboulleux S, Elias D, Baudin E. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. Eur J Endocrinol. 2015;172:R151-R166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Geramizadeh B, Kashkooe A, Malekhosseini SA. Liver Metastasis of Gastrointestinal Neuroendocrine Tumors: A Single Center Experience. Hepat Mon. 2016;16:e37293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, Zamboni G, Weichert W, Pfarr N, Klöppel G. Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018;77:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Cavalcoli F, Rausa E, Conte D, Nicolini AF, Massironi S. Is there still a role for the hepatic locoregional treatment of metastatic neuroendocrine tumors in the era of systemic targeted therapies? World J Gastroenterol. 2017;23:2640-2650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Tanaka H, Matsusaki S, Baba Y, Isono Y, Kumazawa H, Sase T, Okano H, Saito T, Mukai K, Kaneko H. Neuroendocrine tumor G3: a pancreatic well-differentiated neuroendocrine tumor with a high proliferative rate. Clin J Gastroenterol. 2015;8:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Rinke A, Gress TM. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion. 2017;95:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 758] [Article Influence: 47.4] [Reference Citation Analysis (2)] |
| 37. | Engelman ES, Leon-Ferre R, Naraev BG, Sharma N, Sun S, O'Dorisio TM, Howe J, Button A, Zamba G, Halfdanarson TR. Comparison of transarterial liver-directed therapies for low-grade metastatic neuroendocrine tumors in a single institution. Pancreas. 2014;43:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Wang LX, Liu K, Lin GW, Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Chen JX, Rose S, White SB, El-Haddad G, Fidelman N, Yarmohammadi H, Hwang W, Sze DY, Kothary N, Stashek K, Wileyto EP, Salem R, Metz DC, Soulen MC. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc Intervent Radiol. 2017;40:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 41. | Hijioka S, Hosoda W, Mizuno N, Hara K, Imaoka H, Bhatia V, Mekky MA, Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutumi H, Fujiyoshi T, Sato T, Hieda N, Yoshida T, Okuno N, Shimizu Y, Yatabe Y, Niwa Y, Yamao K. Does the WHO 2010 classification of pancreatic neuroendocrine neoplasms accurately characterize pancreatic neuroendocrine carcinomas? J Gastroenterol. 2015;50:564-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D, Walter T; other Knowledge Network members. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 43. | Chung H, Chapman WC. Liver transplantation for metastatic neuroendocrine tumors. Adv Surg. 2014;48:235-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Schaefer B, Zoller H, Schneeberger S. Con: Liver transplantation for expanded criteria malignant diseases. Liver Transpl. 2018;24:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Rossi RE, Burroughs AK, Caplin ME. Liver transplantation for unresectable neuroendocrine tumor liver metastases. Ann Surg Oncol. 2014;21:2398-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Shen YH, Chen S, Zhang WT, Ji Y, Yu L, Sun HC, Qiu SJ, Ren N, Zhou J. Clinical analysis of gastroenteropancreatic neuroendocrine tumor with liver metastasis, compared with primary hepatic neuroendocrine tumor. J Cancer Res Ther. 2014;10 Suppl:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Mazzaferro V, Sposito C, Coppa J, Miceli R, Bhoori S, Bongini M, Camerini T, Milione M, Regalia E, Spreafico C, Gangeri L, Buzzoni R, de Braud FG, De Feo T, Mariani L. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant. 2016;16:2892-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 48. | Giesel FL, Wulfert S, Zechmann CM, Haberkorn U, Kratochwil C, Flechsig P, Kuder T, Schwartz LH, Bruchertseifer F. Contrast-enhanced ultrasound monitoring of perfusion changes in hepatic neuroendocrine metastases after systemic versus selective arterial 177Lu/90Y-DOTATOC and 213Bi-DOTATOC radiopeptide therapy. Exp Oncol. 2013;35:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |