Published online Apr 15, 2019. doi: 10.4251/wjgo.v11.i4.335
Peer-review started: December 26, 2018
First decision: January 11, 2019
Revised: February 13, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 15, 2019
Processing time: 111 Days and 5.8 Hours
Human epidermal growth factor receptor 2 (HER2) is an oncogenic driver, and a well-established therapeutic target in breast and gastric cancers. While the role of HER2 as a prognostic biomarker in colorectal adenocarcinomas (CRCs) remains uncertain, its relevance as a therapeutic target has been established. We undertook the present study to evaluate the frequency of HER2 expression in CRC and to correlate it with various clinicopathological variables.
To correlate HER2 protein expression and HER2 gene amplification with clinicopathological features and survival in surgically resected CRC.
About 1195 consecutive surgically resected CRCs were analyzed by immunohistochemical staining (IHC) to assess HER2 protein expression, and 141 selected tumors were further evaluated by fluorescence in situ hybridization (FISH) to assess HER2 gene amplification. Follow-up information was available for 1058 patients, and using this information we investigated the prevalence of HER2 protein overexpression and gene amplification in a large series of surgically resected CRCs, and evaluated the relationship between overexpression and clinicopathological parameters and prognosis.
HER2 IHC scores of 3+, 2+, 1+, and 0 were seen in 31 (2.6%), 105 (8.8%), 475 (39.7%), and 584 (48.9%) tumors, respectively. HER2 gene amplification was seen in 24/29 tumors with an IHC score of 3+ (82.8%; unreadable in 2/31), 12/102 tumors with an IHC score of 2+ (11.8%; unreadable in 2/104), and 0 tumors with IHC score of 1+ (0/10). HER2 gene amplification was seen in 36/1191 tumors (3.0%; unreadable in 4/1195). Among the tumors with HER2 IHC scores of 3+ and 2+, the mean percentage of tumor cells with positive IHC staining was 90% (median 100%, range 40%-100%) and 67% (median 75%, range 5%-95%), respectively (P < 0.05). Among tumors with IHC scores of 2+, those with HER2 gene amplification had a higher number of tumors cells with positive IHC staining (n = 12, mean 93%, median 95%, range 90%-95%) than those without (n = 90, mean 70%, median 50%, range 5%-95%) (P < 0.05). HER2 gene status was significantly associated with distant tumor metastasis and stage (P = 0.028 and 0.025). HER2 protein overexpression as measured by IHC or HER2 gene amplification as measured by FISH was not associated with overall survival (OS) or disease-specific survival for the overall group of 1058 patients. However, further stratification revealed that among patients with tubular adenocarcinomas who were 65 years old or younger (n = 601), those exhibiting HER2 gene amplification had a shorter OS than those without (mean: 47.9 mo vs 65.1 mo, P = 0.04). Among those patients with moderately to poorly differentiated tubular adenocarcinomas, those with positive HER2 tumor IHC scores (2+, 3+) had a shorter mean OS than those with negative HER2 IHC scores (0, 1+) (47.2 mo vs 64.8 mo, P = 0.033). Moreover, among patients with T2 to T4 stage tumors, those with positive HER2 IHC scores also had a shorter mean OS than those with negative HER2 IHC scores (47.1 mo vs 64.8 mo, P = 0.031).
HER2 protein levels are correlated with clinical outcomes, and positive HER2 expression as measured by IHC confers a worse prognosis in those patients 65 years old or younger with tubular adenocarcinomas.
Core tip: The manuscript investigated the prevalence of human epidermal growth factor receptor 2 (HER2) protein overexpression and gene amplification in a large series of surgically resected colorectal adenocarcinomas (CRCs), and evaluated their relationship with clinicopathological parameters and prognosis. We found that HER2 overexpression [immunohistochemical staining (IHC) score 2+ and 3+] is seen in a small percentage of colorectal adenocarcinomas and HER2 gene amplification occurs in the vast majority of tumors with 3+ IHC score but in a much lower percentage of tumors with 2+ IHC score. In addition, although the prevalence of HER2 overexpression by IHC in CRCs is low, HER2 protein status is correlated with clinical outcomes and positive HER2 expression by IHC confers a worse prognosis in 65 years or younger patients with tubular adenocarcinomas.
- Citation: Wang XY, Zheng ZX, Sun Y, Bai YH, Shi YF, Zhou LX, Yao YF, Wu AW, Cao DF. Significance of HER2 protein expression and HER2 gene amplification in colorectal adenocarcinomas. World J Gastrointest Oncol 2019; 11(4): 335-347
- URL: https://www.wjgnet.com/1948-5204/full/v11/i4/335.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i4.335
Colorectal adenocarcinoma (CRC) is one of the most common malignancies and remains one of the leading causes of cancer-related death in the world[1]. The vast majority of colorectal cancers are adenocarcinomas. Until recently, the role of chemotherapy in treating CRC has been fairly limited, and as such there is a need to develop more effective therapeutic regimes for CRC.
Human epidermal growth factor receptor 2 (HER2) represents a promising therapeutic target. The HER2 gene is a proto-oncogene located on chromosome 17q21 encoding HER2 (ErbB-2), which is involved in cell proliferation and differentiation[2]. The HER2 protein is a transmembrane receptor tyrosine kinase and a member of the family of the epidermal growth factor receptors[3]. Oncogenic activation of HER2 commonly occurs through gene amplification, which results in protein overexpression on the cell membrane that is involved in signal transduction to regulate cell growth[4]. HER2 overexpression/amplification is linked to trastuzumab response in breast/gastric cancers. For breast cancer in particular, HER2 serves as both a prognostic factor and also a therapeutic target, with targeted therapy against HER2 using the monoclonal antibody Trastuzumab (Herceptin) having become the standard of care for patients whose breast carcinomas exhibit HER2 gene amplification[5-7]. A recent randomized phase III trial (ToGA) also revealed that the addition of trastuzumab to chemotherapy improved response rates, median progression-free survival, and overall survival in patients with advanced gastric or gastroesophageal junction cancer with HER2 overexpression[8].
Compared to breast and gastric cancers, the data regarding HER2 protein overexpression and gene amplification in CRCs are limited[9-18]. The numbers of cases in these previous studies were relatively small, and their analyses were primarily reliant upon tissue microarrays[9-18]. In addition, the reported rates of HER2 protein overexpression ranged widely from 3% to 47.4% across studies, indicating that the clinical significance of HER2 in CRC has not yet been fully clarified. In this study, we assessed HER2 overexpression in 1195 CRC patients via immunohistochemical staining (IHC) in 1195 patients and analyzed HER2 gene status via fluorescence in situ hybridization (FISH) in a subset of these patients (all tumors with IHC scores of 2+ and 3+, and some a score of 1+). We then correlated HER2 protein overexpression and HER2 gene status with clinicopathologic features and prognosis to gain insight into the importance of this marker in the context of CRC.
This investigation was performed after approval was obtained from the Ethics Committee of Peking University Cancer Hospital.
A total of 1195 colorectal cancer (545 rectal cancer and 650 colon cancer) patients who underwent curative surgery between April 2009 and March 2012 in the Peking University Cancer Hospital and Institute were included in this study. None of these patients had undergone preoperative radiation or chemotherapy. Clinicopathologic parameters including age, gender, tumor site, tumor type, tumor differentiation, localization and type of the tumor, tumor T stage, lymph node status, and distant metastasis were retrieved from clinical and pathological databases. Follow-up data was available for a total of 1058 CRC patients, and was retrieved from hospital records by interview, telephone, or mailed letters. Follow-up time started on the day of primary tumor surgical operation. The end point for the disease-associated overall survival (OS) analysis was the time of death of the patient or our last review. Disease free survival (DFS) was defined as the time from the date of operation to the date of diagnosis of metastatic disease or recurrence.
IHC was performed with the Ventana pathway rabbit monoclonal antibody to HER2 (clone 4B5; prediluted) on a Ventana Benchmark automated stainer (Ventana, Tucson, Arizona) following the manufacturer’s protocol. Antigen retrieval was performed using Cell Conditioning 1 citrate buffer (pH 6.0; Ventana) for 30 min. Immunostaining was scored by two pathologists using a 4-step scale (0, 1+, 2+, 3+) according to the consensus panel recommendations regarding HER2 scoring for gastric cancer[19,20]. The IHC staining was scored: 0 (no staining or membranous staining in less than 10% of tumor cells), 1+ (faint/barely visible membranous staining in at least 10% of cells or staining in parts of their membrane), 2+ (weak to moderate complete or basolateral membranous staining in at least 10% of tumor cells), 3+ (strong complete or basolateral membraneous staining in at least 10% of tumor cells). HER2 IHC scores of 2+ and 3+ were considered as being “HER2 positive”, while IHC scores of 0 and 1+ were considered as being “HER2 negative”.
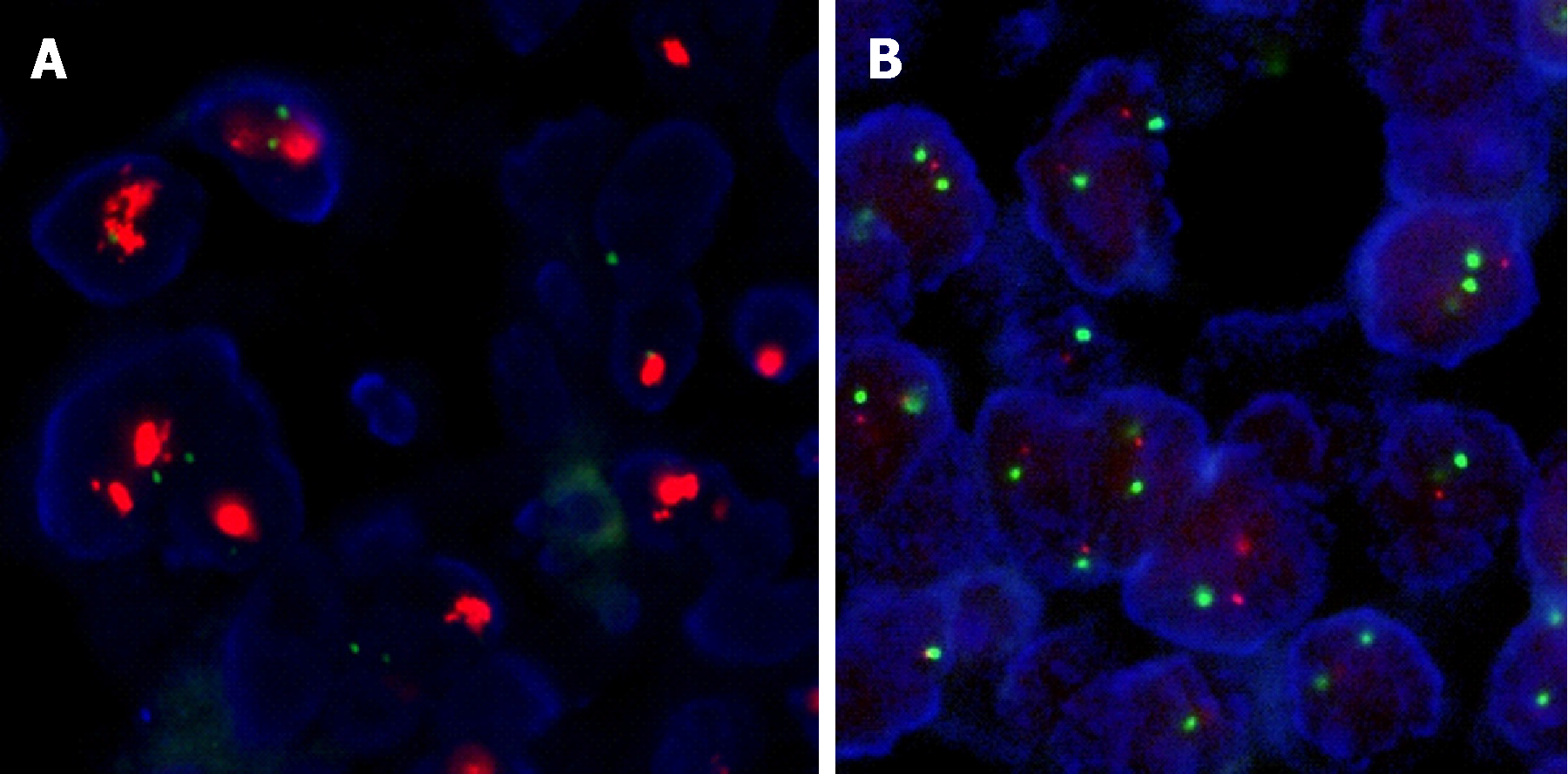
FISH was carried out using the PathVysion HER2 DNA probe kit and procedure (Vysis/Abbott, Abbott Park, Illinois). The kit contains 2 fluorescently labeled DNA probes, HER2 (labeled with Spectrum-Orange) and CEP17 (chromosome 17 enumeration probe labeled with Spectrum-Green). The total numbers of HER2 and CEP17 signals were counted in at least 20 tumor cell nuclei in 2 different areas. FISH staining was evaluated by two different investigators to ensure consistency. The criteria for positive gene amplification were: a HER2/CEP17 signal ratio of 2.0 or higher, or the presence of tight gene clusters as previously reported; otherwise samples were defined as being negative for gene amplification[14].
All statistical analyses were performed using the SPSS software version 20.0 statistical package (SPSS Inc., Chicago, IL, USA). The correlation between HER2 status and patient clinicopathological characteristics was tested via the χ2 test or Fisher’s exact test. Survival curves were fitted using the Kaplan-Meier method, and the differences in survival were assessed by the log rank test. The effects of different factors on patient survival were assessed via multivariate analysis with the Cox proportional hazards regression model, and the hazard ratio and associated 95%CI were calculated for each factor. P-values < 0.05 (two-sided) were considered as being statistically significant.
Among these 1195 CRC patients, 718 were males and 477 were females. The patient ages ranged from 29 to 92 years (mean: 60.8 years). The tumors were staged as pT1 in 32 patients, pT2 in 175 patients, pT3 in 937 patients, and pT4 in 51 patients (Table 1). Lymph node metastasis was evident in 602 patients (N1 in 296 patients, N2 in 306 patients). Follow-up data was available for a total of 1058 CRC patients, including 640 males and 418 females. These patients ranged in age from 24 to 89 years (mean 61 years). 568 patients had colon cancer, while 490 had rectal cancer patients. The histopathological diagnosis was tubular adenocarcinoma in 971 (91.8%) patients and mucinous adenocarcinoma or other types in 87 (8.2%) patients. According to the TNM classification system, 198 (18.7%) of CRC patients had stage I and II tumors, while 860 (81.3%) of patients had stage III and IV disease. Clinicopathological data are summarized in Table 1.
| Clinical / pathological features | n |
| Gender | |
| Male | 718 |
| Female | 477 |
| Age (yr) | |
| ≤ 60 | 578 |
| > 60 | 617 |
| Tumor grade | |
| G1 | 42 |
| G2 | 857 |
| G3 | 296 |
| Tumor stage | |
| pT1 | 32 |
| pT2 | 175 |
| pT3 | 937 |
| pT4 | 51 |
| Nodal status | |
| pN0 | 593 |
| pN1 | 296 |
| pN2 | 306 |
| Tumor type | |
| Tubular carcinoma | 1089 |
| Mucinous carcinoma | 106 |
| Localization | |
| Ascending colon | 273 |
| Transverse colon | 32 |
| Descending colon | 56 |
| Sigmoid | 289 |
| Rectum | 545 |
| Total number | 1195 |
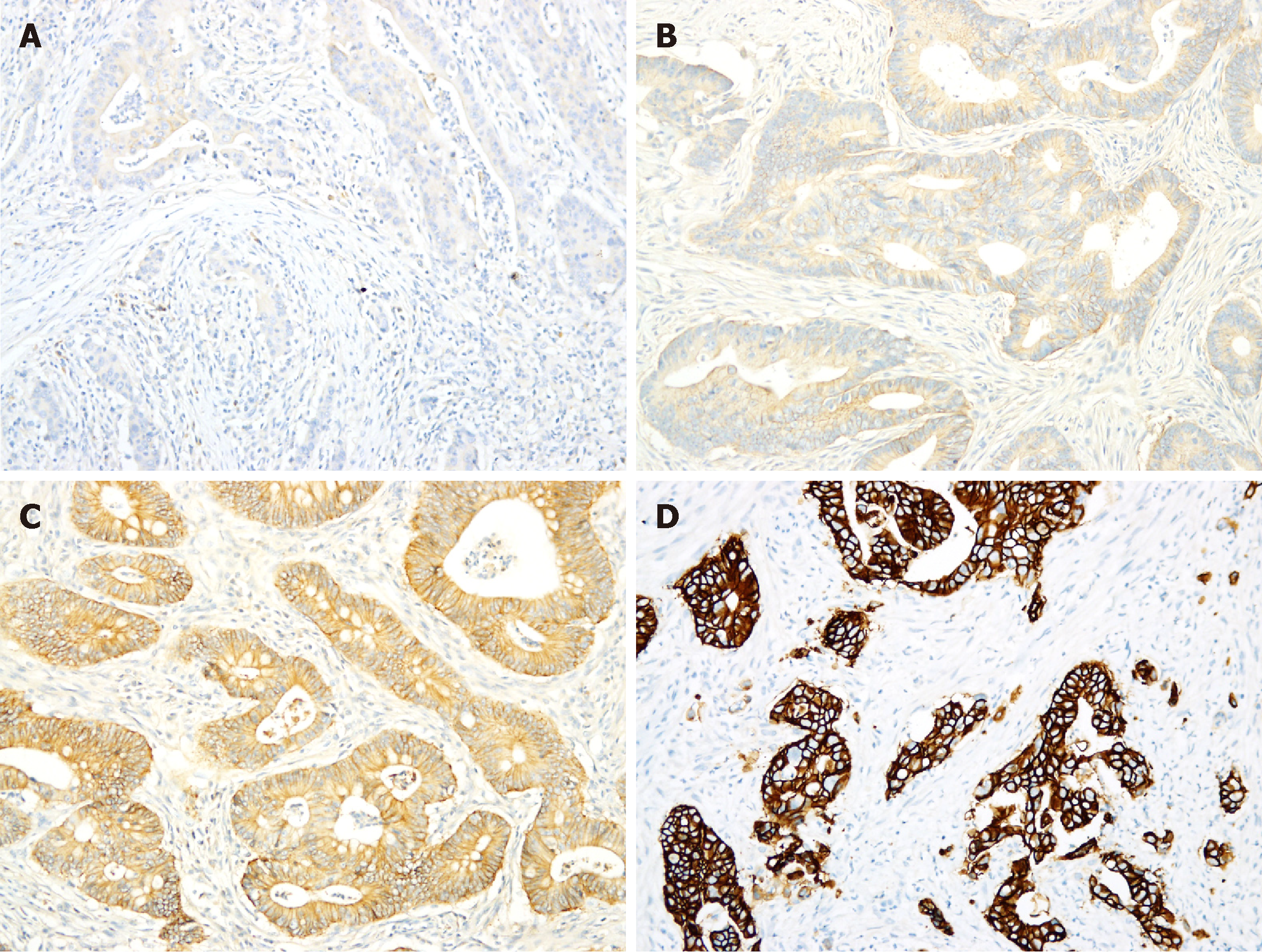
Among these 1195 CRC tumors, HER2 IHC scores of 3+, 2+, 1+ and 0 were observed in 31 (2.6%), 105 (8.8%), 475 (39.7%) and 584 (48.9%) tumors, respectively (Table 2, Figure 1). Among the tumors with IHC 3+ and 2+ scores, the mean percentage of tumor cells with positive staining was 90% (median 100%, range 40%-100%) and 67% (median 75%, range 5%-95%), respectively (P < 0.05).
| Staining pattern | Number of cases | Positive rate |
| IHC 3+ | 31 | 2.6% |
| IHC 2+ | 105 | 8.8% |
| IHC 1+ | 475 | 39.7% |
| IHC 0 | 584 | 48.9% |
All 31 tumors with positive 3+ HER2 staining were further evaluated by FISH to assay for HER2 gene amplification. The FISH results in 29 tumors (29/31; unreadable in 2/31) were evaluable and 24 (24/29, 83%) showed HER2 gene amplification (Table 3, Figure 2). HER2 gene amplification was seen in 12/102 (11.8%; unreadable in 2/104) tumors with HER2 IHC scores of 2+, and 0 (0/10) tumors with IHC scores of 1+ (Table 3, Figure 1). Among the 104 tumors with IHC scores of 2+, those with HER2 gene amplification had a higher number of tumor cells with positive IHC staining (n = 12; mean 93%, median 95%, range 90%-95%) than did those without (n = 102; mean 70%, median 50%, range 5%-95%) (P < 0.05) (Figure 2).
| IHC score | HER2 gene amplification (FISH) | % HER2 gene amplification | |
| Positive | Negative | ||
| 1+ (n = 10) | 0 | 10 | 0% |
| 2+ (n = 102) | 12 | 90 | 11.8% |
| 3+ (n = 29) | 24 | 5 | 82.8% |
HER2 gene amplification was significantly associated with tumor depth of invasion, distant metastasis, and stage (P = 0.001, 0.028, and 0.012, respectively; Table 4), while there was no significant association between HER2 gene status and age, sex, tumor site, grade, histology, or lymph node metastasis (P > 0.05 for all). And we didn’t find any observably differences between right/left-sided tumors in this study (P = 0.514).
| Clinicopathological features | HER2 status | P-value | |
| HER2 negative | HER2 positive | ||
| Gender | 0.441 | ||
| Male | 616 (96.2) | 24 (3.8) | |
| Female | 406 (97.1) | 12 (2.9) | |
| Age (yr) | 0.272 | ||
| ≤ 60 | 501 (96.0) | 21 (4.0) | |
| > 60 | 521 (97.2) | 15 (2.8) | |
| Tumor grade | 0.784 | ||
| 1 | 39 (97.5) | 1 (2.5) | |
| 2 | 773 (96.7) | 26 (3.3) | |
| 3 | 210 (95.9) | 9 (4.1) | |
| Histology | 0.762 | ||
| Tubular | 937 (96.5) | 34 (3.5) | |
| Mucinous/other types | 85 (97.7) | 2 (2.3) | |
| Tumor site | 0.819 | ||
| Colon | 548 (96.5) | 20 (3.5) | |
| Rectal | 474 (96.7) | 16 (3.3) | |
| Left-sided colon | 203 (88.3) | 27 (37.0) | 0.514 |
| Right-sided colon | 292 (86.4) | 46 (63.0) | |
| Depth of invasion | |||
| T1 | 31 (100.0) | 0 (0.0) | 0.039 |
| T2 | 165 (98.8) | 2 (1.2) | |
| T3 | 815 (96.3) | 31 (3.7) | |
| T4 | 11 (78.6) | 3 (21.4) | |
| T1-2 | 196 (99.0) | 2 (1.0) | 0.001 |
| T3-4 | 826 (96.0) | 34 (4.0) | |
| Lymph node metastasis | |||
| N0 | 541 (96.3) | 21 (3.7) | 0.260 |
| N1 | 261 (98.1) | 5 (1.9) | |
| N2 | 220 (95.7) | 10 (4.3) | |
| No | 541 (96.3) | 21 (3.7) | 0.524 |
| Yes | 481 (97.0) | 15 (3.0) | |
| Distant metastasis | 0.028 | ||
| No | 940 (96.9) | 30 (3.1) | |
| Yes | 81 (92.0) | 7 (8.0) | |
| Tumor stage | |||
| (1)I-II | 192 (98.5) | 2 (1.0) | 0.012 |
| III | 749 (96.5) | 27 (3.5) | |
| IV | 81 (92.0) | 7 (8.0) | |
| (3)I-II | 192 (99.0) | 2 (1.0) | 0.044 |
| III-IV | 830 (96.1) | 34 (3.9) | |
A total of 1058 CRC patients with available follow-up data were further assessed for overall survival. Median OS was 49.35 mo (1.1 to 77.0 mo) and median DFS was 48.80 mo (1.0 to 77.0 mo) for the patients without evidence of metastasis before surgery (n = 941).
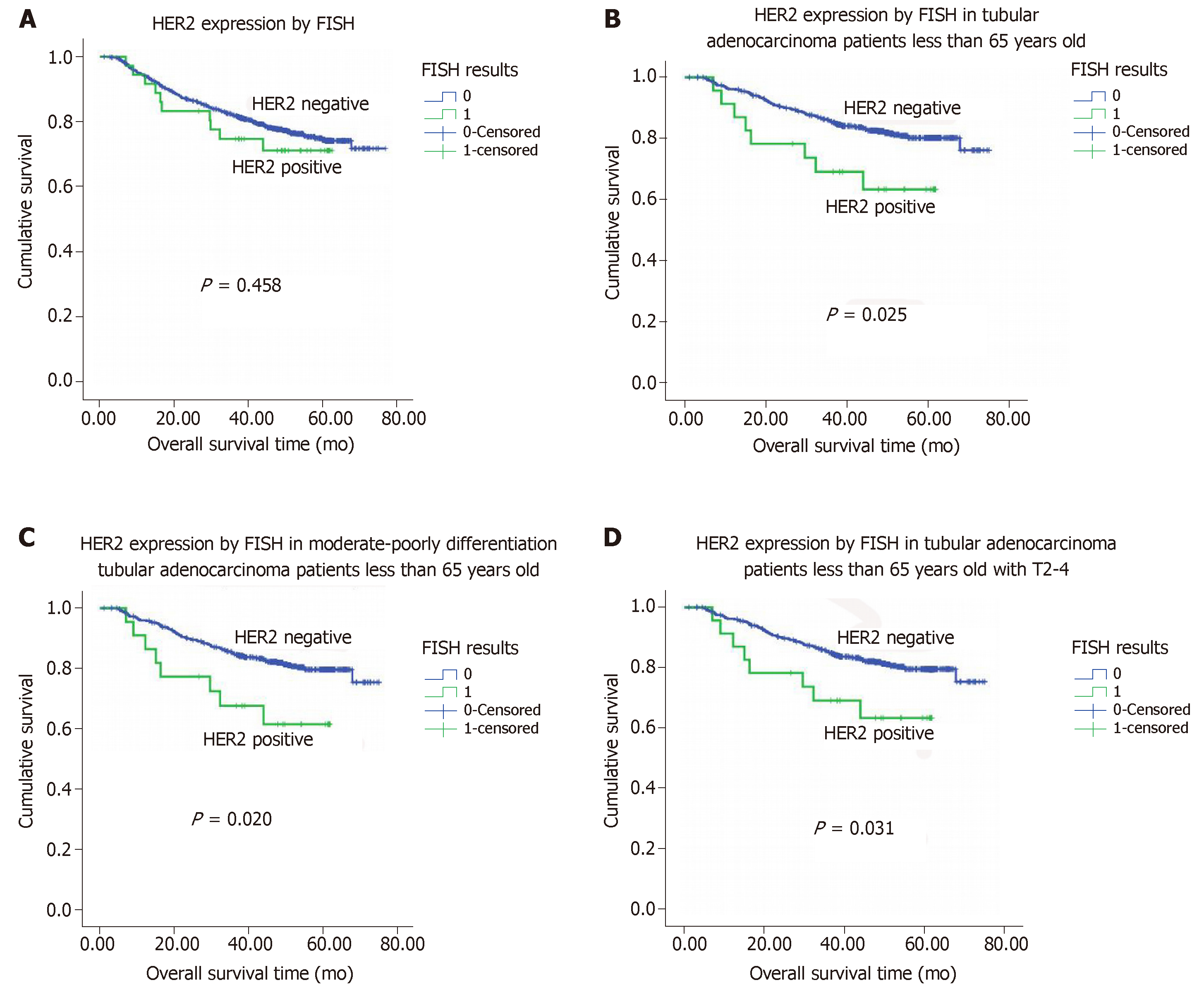
Neither HER2 protein overexpression nor HER2 gene amplification were associated with OS (P = 0.220 and 0.458, respectively; Figure 3A). Tumor grade, histologic type, lymph node metastasis, and tumor stage were each significantly associated with OS (P < 0.05 for all; Table 5). Neither HER2 protein overexpression nor HER2 gene status was associated with DFS (P = 0.320 and 0.662, respectively).
| Clinicopathological features | Patients | Events | Median survival (95%CI), mo | P-value |
| Gender | 0.707 | |||
| Male | 640 | 144 | NA | |
| Female | 418 | 100 | NA | |
| Age (yr) | ||||
| ≤ 60 | 522 | 116 | NA | 0.591 |
| > 60 | 536 | 128 | NA | |
| ≤ 65 | 661 | 138 | NA | 0.027 |
| > 65 | 397 | 106 | NA | |
| Tumor site | 0.457 | |||
| Colon | 568 | 133 | NA | |
| Rectal | 490 | 111 | NA | |
| Tumor grade | 0.000 | |||
| 1-2 | 839 | 176 | NA | |
| 3 | 219 | 68 | NA | |
| Histology | 0.000 | |||
| Tubular | 971 | 205 | NA | |
| Mucinous/ Other types | 87 | 39 | 54.90 | |
| Lymph node metastasis | 0.000 | |||
| No | 562 | 61 | NA | |
| Yes | 496 | 183 | NA | |
| Tumor stage | 0.000 | |||
| I-II | 198 | 13 | NA | |
| III-IV | 860 | 231 | NA | |
| HER2 protein overexpression by IHC | ||||
| IHC (-) | 935 | 221 | NA | 0.220 |
| IHC (+) | 123 | 23 | NA | |
| HER2 gene amplification by FISH | ||||
| Positive | 1022 | 234 | NA | 0.458 |
| Negative | 36 | 10 | NA | |
There was no association between HER2 gene amplification and OS or DFS among the 971 patients with tubular adenocarcinomas, this was not the case among the 797 patients who were 65 years old or younger. Among this group of patients, HER2 protein overexpression was associated with a shorter mean OS for those with tubular adenocarcinoma (47.1 mo for positive vs 65.1 mo for negative, P = 0.025, n = 601; Figure 2B). In a particular subpopulation of patients, those with moderately to poorly differentiated tubular adenocarcinomas (n = 573), the mean OS in those patients whose tumors did not exhibit HER2 gene amplification was significantly longer than those with evidence of HER2 gene amplification (mean: 64.8 mo vs 46.4 mo, P = 0.020; Figure 2C). Moreover, among the subgroup of patients with stage T2 to T4 tubular adenocarcinomas (n = 580), those tumors had HER2 gene amplification had a shorter OS compared to those without HER2 gene amplification (mean: 47.1 mo vs 64.8 mo, P = 0.031; Figure 2D).
In this study we assessed HER2 protein overexpression and HER2 gene amplification in a large series of 1195 colorectal adenocarcinomas. 11.4% (136/1195) of these colorectal adenocarcinomas were positive for HER2 staining by IHC (scores of 2+ and 3+). HER2 gene amplification was identified in 11.8% (12/102) of tumors with HER2 IHC scores of 2+ and in 82.8% (24/29) of tumors with HER2 IHC scores of 3+. No tumors with HER2 IHC scores of 1+ showed evidence of HER2 gene amplification by FISH. Our findings indicate that HER2 protein overexpression occurs in a small percentage of colorectal adenocarcinomas, similarly to what has been reported in previous studies. There are only 9 prior studies regarding HER2 status in colorectal adenocarcinomas in the English literature[9-17]. Three of these nine studies used tissue microarrays (104, 518, and 1851 patients, respectively) and the remaining 6 used full tissue blocks (ranging from 138 to 317 patients)[9-17]. Our study is the largest to date to employ full tumor tissue blocks for analysis (1195 patients). The reported rates of HER2 protein overexpression in the literature ranged from 2.7% to 15.5%, with the exception of one study in which the authors reported 47.4% positive HER2 staining in 137 colorectal adenocarcinomas[9-17]. One explanation for this dramatic range in HER2 overexpression positivity across studies may be that antibodies used for staining varied among research groups. The HER2 antibody used in the study with highest positive HER2 staining (47.4%) was a polyclonal rabbit antibody. Even though those authors demonstrated a high HER2 overexpresssion rate, the percentage of cases exhibiting HER2 gene amplification was only 8% in those with 3+ HER2 staining, and 0% in 38 cases with 2+ HER2 staining, raising questions regarding the validity of the IHC scoring. The positive rates of HER2 protein staining in studies using the DAKO HER2 antibody were 2.7% to 15.5%, while it was 8% in the study using Ventana pathway antibody by Kavanagh et al[14] and was 11.4% in our study using this same Ventana pathway.
Our study also indicates that there is a high concordance between HER2 IHC 3+ staining and HER2 gene amplification in colorectal adenocarcinomas. In our study 83% of tumors (24/29) with 3+ HER2 staining exhibited HER2 gene amplification. There were 6 other studies in the literature on this topic, however the number of cases analyzed in 5 of these 6 studies was small (less than 10). Marx et al[15] found that 100% of tumors (27/27) with 3+ HER2 staining (as measured with the DAKO antibody) harbored HER2 gene amplifications. In the study by Park et al[10], only 2 of 27 tumors with 3+ HER2 staining showed evidence of HER2 gene amplification, however, as described above, the IHC staining in that study was performed using a polyclonal antibody of uncertain reliability. In 3 other smaller studies (2-6 cases with 3+ HER2 staining), this concordance rate with gene amplification was high (100%). Variations in the antibodies used likely led to differential staining and scoring, contributing to the disagreement in HER2 gene amplification in tumors with 3+ HER2 scores. Nevertheless, these studies, including ours, have shown that HER2 IHC 3+ staining is highly predictive of HER2 gene amplification in colorectal adenocarcinomas. In contrast, the percentage of tumors with HER2 IHC 2+ staining showing evidence of HER2 gene amplification was highly variable. In our study, such amplification was only evident in 20% of tumors, similar to what has been reported by Kavanagh et al[14] (2/9 or 22%, using the Ventana Pathway antibody) and Nathanson et al[9] (1/3 or 33%, using the DAKO antibody). In contrast, Marx et al[15] observed HER2 gene amplification in 75% of tumors with HER2 scores of 2+ (using the DAKO antibody). In the study by Park et al[10], using the Zymed antibody, no gene amplification events were observed in such tumors. In the study by Ooi et al[11], 2/2 tumors with 2+ HER2 staining showed evidence of HER2 gene amplification (using an antibody from Nicheri, Japan). Similarly, variations in the antibodies used likely led to differential staining and scoring, contributing to the observed high levels of reported variation in HER2 gene amplification in tumors with 2+ HER2 scores.
The high concordance between HER2 IHC 3+ staining and HER2 gene amplification indicates that gene amplification is the major factor accounting for HER2 protein overexpression in colorectal adenocarcinomas with HER2 IHC scores of 3+. However, HER2 gene amplification was seen in only 12/102 (11.8%) tumors with HER2 IHC scores of 2+, and among these tumors, those with evidence of HER2 gene amplification had a higher number of tumor cells with positive IHC staining (n = 12, mean 93%) than those without (n = 90, mean 70%) (P < 0.05). Our study thus confirmed reported results regarding colorectal cancer, and these results were similar to those observed in breast cancer where IHC 3+ HER2 overexpression is highly correlated with HER2 gene amplification, whereas 2+ staining is equivocal for gene amplification[21-24].
In our present study, HER2 gene amplification was higher in patients with a more advanced disease stage or distant metastases. This suggests that HER2 may play some role in tumor progression and would thus be a valuable prognostic factor for certain CRC patients. In our study, the significance of HER2 gene amplification was best observed in the subpopulation of patients who were 65 years or younger with tubular adenocarcinomas. However, we did not observe a significant difference in prognosis for all colorectal carcinoma patients, regardless of HER2 amplification or HER2 expression status.
Although we found a statistically significant association between HER2 gene amplification and tumor depth of invasion, advanced stage, and distant metastasis, several other studies have failed to show such associations[15,19,25,26]. Those studies, however, reported a correlation between HER2 gene amplification and high grade histology, higher tumor stage, and positive nodal status[27,28]. The role of HER2 as a prognostic factor in CRCs is still controversial, as some of the earlier studies have failed to identify any specific association with prognosis[28,29] while others reported a correlation between HER2 gene amplification and poorer survival[30]. Similar observations have been made for gastric cancer, although a larger number of studies have supported the association between HER2 overexpression and poorer survival[31]. Possible explanations for such discrepancies include: Antibody resources, detection methods, scoring systems, and patient populations (different ethnic groups with different genetic backgrounds). It has been clearly demonstrated that HER2 gene amplification differs significantly between right/left-sided and rectal carcinomas. We were not able to produce similar results, potentially due to differences in groups analyzed, HER2 testing methods, and tumor biological characteristics[32,33].
In summary, we investigated HER2 protein status and HER2 gene status in a large series of colorectal adenocarcinomas. Our results show that HER2 protein overexpression is evident in 11.4% of colorectal adenocarcinomas. HER2 IHC scores of 3+ are highly correlated with HER2 gene amplification, while this correlation is weaker in tumors with IHC 2+ staining. We also observed an association between HER2 gene amplification and prognosis in patients with tubular adenocarcinomas who were 65 years or younger. Our study indicates that a certain population of patients with colorectal adenocarcinomas may benefit with HER2-targeted therapy.
The vast majority of colorectal cancers are adenocarcinomas. Until recently, the role of chemotherapy in treating colorectal adenocarcinomas (CRCs) has been fairly limited, and as such there is a need to develop more effective therapeutic regimes for CRC. Human epidermal growth factor receptor 2 (HER2) is an oncogenic driver; it’s a well-established therapeutic target in breast and gastric cancers.
The role of HER2 as a prognostic biomarker in CRCs remains uncertain, but its relevance as a therapeutic target has been established.
In this study, the authors aim to evaluate the frequency of HER2 expression in CRC and to correlate it with various clinicopathological variables.
In this study, to assess HER2 protein expression, 1195 consecutive surgically resected CRCs were analyzed by immunohistochemical staining (IHC). And to assess HER2 gene amplification, 141 selected tumors were further evaluated by fluorescence in situ hybridization (FISH). The authors investigated the prevalence of HER2 protein overexpression and gene amplification in a large series of surgically resected CRCs, and evaluated the relationship between overexpression and clinicopathological parameters and prognosis.
HER2 gene amplification was seen in 24/29 tumors with an IHC score of 3+, 12/102 tumors with an IHC score of 2+, and 0 tumors with IHC score of 1+ (0/10). HER2 gene amplification was seen in 36/1191 tumors. Among the tumors with HER2 IHC scores of 3+ and 2+, the mean percentage of tumor cells with positive IHC staining was 90% and 67%. Among tumors with IHC scores of 2+, those with HER2 gene amplification had a higher number of tumors cells with positive IHC staining than those without. HER2 gene status was significantly associated with distant tumor metastasis and stage. HER2 protein overexpression as measured by IHC or HER2 gene amplification as measured by FISH was not associated with overall survival (OS) or disease-specific survival for the overall group of 1058 patients. Among those patients with moderately to poorly differentiated tubular adenocarcinomas, those with positive HER2 tumor IHC scores (2+, 3+) had a shorter mean OS than those with negative HER2 IHC scores (0, 1+).
HER2 protein levels are correlated with clinical outcomes, and positive HER2 expression confers a worse prognosis in patients 65 years old or younger with tubular adenocarcinomas.
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8236] [Article Influence: 457.6] [Reference Citation Analysis (11)] |
| 2. | Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 577] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8336] [Cited by in RCA: 8625] [Article Influence: 221.2] [Reference Citation Analysis (0)] |
| 4. | Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (1)] |
| 5. | Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R; Herceptin Adjuvant (HERA) Trial Study Team. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 7. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3742] [Cited by in RCA: 3728] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 8. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5532] [Article Influence: 345.8] [Reference Citation Analysis (3)] |
| 9. | Nathanson DR, Culliford AT 4th, Shia J, Chen B, D'Alessio M, Zeng ZS, Nash ZM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer. 2003;105:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Park DI, Kang MS, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Han WK, Kim H, Ryu SH, Sepulveda AR. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis. 2007;22:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Ooi A, Takehana T, Li X, Suzuki S, Kunitomo K, Iino H, Fujii H, Takeda Y, Dobashi Y. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol. 2004;17:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T, Troetschel M, Walko C, Day R, Chen HX, Finkelstein S. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Al-Kuraya K, Novotny H, Bavi P, Siraj AK, Uddin S, Ezzat A, Sanea NA, Al-Dayel F, Al-Mana H, Sheikh SS, Mirlacher M, Tapia C, Simon R, Sauter G, Terracciano L, Tornillo L. HER2, TOP2A, CCND1, EGFR and C-MYC oncogene amplification in colorectal cancer. J Clin Pathol. 2007;60:768-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kavanagh DO, Chambers G, O'Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Atanackovic D, Bokemeyer C, Fiedler W, Terracciano L, Sauter G, Izbicki JR. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Kim JY, Lim SJ, Park K. Cyclooxygenase-2 and c-erbB-2 expression in colorectal carcinoma assessed using tissue microarrays. Appl Immunohistochem Mol Morphol. 2004;12:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kountourakis P, Pavlakis K, Psyrri A, Rontogianni D, Xiros N, Patsouris E, Pectasides D, Economopoulos T. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J. 2006;12:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 21. | Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W, Höfler H, Kreipe HH. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Hoang MP, Sahin AA, Ordòñez NG, Sneige N. HER-2/neu gene amplification compared with HER-2/neu protein overexpression and interobserver reproducibility in invasive breast carcinoma. Am J Clin Pathol. 2000;113:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol. 1999;17:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, Untch M, Löhrs U. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001;19:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Schuell B, Gruenberger T, Scheithauer W, Zielinski CH, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer. 2006;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Ingold Heppner B, Behrens HM, Balschun K, Haag J, Krüger S, Becker T, Röcken C. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111:1977-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Seo AN, Kwak Y, Kim DW, Kang SB, Choe G, Kim WH, Lee HS. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One. 2014;9:e98528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Conradi LC, Styczen H, Sprenger T, Wolff HA, Rödel C, Nietert M, Homayounfar K, Gaedcke J, Kitz J, Talaulicar R, Becker H, Ghadimi M, Middel P, Beissbarth T, Rüschoff J, Liersch T. Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol. 2013;37:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Drecoll E, Nitsche U, Bauer K, Berezowska S, Slotta-Huspenina J, Rosenberg R, Langer R. Expression analysis of heat shock protein 90 (HSP90) and Her2 in colon carcinoma. Int J Colorectal Dis. 2014;29:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 32. | Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, Philip PA, Shields AF, Lenz HJ, Marshall JL. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356-86368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, Morris VK, Advani S, Menter DG, Eng C, Shaw K, Broaddus R, Routbort MJ, Liu Y, Morris JS, Luthra R, Meric-Bernstam F, Overman MJ, Maru D, Kopetz S. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin Cancer Res. 2018;24:1062-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jorgensen JT, Sunakawa Y S-Editor: Wang JL L-Editor: A E-Editor: Wu YXJ