Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.404
Peer-review started: January 16, 2019
First decision: March 14, 2019
Revised: March 29, 2019
Accepted: April 19, 2019
Article in press: April 19, 2019
Published online: May 15, 2019
Processing time: 119 Days and 17.9 Hours
Pathological manifestations of hepatic tumours are often associated with prognosis. Although surgical specimens (SS) can provide more information, currently, pre-treatment needle core biopsy (NCB) is increasingly showing important value in understanding the nature of liver tumors and even in diagnosis and treatment decisions. However, the concordance of the clinicopathological characteristics and immunohistochemical (IHC) staining between NCB and SS from patients with hepatic tumours were less concerned.
To introduce a more accurate method for interpreting the IHC staining results in order to improve the diagnostic value of hepatic malignancy in NCB samples.
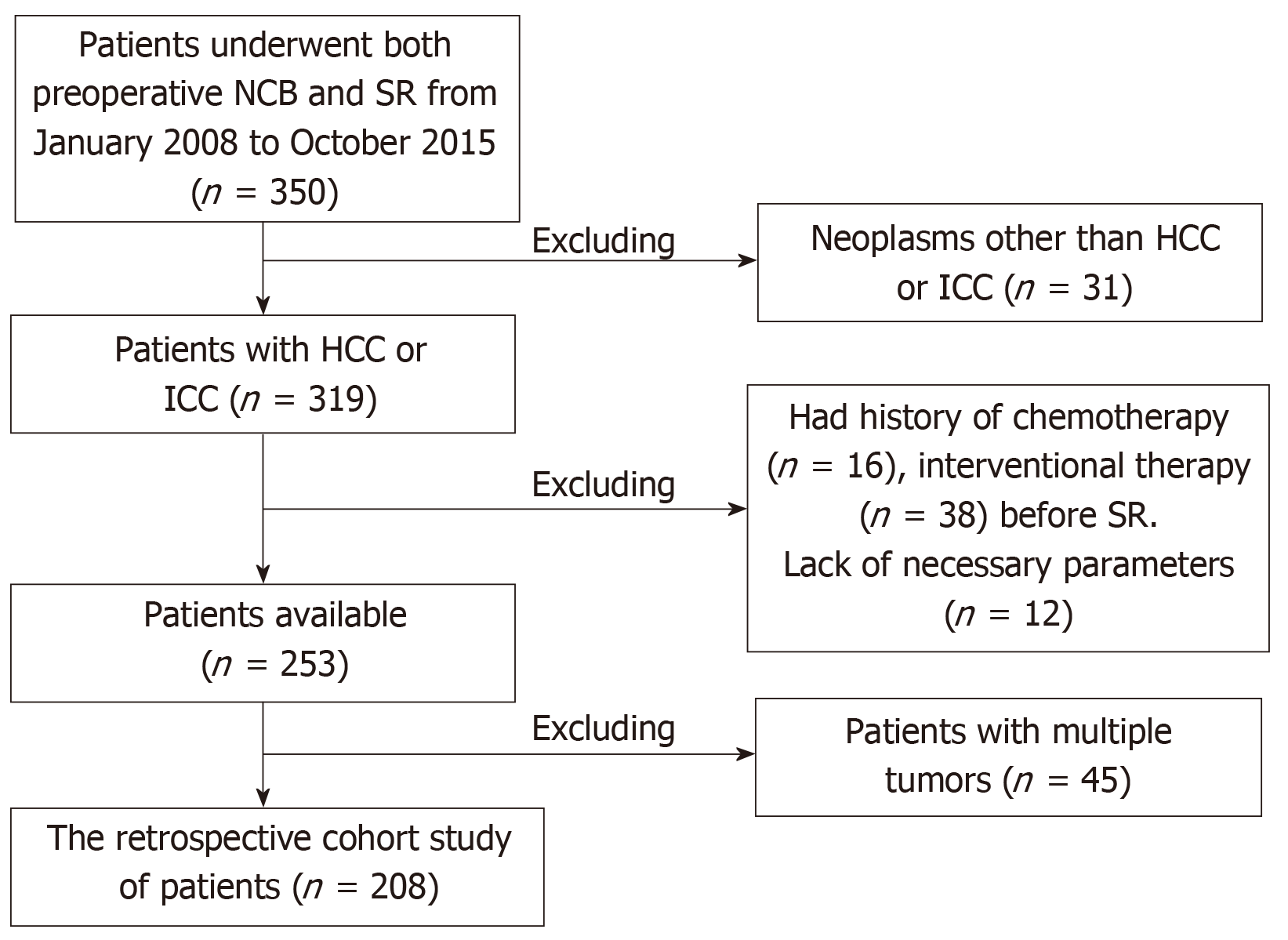
A total of 208 patients who underwent both preoperative NCB and surgical resection for hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC) between 2008 and 2015 were enrolled in this study. The expression of CK19, GPC3, and HepPar1 were detected by IHC staining. Clinicopathological, NCB, and surgical data were collected and analysed using χ2 and kappa statistics.
Morphologically, the presence of compact tumour nests or a cord-like structure in NCB was considered the primary cause of misdiagnosis of HCC from ICC. The kappa statistic showed a moderate agreement in histomorphology (k = 0.504) and histological grade (k = 0.488) between NCB and SS of the tumours. A 4-tier (+++, ++, +, and -) scoring scheme that emphasized the focal neoplastic cell immunoreactivity of tumour cells revealed perfect concordance of CK19, GPC3 and HepPar1 between NCB and SS (k = 0.717; k = 0.768; k = 0.633). Furthermore, with the aid of a binary classification derived from the 4-tier score, a high concordance was achieved in interpreting the IHC staining of the three markers between NCB and final SS (k = 0.931; k = 0.907; k = 0.803), increasing the accuracy of NCB diagnosis C (k = 0.987; area under the curve = 0.997, 95%CI: 0.990-1.000; P < 0.001).
These findings imply that reasonable interpretation of IHC results in NCB is vital for improving the accuracy of tumour diagnosis. The simplified binary classification provides an easy and applicable approach.
Core tip: Pathological manifestations of hepatic tumours are often associated with prognosis. The present study was designed to evaluate the concordance of the clinicopathological characteristics and staining of three biomarker between the needle core biopsy (NCB) and surgery specimen from patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma. Our results indicated that reasonable interpretation of immunohistochemical staining results in NCB is vital for improving the accuracy of tumour diagnosis. The simplified binary classification provides an easy and applicable approach to improve the diagnostic value of hepatic malignancy in NCB samples.
- Citation: Wu JS, Feng JL, Zhu RD, Liu SG, Zhao DW, Li N. Histopathological characteristics of needle core biopsy and surgical specimens from patients with solitary hepatocellular carcinoma or intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2019; 11(5): 404-415
- URL: https://www.wjgnet.com/1948-5204/full/v11/i5/404.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i5.404
Liver malignancies including hepatocellular carcinoma (HCC) arising from hepatocytes and cholangiocarcinoma (CCA) arising from epithelial cells of the bile ducts are the fifth most commonly diagnosed tumours and the second most frequent cause of cancer-related deaths in males worldwide[1,2]. The mostly accepted view is that HCC and CCA are different diseases and have relatively independent characteristics in diseased populations and underlying diseases. In recent years, and perhaps until now, we have had somewhat naïve hopes of fitting the complex disease into a tidy and easily understood model. Currently, we classify malignancies mainly according to the anatomical site of the tumour, but the diagnosis of CCA is clinically difficult to identify with HCC, especially in tumour tissues obtained by needle core biopsy (NCB), which involves the combination of a variety of methods including imaging and biopsy pathology as well as Immunohistochemistry (IHC) techniques[3,4] based both on surgical specimens (SS) and NCB. To date, unfortunately, most CCA patients are usually diagnosed at a late stage of the disease. The overall 5-year survival of CCA is poor, and there is no sufficiently sensitive and specific biomarker available to facilitate the diagnosis or predict the effect of therapy.
With the in-depth understanding of the nature of this disease, simple anatomical division has not been able to explain the clinical manifestations of liver malignancies, and perhaps the whole course of the clinical manifestation has been determined at the “origin” of the disease. International efforts of clinicians and scientists are helping to identify the genetic drivers of liver malignancy progression, which will unveil early diagnostic markers and direct the development of individualized therapies. Researchers must also face unprecedented challenges to distinguish the true genetic driver changes that are critical for tumour development to identify the most promising therapeutic target for liver malignancies.
Some novel diagnostic biomarkers, including cytokeratin-19 (CK19), glypican-3 (GPC3), and hepatocyte paraffin-1 (HepPar1), are considering to be related to the prognosis of patients with PCL as predictive markers based on pathological sections[5-8], which indicated that combining NCB, IHC, and imaging technology might help provide suggestions concerning patients’ medical treatment selection and optimize therapeutic strategies at the time of diagnosis[9-12]. The purpose of this study was to compare the pathological characteristics of SS and NCB and attempt to improve the diagnostic rate of puncture specimens by immunohistochemical (IHC) scoring. It is hoped that our scoring system can be used in the future to facilitate early and accurate diagnoses in more patients.
The current study was conducted in accordance with the Declaration of Helsinki. All patients provided written, informed consent. The study was approved by the Local Ethics Committee. Between January 2008 and October 2015, 350 patients who underwent both preoperative NCB and surgical resection at Beijing You-An Hospital, Capital Medical University (163/350), Xijing Hospital, Fourth Military Medical University (116/350) and The Second Hospital, Hebei Medical University (71/350) were enrolled. All authors had evaluated original data and approved the final manuscript.208 patients were enrolled in the cohort for the present study (Figure 1).
NCBs were performed under local anaesthesia with 2% lidocaine[13]. An automated biopsy gun (18-gauge core biopsy needle) was used to procure a minimum 1.5-cm-core biopsy specimen. All NCBs were performed using a similar similar fashionstand-alone protocol with computed tomography (CT) scans or ultrasound guidance to document the needle position within the lesion. In patients with a solitary nodule diameter greater than 5 cm, three different biopsies were performed within the lesion. When the tumour diameter was between 2-5 cm, two passes were usually performed, whereas when the tumour diameter was 2 cm or less, one pass was performed. The biopsy specimens were fixed in 10% neutral buffered formalin. All surgeries were performed by three independent groups of doctors. Criteria to evaluate the indication for surgical resection based on the liver function and tumour status have been described previously[14]. The formalin-fixed tissues ere processed, sectioned at a 5-μm thickness and stained with haematoxylin and eosin (H and E) technique.
Monoclonal antibody (clone BA17; 1:100) and mouse anti-human GPC3 monoclonal antibody (clone 1G12; 1:200) were purchased from the Zeta Company. Mouse anti-human monoclonal antibody HepPar1 (clone OCH1E5; 1:200) was purchased from Zymed Laboratories, Inc. The sections were steamed for 20 min in citrate target retrieval buffer (pH 6.0). Evidence for cytoplasmic staining of adjacent interlobular duct epithelia served as an internal positive control for CK19, yolk sac tumour tissue was used as a positive control sample for GPC3, and normal hepatocytes were used as positive control for HepPar1. Negative controls were established by substitution of the primary antibodies with non-immunized serum, resulting in the absence of signal detection.
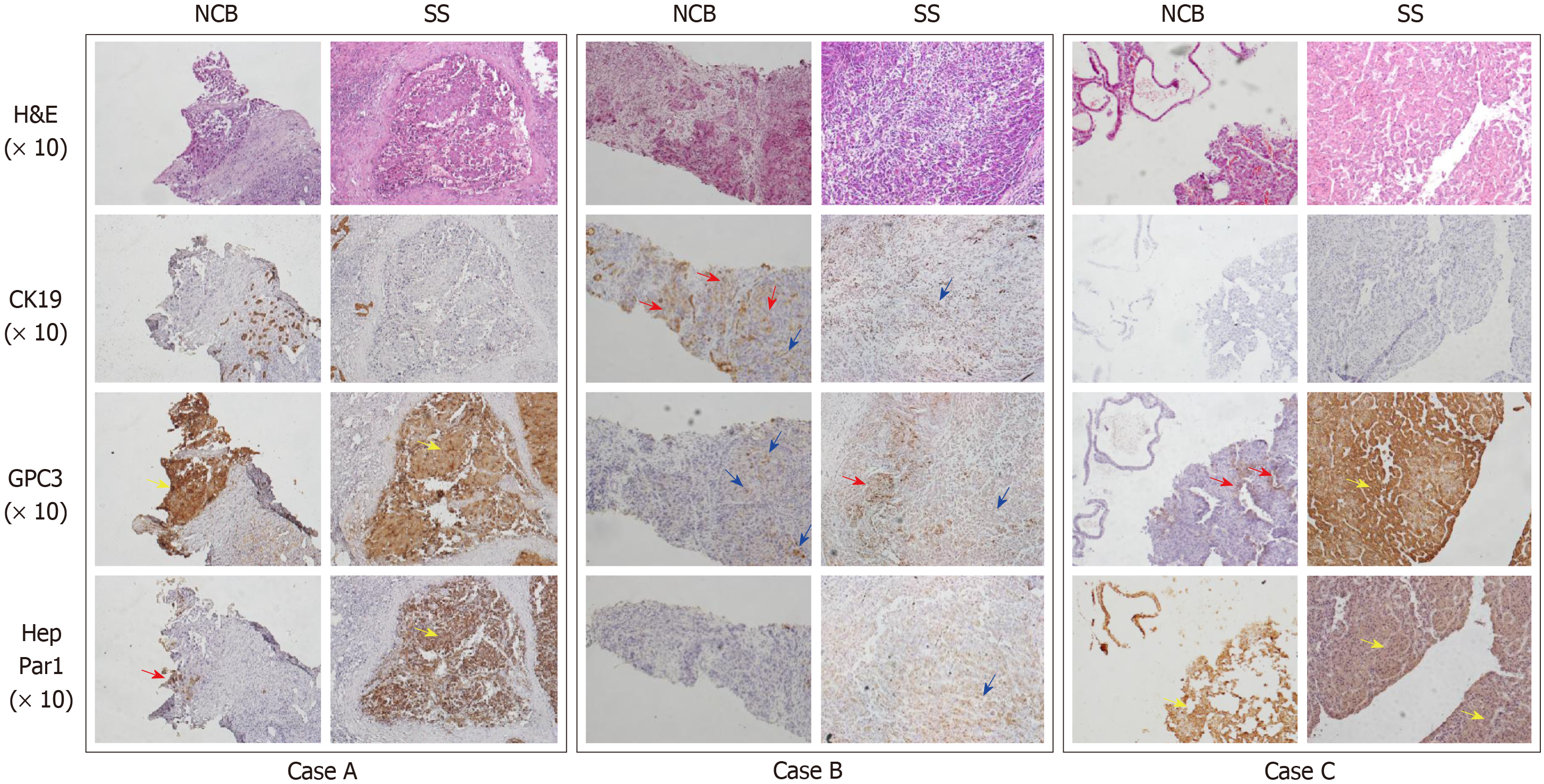
Semi-quantitative scoring methods are widely used to convert the subjective perception of IHC marker expression by histopathologists into quantitative data, which are then used for statistical analyses and the establishment of conclusions. In the current study, two major approaches were used in the interpretation of IHC. The first approach was described by Sabattini et al[15]: “-” was denoted for a score of less than 10% positively stained tumour cells or no visible staining; “+” score was denoted for 10%–49% positive tumour cells; “++” was established for more than 50% positively stained tumour cells. The second approach was a four-tier semi-quantitative score method as described previously[16]: “-” for no labelling; “+” for positive cells scattered individually across the microscopic field; “++” for at least one positive cluster; and “+++” for numerous positive clusters present within the tumour (Figure 2). Considering the advantages of two-tier grading in diagnostic decisions, combining all positive reports of the three- or four-tier grading system into one group was further proposed.
In case A, HepPar-1 showed positive expression in one cluster (++) and GPC3 showed positive staining of approximately the entire tumour nest (+++) in NCB. However, both markers showed massive positive staining (+++) across all microscopic fields in SS. In case B, CK19 showed scattered positive cells or clusters (+++) in NCB. Although GPC3 showed scattered positive staining (+) in NCB, scattered positive individuals or positive clusters (+++) in SS could be observed. In case C, GPC3 expression showed two positive clusters around the sinusoid-like structures (++) in NCB, whereas massive positive clusters were observed across the microscopic field (+++) in SS.
The biopsy and SS were assessed independently and blindly by two investigators according to WHO criteria[17]. When two pathologists reached two different conclusions, a consensus was essential for the final results. Surgically resected specimens were used as the gold standard for diagnosis or histological grading. The ambiguous morphological appearances in NCB were diagnosed according to the above-described different scoring methods used for CK19, GPC3[18], and HepPar1 expression. To investigate the accuracy of diagnosis in NCB, diagnoses based on a single morphological evaluation or combining IHC and morphological observations in NCB were compared to the gold standard.
The degree of pathological differentiation of HCC was scored using the modified nuclear grading scheme outlined by Edmondson and Steiner[19]: G1–G2: well differentiated; G3: moderately differentiated; G4: poorly differentiated. In all cases, the tumour grade was defined by the poorest degree of differentiation identified within the tumour upon pathological analysis of the entire specimen.
Analyses were performed by IBM SPSS (version 22.0, SPSS, Chicago, IL). The data were expressed as the mean ± SD. χ2 test and Student’s t test were used to compare the distribution of categorical and continuous variables, respectively. To evaluate the ability of preoperative NCB to predict the final surgical pathological diagnosis of the tumours, a receiver operating characteristic curve analysis was used, and the area under the curve (AUC) was calculated to assess the performance of preoperative NCB. The similarities in expression of the biomarkers between NCB and SS were assessed using the kappa statistic: Kappa values < 0 indicated “no agreement”, 0–0.20 “slight”, 0.21–0.40 “fair”, 0.41–0.60 “moderate”, 0.61–0.80 “substantial”, and 0.81–1 “almost” perfect agreement[20]. P < 0.05 was considered statistically significant.
The clinicopathological characteristics of the 208 patients are summarized in Table 1. The participants included 177 males and 31 females, with mean age of 54.37 ± 11.1 (26–84 and a mean tumour diameter of 4.6 ± 2.5 (1.8–15) cm. One hundred seventy-three patients (83.3%) exhibited cirrhosis, 192 (92.3%) had viral hepatitis B, and 12 (5.8%) presented hepatitis C infection, which accounted for the vast majority of the enrolled patients (98.1%). Complications of NCB were not recorded in this study.
| Variable | Value | |
| Age (yr) | Median | 55 |
| Range | 26-84 | |
| means ± SD | 54.4 ± 11.1 | |
| Sex, n (%) | ||
| Male | 177 (85.1) | |
| Female | 31 (14.9) | |
| Cirrhosis, n (%) | ||
| Yes | 173 (83.2) | |
| No | 35 (16.8) | |
| Aetiology, n (%) | ||
| HBV infection | 192 (92.3) | |
| HCV infection | 12 (5.8) | |
| alcohol abuse | 2 (1.0) | |
| Primary biliary cirrhosis | 1 (0.5) | |
| Schistosoma infection | 1 (0.5) | |
| Tumour diameter (cm) | ||
| Median | 4.0 | |
| Range | 1.8-15 | |
| means ± SD | 4.6 ± 2.5 | |
In this study, the kappa statistic showed that the agreement in histological subtypes between NCB and SS was 0.504 (P < 0.001) (Table 2). The result indicated that the degree of histological concordance between NCB and SS was moderate. Although histological disagreements between NCB and SS were also observed, most of the histological subtypes of PCL did not impact the accuracy of the NCB diagnosis, except the compact tumour nests/disordered cell mass and the cord-like structure in a fibrous stroma. Based solely on histomorphology, 7 (9.6%) intrahepatic cholangiocarcinoma (ICC) cases were misdiagnosed with HCC and 10 (6.4%) HCC cases were misdiagnosed with ICC among the NCB samples.
| SS | NCB | |||||||
| A | B | C | D | E | F | G | H | |
| A | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 16 | 51 | 1 | 0 | 1 | 0 | 0 | 0 |
| C | 2 | 14 | 36 | 0 | 0 | 0 | 0 | 0 |
| D | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 |
| E | 0 | 5 | 4 | 0 | 3 | 0 | 0 | 0 |
| F | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| G | 0 | 0 | 21 | 1 | 0 | 0 | 11 | 9 |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Kappa value | 0.504 | |||||||
| P value | < 0.001 | |||||||
GPC3 and HepPar1 are routine diagnostic markers for HCC.CK19 is also expressed in some subtypes of HCC[21,22] and hepatoblastoma[7], indicating that these tumours might originate from hepatic progenitor cells or hepatoblasts.
The four- and three-tier score method demonstrated a kappa value for consistency between NCB and SS of 0.717 (substantial) and 0.841 (almost perfect) for CK19, 0.768 (substantial) and 0.714 (substantial) for GPC3, and 0.633 (substantial) and 0.619 (substantial) for HepPar1, respectively. When the simplified two-tier score method (positive and negative) was used, the corresponding kappa value for CK19 was 0.931 and 0.979, for GPC3 was 0.907 and 0.933, and for HepPar1 was 0.803 and 0.874, in the 4-tier and 3-tier origin groups, respectively (Tables 3-5). The results indicated that both the 4-tier and 3-tier-based binary classifications had nearly perfect consistency in the interpretation of the IHC results between NCB and SS. Therefore, the two binary classification methods were used in further analyses.
| NCB | SS | |||||||||||
| 4-tier | 4-tier-based binary classification | 3-tier | 3-tier-based binary classification | |||||||||
| +++ | ++ | + | - | + | - | ++ | + | - | + | - | ||
| 4-tier | +++ | 52 | 1 | 0 | 0 | |||||||
| ++ | 3 | 2 | 0 | 0 | ||||||||
| + | 3 | 21 | 4 | 0 | ||||||||
| - | 0 | 0 | 7 | 115 | ||||||||
| 4-tier-based binary classification | + | 86 | 0 | |||||||||
| - | 7 | 115 | ||||||||||
| 3-tier | ++ | 35 | 8 | 0 | ||||||||
| + | 7 | 22 | 0 | |||||||||
| - | 0 | 2 | 134 | |||||||||
| 3-tier-based binary Classification | + | 72 | 0 | |||||||||
| - | 2 | 134 | ||||||||||
| Kappa value | 0.717 | 0.931 | 0.814 | 0.979 | ||||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||
| NCB | SS | |||||||||||
| 4-tier | 4-tier-based binary classification | 3-tier | 3-tier-based binary classification | |||||||||
| +++ | ++ | + | - | + | - | ++ | + | - | + | - | ||
| 4-tier | +++ | 62 | 1 | 0 | 0 | |||||||
| ++ | 18 | 25 | 2 | 0 | ||||||||
| + | 2 | 2 | 15 | 0 | ||||||||
| - | 0 | 1 | 8 | 72 | ||||||||
| 4-tier-based binary classification | + | 127 | 0 | |||||||||
| - | 9 | 72 | ||||||||||
| 3-tier | ++ | 42 | 5 | 0 | ||||||||
| + | 25 | 26 | 5 | |||||||||
| - | 0 | 2 | 103 | |||||||||
| 3-tier-based binary classification | + | 98 | 5 | |||||||||
| - | 2 | 103 | ||||||||||
| Kappa value | 0.768 | 0.907 | 0.714 | 0.933 | ||||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||
| NCB | SS | |||||||||||
| 4-tier | 4-tier-based binary classification | 3-tier | 3-tier-based binary classification | |||||||||
| +++ | ++ | + | - | + | - | ++ | + | - | + | - | ||
| 4-tier | +++ | 79 | 0 | 0 | 0 | |||||||
| ++ | 15 | 1 | 0 | 0 | ||||||||
| + | 5 | 12 | 12 | 0 | ||||||||
| - | 1 | 5 | 13 | 65 | ||||||||
| 4-tier-based binary classification | + | 124 | 0 | |||||||||
| - | 19 | 65 | ||||||||||
| 3-tier | ++ | 35 | 26 | 0 | ||||||||
| + | 12 | 32 | 7 | |||||||||
| - | 0 | 6 | 90 | |||||||||
| 3-tier-based binary classification | + | 105 | 7 | |||||||||
| - | 6 | 90 | ||||||||||
| Kappa value | 0.633 | 0.803 | 0.619 | 0.874 | ||||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||
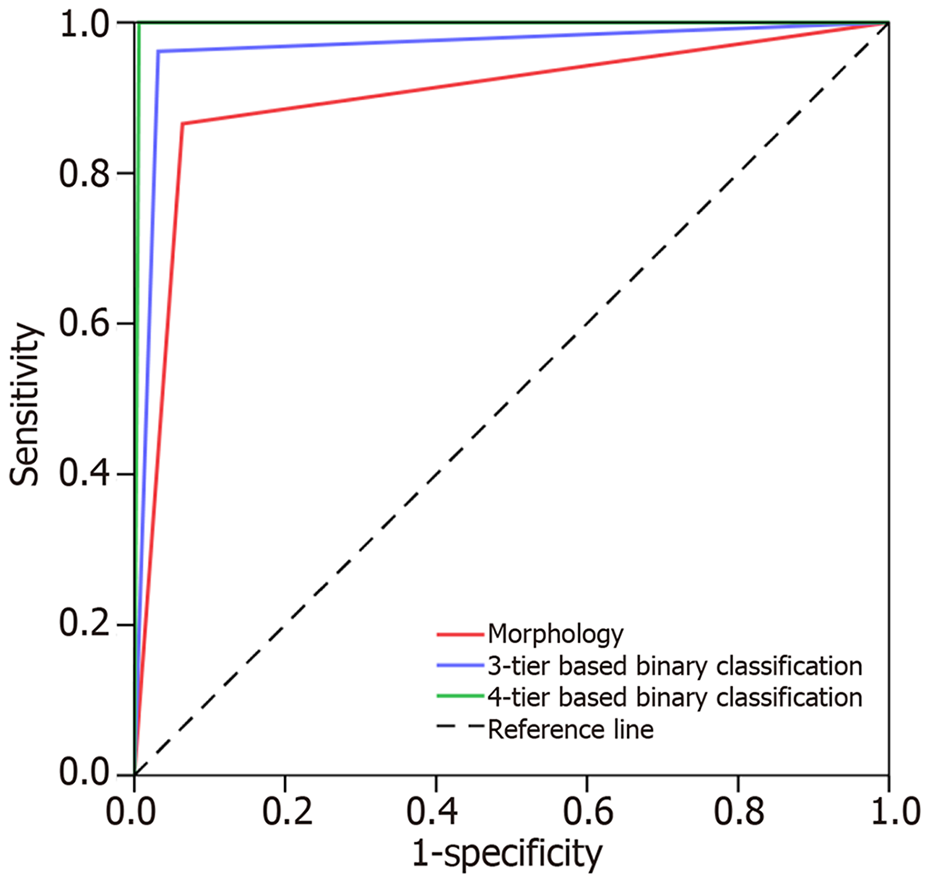
Based solely on morphology, the sensitivity, specificity, and accuracy of NCB in PCL diagnosis were 86.5%, 93.6%, and 91.8%, respectively. The AUC value of NCB was 0.901 (95%CI: 0.842-0.959; P < 0.001). The kappa statistic showed that the agreement between the NCB morphological diagnosis and gold standard was 0.786 (P < 0.001). When the 3-tier based binary classification was used, 7 (3.4%) cases were mis-diagnosed in NCB. The sensitivity, specificity, and accuracy of the diagnostic method in NCB were 96.2%, 96.8%, and 96.6%, respectively. The AUC value was 0.965 (95%CI: 0.931-0.999; P < 0.001). The kappa statistic showed that the agreement between the NCB and SS diagnosis was 0.912 (P < 0.001). However, when the 4-tier based binary classification was used, only 1 (0.5%) case was misdiagnosed in NCB. The sensitivity, specificity, and accuracy of the method in NCB were 99.4%, 100%, and 99.5%, respectively. The AUC value was 0.997 (95%CI: 0.990-1.000; P < 0.001). The kappa statistic showed that the agreement between NCB and SS diagnosis was 0.987 (P < 0.001) (Figure 3 and Table 6).
| NCB diagnosis | SS diagnosis (gold standard) | ||
| HCC | ICC | ||
| Morphology | HCC | 146 | 7 |
| ICC | 10 | 45 | |
| Kappa value | 0.786 | ||
| P value | < 0.001 | ||
| 3-tier-based binary classification | HCC | 151 | 2 |
| ICC | 5 | 50 | |
| Kappa value | 0.912 | ||
| P value | < 0.001 | ||
| 4-tier-based binary classification | HCC | 155 | 0 |
| ICC | 1 | 52 | |
| Kappa value | 0.987 | ||
| P value | < 0.001 | ||
The degree of concordance between NCB and the final SS tumour three-tier grade is shown in Table 7. In NCB, the HCC cases classified as well, moderately, and poorly differentiated represented 37 (17.8%), 99 (47.6%), and 72 (34.6%) of all cases, respectively. In contrast, in SS, there was a significantly lower proportion of well-differentiated HCC and a higher proportion of poorly differentiated HCC (well: 18/208, 8.7%; moderately: 93/208, 44.7%; poorly: 97/208, 46.6%) (P < 0.05). The corresponding kappa statistic for concordance was 0.488 (P < 0.001). Considering the significance of poorly differentiated HCC in prognostic predictions in comparison to individuals with well and moderately differentiated HCC, we further combined the well and moderately differentiated HCC into one group. The degree of consistency of the two-tier grade (well + moderately vs poorly) between SS and NCB was then increased but nevertheless remained at the moderate level (k = 0.558).
| SS | NCB | ||||
| 3-tier | 2-tier | ||||
| Well | Moderately | Poorly | Well and moderately | Poorly | |
| 3-tier | |||||
| Well | 15 | 3 | 0 | ||
| Moderately | 17 | 66 | 10 | ||
| Poorly | 5 | 30 | 62 | ||
| 2-tier | |||||
| Well + moderately | 101 | 10 | |||
| Poorly | 35 | 62 | |||
| Kappa value | 0.488 | 0.588 | |||
| P value | < 0.001 | < 0.001 | |||
The accurate diagnosis of PCL is very important prior to surgical procedures, as well as for prognostication and information regarding future treatment decisions. Despite significant advances in non-invasive techniques, such as radiological imaging and serum tumour biomarker detection, preoperative NCB should be one of the most important approaches in diagnosis of PLC, especially the differentiation of HCC and ICC. In the present study, the concordance of the clinicopathological characteristics and CK19, GPC3, and HepPar1 IHC staining between NCB and SS in patients with solitary HCC or ICC was investigated. Our results showed different degrees of discrepancy in the histomorphology and CK19, GPC3, and HepPar1 detection of tumours between NCB and SS, which could impact the diagnostic accuracy and predispose patients towards an underestimated tumour grade and malignancy potential.
Morphological characteristics have been the source for the pathological diagnosis. Microscopically, the typical pathological patterns of HCC include trabecular, acinar, and pseudo-glandular features, whereas ICC shows adenocarcinomatous structures characterized by tubular complexes and a moderate amount of fibrous stroma[23]. However, HCC and ICC occasionally share overlapping morphological appearances, which can pose challenges in the differential diagnosis. In this study, the morphological observation led to a misdiagnosis rate of 8.2% in NCB, which was attributed to the presence of compact tumour nests or cord-like structures in tissues[24]. With the aid of IHC staining, the diagnostic accuracy in NCB significantly improved.
Although IHC can remarkably aid in pathological diagnosis, the heterogeneous expression of biomarkers in one tumour can directly interfere with the interpretation of IHC results and determine the variability of the achieved results, especially in tumour tissues obtained with needle biopsy[3,25-28]. Semi-quantitative scoring based on the proportion of positively stained tumour cells is widely used to convert IHC staining into positive or negative results, although varied cut points of 10%, 25% or 50% positive immunoreactivity in the specimen were used by different groups[29]. The usage of these methods led to a negligible significance of focal neoplastic cell immunoreactivity within a limited sample of carcinomas. In the current study, according to the 3-tier scoring, some cases with scattered positive tumour cells in CNB can be interpreted as negative, and therefore, a diametrically opposing positive or negative result can be obtained. In CNB diagnosis integrating CK19, GPC3 and HepPar1 IHC detection, semi-quantitative scoring based on the proportion of positively stained tumour cells should be avoided.
In comparison to 3-tier scoring, 4-tier scoring and the further proposed binary classification derived from 4-tier scoring showed a high concordance in interpreting the IHC staining of CK19, GPC3, and HepPar1 between NCB and SS, which also showed a decisive role in increasing the accuracy of diagnosis in CNB. Therefore, this simplified binary classification can be used as an easy and applicable approach in preoperative CNB diagnosis.
During the past decade, several studies have emphasized the significance of histological grading in the risk of recurrence or metastasis in HCC after liver resection or transplantation[30]. Therefore, the degree of concordance of NCB grade to the gold standard by SS will directly determine the safety and reliability of preoperative NCB grading in the evaluation of prognosis of patients and the treatment decision. Nevertheless, in the present study, the consistency of the three-tier histological grading between SS and NCB was moderate (k = 0.488). Since several previous studies have reported that poor differentiation of HCC was the independent prognostic indicator, we further compared the consistency of the histological grade between SS and NCB in a two-tier grading method (well and moderately vs. poorly). Our result showed that the degree of consistency of the two-tier histological grading between SS and NCB was increased; however, it continued at the moderate level (k = 0.558), which was similar to the results of another previous study (k = 0.380)[31]. Notably, although the agreement between SS and NCB histological grading was not as perfect as expected, the presence of the poorly differentiated region in NCB can still be valuable in prognosis prediction because the histological grading of tumours has been determined according to the worst differentiation clusters or regions microscopically.
There were some limitations in this study. Even with the aid of the 4-tier based binary classification, 0.48% (1/208) patients were misdiagnosed as preoperative NCB in this cohort, which indicated that the utility of the three markers in NCB had diagnostic limitations and that the combined utility of other biomarkers would be necessary to further improve the diagnostic accuracy. In addition, this study also does not address potential adverse events following NCB. Third, the present study investigated the concordance of detective indexes in patients with a solitary tumour between NCB and SS. The degree of consistency of the indicators in patients with a multifocal tumour between NCB and SS necessitates further investigation, which will be the focus of our future studies.
In conclusion, the present study suggested that in preoperative NCB, the presence of compact tumour nests or a cord-like structure alone were the main causes of misdiagnosis of HCC from ICC. Combining the detection of CK19, GPC3, and HepPar1 can improve the accuracy of diagnosis. However, focal neoplastic cell immunoreactivity of these markers in NCB should not be neglected. The concordance of histological grade between NCB and the SS was moderate. These findings imply that reasonable interpretation of IHC staining results and evaluation of the histo-morphology in NCB are vital for improving the accuracy of tumour diagnosis, as well as prognostic prediction.
Pathological manifestations of hepatic tumours are often associated with prognosis. Although surgical specimens (SS) can provide more information, currently, pre-treatment needle core biopsy (NCB) is increasingly showing important value in understanding the nature of liver tumors and even in diagnosis and treatment decisions. However, the concordance of the clinicopathological characteristics and immunohistochemical (IHC) staining between NCB and SS from patients with hepatic tumours were less concerned.
The present study was designed to evaluate the concordance of the clinicopathological characteristics and the novel biotic marker of CK19, GPC3, and HepPar1 staining between the NCB and SS from patients with hepatocellular carcinoma (HCC) or intrahepatic cho-langiocarcinoma (ICC).
We want to introduce a more accurate method for interpreting the immunohistochemical staining results to improve the diagnostic value of hepatic malignancy in NCB samples.
A total of 208 patients who underwent both preoperative NCB and surgical resection for HCC or ICC between 2008 and 2015 were enrolled in this study. The expression of CK19, GPC3, and HepPar1 were detected by IHC staining. Clinicopathological, NCB, and surgical data were collected and analysed using χ2 and kappa statistics.
Morphologically, the presence of compact tumour nests or a cord-like structure in NCB was considered the primary cause of misdiagnosis of HCC from ICC. The kappa statistic showed a moderate agreement in histomorphology (k = 0.504) and histological grade (k = 0.488) between NCB and SS of the tumours. A 4-tier (+++, ++, +, and -) scoring scheme that emphasized the focal neoplastic cell immunoreactivity of tumour cells revealed perfect concordance of CK19, GPC3 and HepPar1 between NCB and SS (k = 0.717; k = 0.768; k = 0.633). Furthermore, with the aid of a binary classification derived from the 4-tier score, a high concordance was achieved in interpreting the IHC staining of the three markers between NCB and final SS (k = 0.931; k = 0.907; k = 0.803), increasing the accuracy of NCB diagnosis C(k = 0.987; area under the curve = 0.997, 95%CI: 0.990-1.000; P < 0.001).
Our findings imply that reasonable interpretation of IHC staining results in NCB is vital for improving the accuracy of tumour diagnosis. The simplified binary classification provides an easy and applicable approach.
Although the binary classification can significantly improve the accuracy of diagnosis of HCC or ICC, it is unclear whether the method can be transferred to patients with other tumors. In addition, the degree of consistency of the indicators in patients with a multifocal tumor between NCB and SS necessitates further investigation, which will be the focus of our future studies.
We thank Wenyan Song and Cuiyu Jia for their assistance with the data collection.
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 2. | Feng J, Wu J, Zhu R, Feng D, Yu L, Zhang Y, Bu D, Li C, Zhou Y, Si L, Liu Y, Liang Z, Xu J, Wu T. Simple Risk Score for Prediction of Early Recurrence of Hepatocellular Carcinoma within the Milan Criteria after Orthotopic Liver Transplantation. Sci Rep. 2017;7:44036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mafficini A, Amato E, Fassan M, Simbolo M, Antonello D, Vicentini C, Scardoni M, Bersani S, Gottardi M, Rusev B, Malpeli G, Corbo V, Barbi S, Sikora KO, Lawlor RT, Tortora G, Scarpa A. Reporting tumor molecular heterogeneity in histopathological diagnosis. PLoS One. 2014;9:e104979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | McGahan JP, Bishop J, Webb J, Howell L, Torok N, Lamba R, Corwin MT. Role of FNA and Core Biopsy of Primary and Metastatic Liver Disease. Int J Hepatol. 2013;2013:174103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Feng J, Zhu R, Chang C, Yu L, Cao F, Zhu G, Chen F, Xia H, Lv F, Zhang S, Sun L. CK19 and Glypican 3 Expression Profiling in the Prognostic Indication for Patients with HCC after Surgical Resection. PLoS One. 2016;11:e0151501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Feng J, Chen J, Zhu R, Yu L, Zhang Y, Feng D, Kong H, Song C, Xia H, Wu J, Zhao D. Prediction of early recurrence of hepatocellular carcinoma within the Milan criteria after radical resection. Oncotarget. 2017;8:63299-63310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Yun WJ, Shin E, Lee K, Jung HY, Kim SH, Park YN, Yu E, Jang JJ. Clinicopathologic implication of hepatic progenitor cell marker expression in hepatoblastoma. Pathol Res Pract. 2013;209:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Soriano A, Varona A, Gianchandani R, Moneva ME, Arranz J, Gonzalez A, Barrera M. Selection of patients with hepatocellular carcinoma for liver transplantation: Past and future. World J Hepatol. 2016;8:58-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Paradis V, Kudo M, Zucman-Rossi J. Tissue biomarkers as predictors of outcome and selection of transplant candidates with hepatocellular carcinoma. Liver Transpl. 2011;17 Suppl 2:S67-S71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ofuji K, Saito K, Yoshikawa T, Nakatsura T. Critical analysis of the potential of targeting GPC3 in hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Berretta M, Cavaliere C, Alessandrini L, Stanzione B, Facchini G, Balestreri L, Perin T, Canzonieri V. Serum and tissue markers in hepatocellular carcinoma and cholangiocarcinoma: clinical and prognostic implications. Oncotarget. 2017;8:14192-14220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci. 2011;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Daly B, O'Kelly K, Klassen D. Interventional procedures in whole organ and islet cell pancreas transplantation. Semin Intervent Radiol. 2004;21:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Cucchetti A, Cescon M, Trevisani F, Pinna AD. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol. 2012;18:6398-6408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G, Pileri SA. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506-511. [PubMed] |
| 16. | Marcu M, Radu E, Sajin M. Neuroendocrine differentiation in prostate adenocarcinoma biopsies and its correlation to histological grading. Curr Health Sci J. 2010;36:37-42. [PubMed] |
| 17. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system, Forth ed. Lyon: International Agency for Research on Cancer 2010; 205-227. |
| 18. | The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 597] [Article Influence: 35.1] [Reference Citation Analysis (2)] |
| 19. | Shin E, Yu YD, Kim DS, Won NH. Adiponectin receptor expression predicts favorable prognosis in cases of hepatocellular carcinoma. Pathol Oncol Res. 2014;20:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 21. | Lu XY, Xi T, Lau WY, Dong H, Zhu Z, Shen F, Wu MC, Cong WM. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Ann Surg Oncol. 2011;18:2210-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Lee Y, Park H, Lee H, Cho JY, Yoon YS, Choi YR, Han HS, Jang ES, Kim JW, Jeong SH, Ahn S, Kim H. The Clinicopathological and Prognostic Significance of the Gross Classification of Hepatocellular Carcinoma. J Pathol Transl Med. 2018;52:85-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Strumfa I. Liver Biopsy - Indications, Procedures, Results. 1st ed. Croatia: In Tech 2012; 115-159. |
| 24. | Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagn Pathol. 2012;7:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | van Dekken H, Verhoef C, Wink J, van Marion R, Vissers KJ, Hop WC, de Man RA, IJzermans JN, van Eijck CH, Zondervan PE. Cell biological evaluation of liver cell carcinoma, dysplasia and adenoma by tissue micro-array analysis. Acta Histochem. 2005;107:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002;30:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 317] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Hübner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44:507-511, 514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 599] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 28. | Cheek E, Rajkumar C. How to present statistics in medical journals. Age Ageing. 2014;43:306-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol. 2014;9:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 30. | Wang L, Wang J, Zhang X, Li J, Wei X, Cheng J, Ling Q, Xie H, Zhou L, Xu X, Zheng S. Diagnostic Value of Preoperative Needle Biopsy for Tumor Grading Assessment in Hepatocellular Carcinoma. PLoS One. 2015;10:e0144216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Chen J, Wu M, Liu R, Li S, Gao R, Song B. Preoperative evaluation of the histological grade of hepatocellular carcinoma with diffusion-weighted imaging: a meta-analysis. PLoS One. 2015;10:e0117661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dueland S, Ko E, Kositamongkol P S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ