Published online Mar 15, 2019. doi: 10.4251/wjgo.v11.i3.208
Peer-review started: October 2, 2018
First decision: November 15, 2018
Revised: December 6, 2018
Accepted: January 9, 2019
Article in press: January 10, 2019
Published online: March 15, 2019
Processing time: 164 Days and 10.3 Hours
Quality control in colon cancer surgery is an ongoing debate ever since standardization proved to be highly efficient in improving survival in rectal cancer. Complete mesocolic excision (CME) is widely acclaimed as the new gold-standard in colon cancer resections, thus it is imperative to establish quality criteria of CME in order to make it easily understood and verified by surgeons worldwide. One simple and reproducible tool could be the measurement of arterial stumps postoperatively and a straightforward way to test its reliability is to test it in a comparative study between CME and non-CME surgery.
To validate arterial stump measurement as a surgical quality tool by comparing CME with conventional radical colectomies.
This was a retrospective study, carried out on a prospective database. We collected data from two groups of patients, divided according to standard CME with D2 central vascular ligation (group A) and non-standardized surgery (group B). The two groups were compared with regard to the arterial stump length after right- and left-sided colectomies for colon cancer. The actual stump lengths of the ileocolic artery (ICA) and inferior mesenteric artery (IMA) were compared with their theoretical best D2 position of predicted ligation levels (D2PLLs) for calculating the potential for improvement. Measurements on follow-up computed tomography scans were carried out by three observers. Pathological data were recorded (specimen length, lymph node yield) and correlated with stump length.
We analysed 58 colectomies. The stump lengths (mean ± SD) in group A were 16.97 ± 4.77 mm for ICA and 31.70 ± 15.71 mm for IMA, whereas group B had 49.93 ± 20.29 mm for ICA and 67.24 ± 28.71 mm for IMA. Shorter lengths were obtained in group A, by a mean difference of 35.66 mm (χ2 = 27.38, P < 0.001), which was significant for all types of colectomies. Except for a 5.85 ± 4.71 mm difference for right colectomies, all the ligations from group A significantly reached their potential height (0.26 ± 12.18 mm from D2PLL; χ2 = 0.005, P = 0.944). Apart from three left colectomies, group B failed to reach D2PLL, by a mean difference of 32.14 ± 26.15 mm (χ2 = 21.77, P < 0.001). The calculated improvement potentials were significantly shorter in group A than in group B, by a mean of 31.88 mm (χ2= 22.13, P < 0.001). The large spread of results in group B showed that there is significant variability (P = 0.004) when compared to standard surgery. Significant correlations were found between stump length, specimen length and number of lymph nodes (P = 0.018 and P = 0.008 respectively). No statistical difference was found between observers’ measurements (P = 0.866).
Arterial stump monitoring is a significant step in defining surgical quality, as longer stumps contain residual mesocolic tissue and correlate with major prognostic factors.
Core tip: This is the first study to assess arterial stump measurement as a tool for comparative analysis between complete mesocolic excision and non-standardized colonic surgery. We demonstrated its benefit as a tool for evaluating surgical quality, mainly due to its correlation with a major prognostic factor. It is a simple and reproducible measurement on routine computed tomography scans without metallic markers. We showed that the central vascular ligation can be performed without extensive D3 lymphatic dissection.
- Citation: Livadaru C, Morarasu S, Frunza TC, Ghitun FA, Paiu-Spiridon EF, Sava F, Terinte C, Ferariu D, Lunca S, Dimofte GM. Post-operative computed tomography scan – reliable tool for quality assessment of complete mesocolic excision. World J Gastrointest Oncol 2019; 11(3): 208-226
- URL: https://www.wjgnet.com/1948-5204/full/v11/i3/208.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i3.208
The surgical treatment of colon cancer is the only treatment with a curable visa. Complete mesocolic excision (CME), as Hohenberger et al[1] initially presented it in 2009, is based on three principles. First and foremost, the mobilization of the colon needs to strictly respect the avascular plane of dissection; this ensures removal of the colonic tumour together with a sealed, embryologically-defined mesocolon, which contains all lymphovascular potential routes of tumour dissemination. The second principle refers to the central vascular ligation (CVL), which ensures vertical removal of the entire mesocolic root and apical lymph nodes (LNs). The third principle involves clearing the LNs in the pericolic station, with the advised longitudinal resection margin being up to 5 cm proximally and 10 cm distally from the tumour, depending on the feeding vessels[1,2].
CME has proved to be a reliable technique for standardizing colon surgery; however, the debate on defining the recommended point of CVL has not reached unanimity[2-5]. Studies that comparatively analysed CME with conventional surgery showed more surgical complications in CME. Although no randomized trials exist to support this, it seems that attempting a true central ligation causes extra risks, unlike the fascial plane dissection[6-8]. Complete mesenterectomy at the root of the superior mesenteric artery (SMA) poses danger for splanchnic nerves laceration, which will cause refractory diarrhoea[6]. Significantly more injuries to the spleen and the superior mesenteric vein (SMV) have been correlated to central ligation[7], and the high variability of venous collaterals giving rise to the gastrocolic trunk of Henle has been shown to add further haemorrhagic risk[8,9]. On the left side, ligation of the inferior mesenteric artery (IMA) in proximity to the aorta can ensue permanent urological disorders and faecal incontinence in some patients, while sexual dysfunction persists in most patients[2,10,11].
Recently, the theory of tailoring central ligation to the crossing variations between the ileocolic artery (ICA) and SMV has been raised[12,13]. Additionally, the recommendation of a 10 mm safe margin from the arterial origin has been put forward in the CME consensus[2]; although, this stump length could also be obtained when performing a D2 lymphadenectomy, considering that the ICA runs in front of the SMV[13]. Further insight was given by Japanese research groups, who stated that the lymphatic drainage of the right colon ends in front of the SMV, making dissection beyond it unwarranted. They advocate for a full clearance alongside the anterior aspect of the SMV, without reaching beyond it[2].
Regardless of the continuing debate, some surgical societies have understood the value of standardization and implemented the central ligation as a guideline procedure; these include the French Society of Digestive Surgery[14] and the American Society of Colon and Rectal Surgeons[15,16]. Furthermore, the Japanese recommend at least D2 resections for cT2 colon cancer[17]. The Norwegian Health Directive expects a standard CME with complete D2 dissection to be performed on all interventions with curative intent and advises additional D3 lymphadenectomy whenever possible[18], by following the evidence on increased 5-year overall survival from 82.1% to 89.1% as reported by Hohenberger et al[1].
Nevertheless, if the surgery does not closely respect the embryologic cleavage plan, contamination with malign cytology through the disrupted mesocolon could render null any potential benefit of the central ligation[1]. Consequently, analysing surgical specimens is crucial for evaluating the quality of CME. In this way, the integrity of the mesocolon and the number of LNs can be accurately measured for stratifying the survival outcomes, as the difference between mesocolic plane and muscularis propria planes of resection translates to a 27% increase in 5-year overall survival for stage III disease[19]. Another indicator, proposed by West et al[20,21], is measurement of the distance from the CVL to the bowel margin; however, this does not directly reflect the residual stump length and accompanying LNs because the main pedicle’s length is variable and thus the arterial stump may be longer than the pathologist suggests[22,23].
As a shorter arterial stump would imply fewer residual nodes, Hohenberger et al[24] applied angiography to patients with local recurrence of colorectal cancer to visualize the residual IMA. Thus, they appreciated that long stumps illustrated an incomplete primary lymphadenectomy which had caused the local cancer recurrence[24]. Recent studies, however, proposed a simpler technique to evaluate the point of CVL—by measuring the arterial stumps on post-operative computed tomography (CT) scans[22,25,26]. Their results showed promise in evaluating the quality of colon resections, thus calling for further studies to be made for validation of the technique.
Our aim was to analyse the existing variation in the technique of radical colectomy and to demonstrate the utility of the arterial stump as a tool for evaluating surgical quality. Our first objective was to compare the arterial stump lengths from our own CME series without local recurrences[27], with a different series of conventional radical colectomies. Our second objective was to calculate the improvement potential in each group, by comparing the actual lengths with their theoretical best position of D2 and D3 predicted ligation levels (D2PLL and D3PLL respectively).
This study was designed as a retrospective analysis and conducted on a prospectively maintained database of patients treated at the Regional Oncology Institute (IRO) in Iasi, Romania.
The study was approved by the Institutional Ethics Committee of the IRO, and patients had given consent for inclusion in the research database.
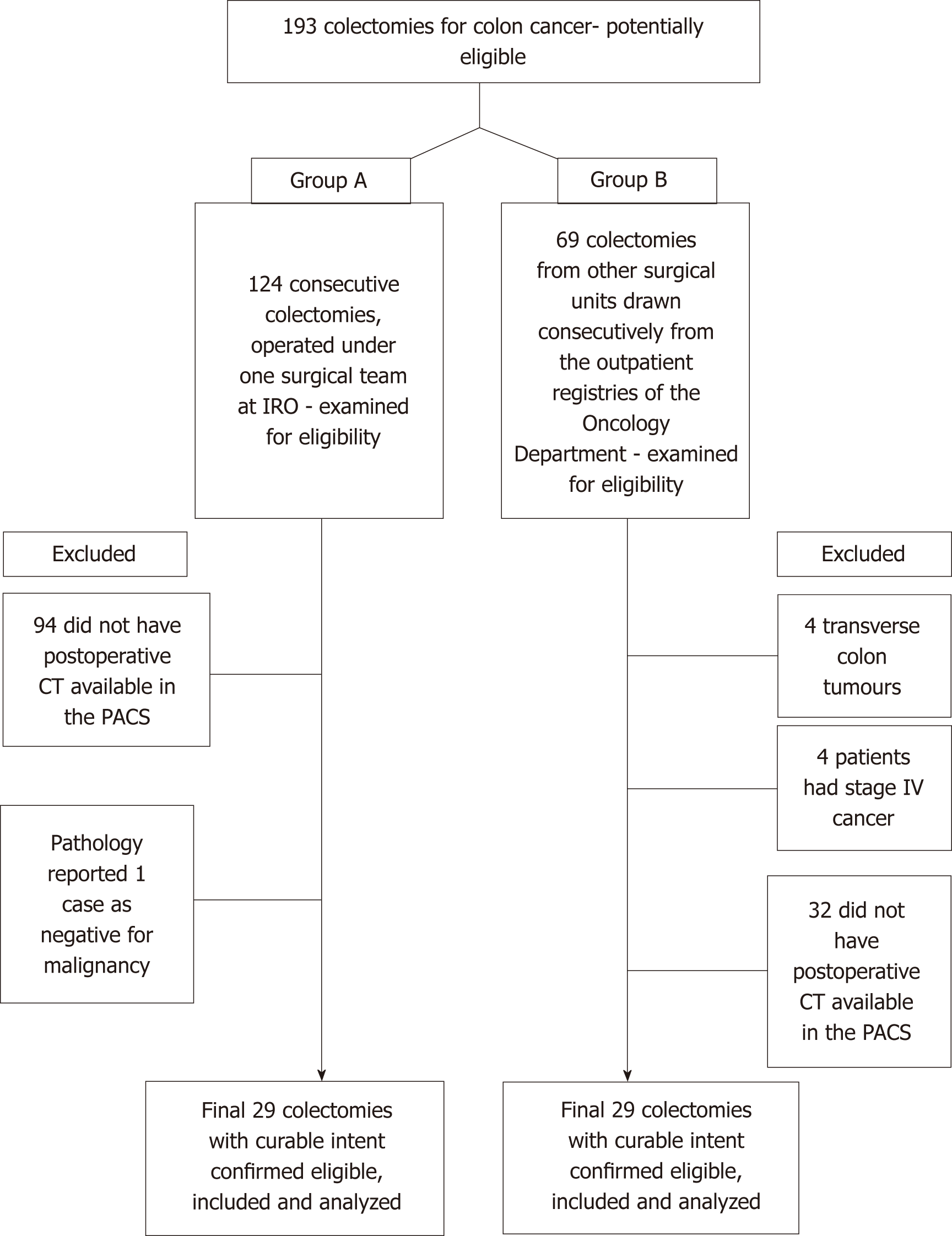
Two groups of adult patients (groups A and B, see below) were included in consecutive order. All underwent surgery with curative intent for colon cancer (stages I-III UICC 7th edition) and had at least one post-operative good quality contrast-enhanced CT scan that was available for re-evaluation.
Group A patients were operated on at the 2nd Surgery Department of IRO between the dates of March 2012 and February 2018; all surgeries were performed by one surgical team. Group B patients were operated on in other surgical units between the dates of July 2007 and February 2018, and are currently being followed up at the Medical Oncology Outpatient Clinic in our institution.
Exclusion criteria for both groups were stage IV colonic tumours, palliative colectomies, and tumours in the transverse colon.
Colectomies in group A patients were performed according to CME principles with the following adjustments to the high vascular ligation site: (1) Ligation of the ICA at the right side of the SMV for right colectomies (D2 resection); (2) Ligation of the IMA at roughly 1 cm from its origin in left colectomies; or (3) Low ligation of the IMA in segmental sigmoidectomy, distal to the branching point of the left colic artery (LCA) but with subsequent LN dissection along the IMA trunk, up to its origin from the aorta (D3 lymphadenectomy).
Group B colectomies were non-CME surgical procedures, but were performed with intent of oncological radicality. Surgeons involved in this group represent a heterogenous group working in district hospitals without a major interest in colonic cancer surgery. There was no additional selection, except for availability of the post-operative CT scan and curative intent of the surgical procedure.
The individual data for group A patients were gathered from a prospectively maintained electronic database, containing all the patients who had undergone colectomies performed by the same surgical team. The individual data for group B patients were obtained from the registries of the Outpatient Clinic of Medical Oncology at IRO. All these patients were cross-referenced in the hospital’s electronic registry, for confirmation of data. Only patients with an available post-operative abdominal CT scan were included in the study and the images were retrieved from the Picture Archiving and Communication System (known as “PACS”) for analysis.
The majority of patients (n = 51) had undergone spiral CT scans in our radiology department, by means of a BrightSpeed 16SL scanner with 16 detectors (GE Healthcare, Waukesha, WI, United States). The protocol included 16 mm x 1.25 mm increment, pitch of 1.75, table speed of 35 mm/rot, 120 kV and 260 mA, with slight variations according to patient particularities. Contrast media was injected (816.5 mg/mL of Iomeron 400®; Bracco Imaging, Milano, Italy) with a power injector at a rate of 2.5 mL/s. Scans were obtained in the arterial and venous phases, with an acquisition delay of 15 sec and 50 sec after the bolus threshold (150 HU). Datasets were reconstructed with a 1.25–3 mm section thickness, and the reconstruction interval varied between 1.3 mm and 5 mm. A minority of CTs (n = 7) originated in private radiology departments, but biases were minimised as the acquisition protocols and reconstruction intervals were similar and only the reconstructed thickness varied from 1.25 mm to 5 mm, accepted as standard for colorectal follow-up.
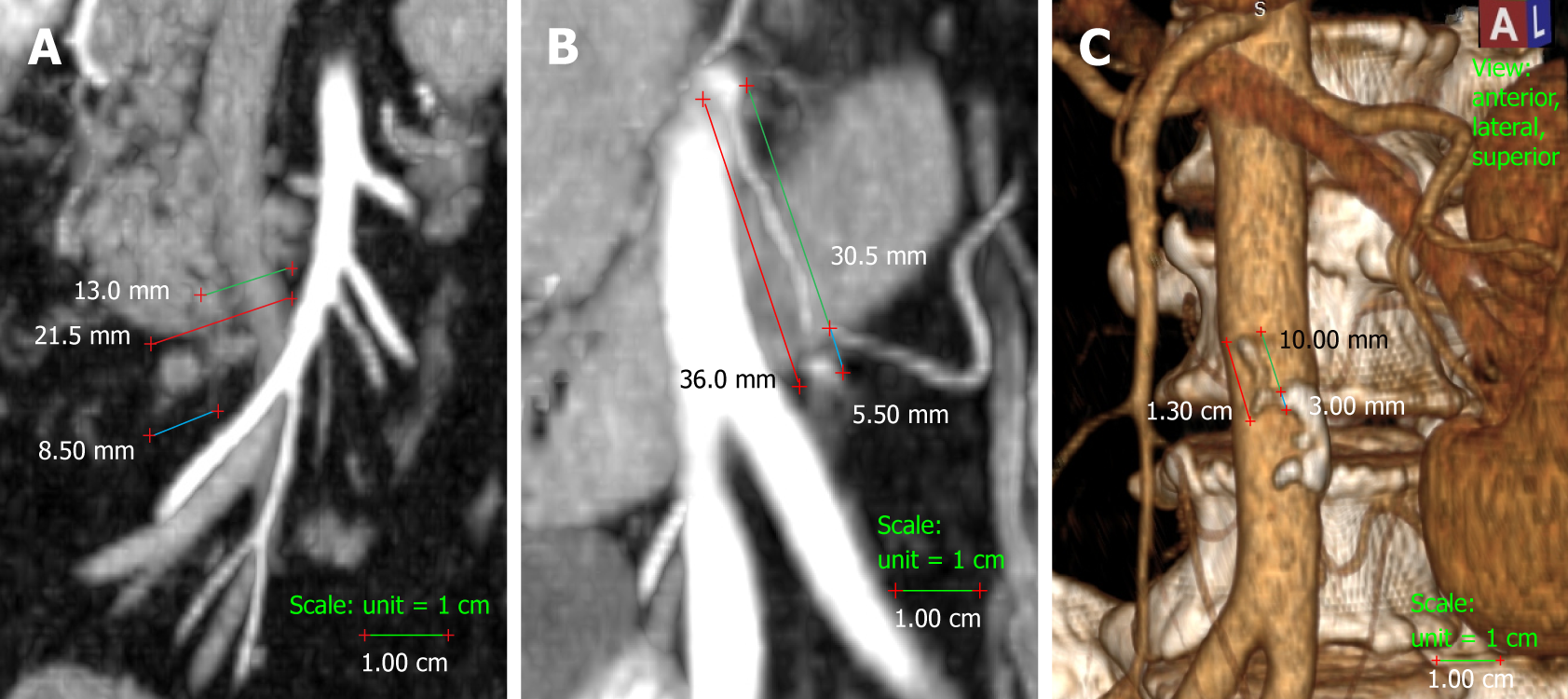
No metal clips were used on the arterial stumps. We examined the CT scans from both groups using a dedicated radiology imaging software (RadiAnt DICOM Viewer v. 4.2.1; Medixant, Poznan, Poland) with 3D reconstructions in multiple planes and a maximum intensity projection of 10 mm. Lengths were measured with the software’s dedicated ruler[28].
On the right side, we only chose the ICA because it is the most constant collateral of the SMA[9]. It was defined as the last vessel arising from the SMA to the right side, at the level of the 5th and 6th jejunal artery emergence, and its trajectory is anterior, inferior and to the right, thus always making an acute angle with the SMA stem[29]. The IMA was defined as originating at the level of the 3rd lumbar vertebral body and descending on a discrete left trajectory[30]. Arterial lumen continuity could be verified by applying negative contrast in different oblique planes, and the stumps were frequently identified through the presence of a granuloma on their tip[26]. Additionally, the anatomical crossing of the ICA (anteriorly or posteriorly) in relation to the SMV was registered.
We measured the presumed and actual arterial stump lengths on all the colectomies included in the study. In right colectomies, the actual arterial length was measured as the length from the origin of the ICA to the ligation point. The presumed arterial length was measured as the length from the origin of ICA to the right-hand side of the SMV, as this is the standard length for D2 ligation (Figure 1). In left-sided colectomies, the ligation point was analysed in relation to the IMA and the LCA. The actual stump length was measured from the origin of the IMA to the ligation point, while the presumed stump length was measured from the IMA origin to immediately distal to the LCA origin, as is done for standard D2 ligation with preservation of the LCA. The D2 improvement potential (D2IP) was defined as the difference between actual stump lengths and the D2PLL (Figure 1). When we compared this with the D3 central ligation, a value of 10 mm was considered as the presumed stump length in left colectomies[2]. The D3 improvement potential (D3IP) was calculated only for the IMA and represents the difference between actual stump lengths and the 10 mm safe length (D3PLL) recommended by consensus[2] (Figure 1).
Measurements were performed by the main author (Livadaru C) and validated independently by two radiologists (Paiu-Spiridon EF and Sava F) from the Radiology Department of IRO, in order to access inter-observer variations. The quantitative parameter (arterial length) was expressed as the average between the three observers.
Statistical analysis was carried out with SPSS v20.0 (IBM Corp, Armonk, NY, United States). Descriptive statistics were performed on all collected data. For verifying the normal distribution of all the data, we used the Shapiro-Wilk test. Only homogenous data were averaged. The inter-observer agreement between the three sets of measurements was confirmed using the Kruskal-Wallis test. We applied the independent Student’s t-test for comparing the mean arterial length between groups A and B. We used the paired t-test to verify whether or not the actual residual lengths of the arteries differed from the D2 and D3 presumed lengths. Furthermore, by using the paired t-test we calculated a specific value that quantified the potential for improvement of the ligation height (defined as the statistically significant difference between the mean actual and presumed stump length). By using the independent t-test, we also compared the potentials for improvement found in each group. Levene’s test was used to compare the actual stump length variation between groups. Whenever the data showed a non-normal distribution, non-parametric tests such as the Kruskal-Wallis were used. A P-value of < 0.05 was considered statistically significant for all tests.
From the 124 consecutively registered patients in group A, 29 colectomies were selected after applying the exclusion criteria. Similarly, for group B, the search in the Oncology Outpatient Clinic registries provided 69 consecutive cases, from which 29 colectomies were selected after applying the inclusion criteria (Figure 2). When stratified for type of colectomy, the stump measurements were normally distributed (Shapiro-Wilk’s test), but overall, when all measurements were considered for each group, they were non-normally distributed - a fact explained by the differences in category between the ICA and IMA. The median time from surgery to post-operative CT scan was 13.5 mo [inter-quartile range (IQR): 6.0-27.0]. The two groups had similar mean time intervals from surgery to the CT scan (18 mo vs 27 mo; t = -1.23, P = 0.227). Patient demographics are listed in Table 1.
| Characteristic | Group A | Group B |
| Age (yr) | ||
| Mean | 64 | 59 |
| Range | 49-80 | 40-76 |
| Sex | ||
| Male | 14 | 16 |
| Female | 15 | 13 |
| Type of colectomy | ||
| Right colectomy | 12 | 10 |
| Left-sided colectomies | 17 | 19 |
| Left colectomy | 4 | 16 |
| Sigmoidectomy | 13 | 3 |
| pT stage | ||
| pTis | 1 | 3 |
| pT2 | 3 | 17 |
| pT3 | 19 | 9 |
| pT4 | 6 | |
| pN stage | ||
| pNx | 1 | |
| pN0 | 13 | 14 |
| pN1 | 11 | 9 |
| pN2 | 4 | 6 |
| M stage | ||
| M0 | 29 | 29 |
| M1 | 0 | 0 |
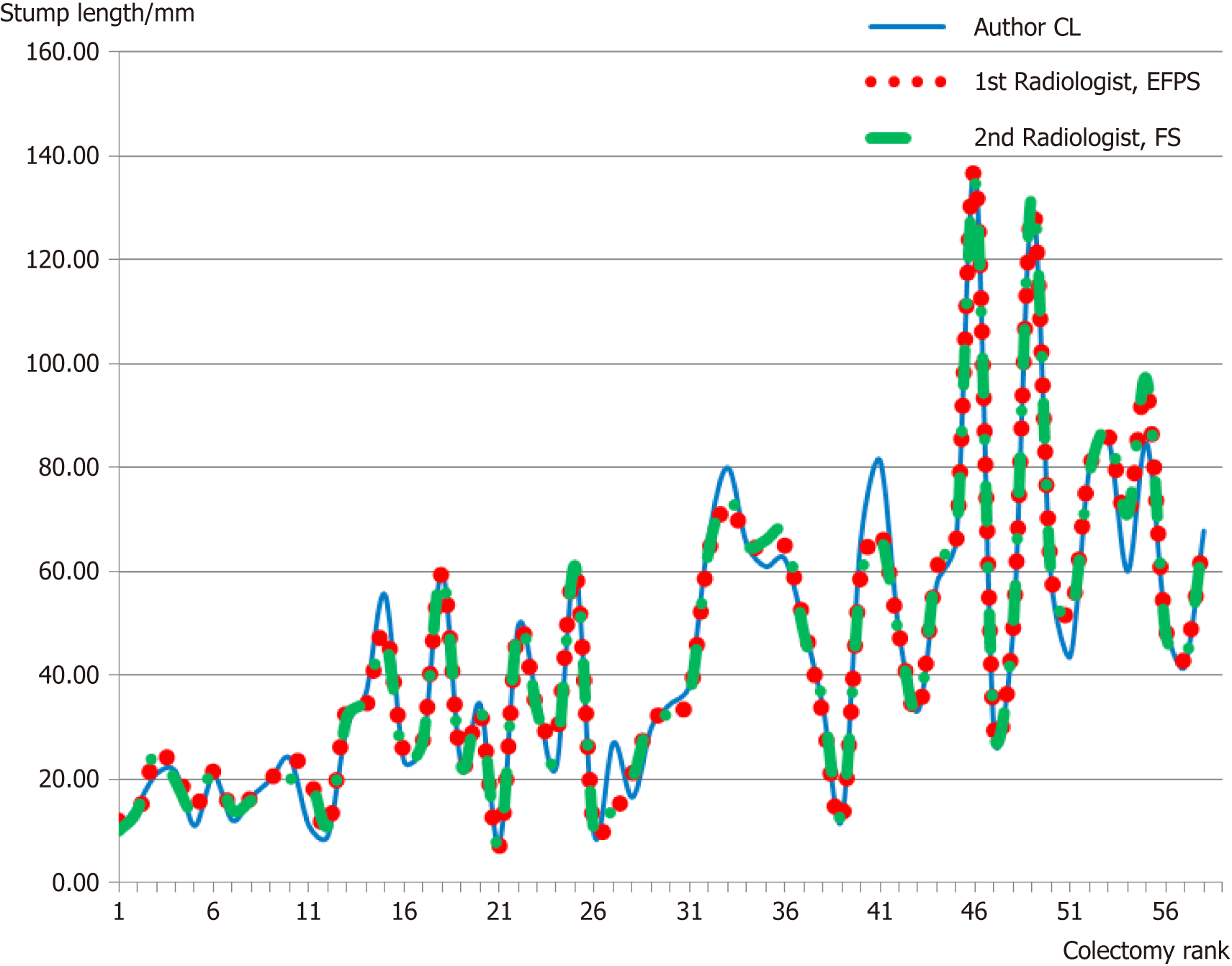
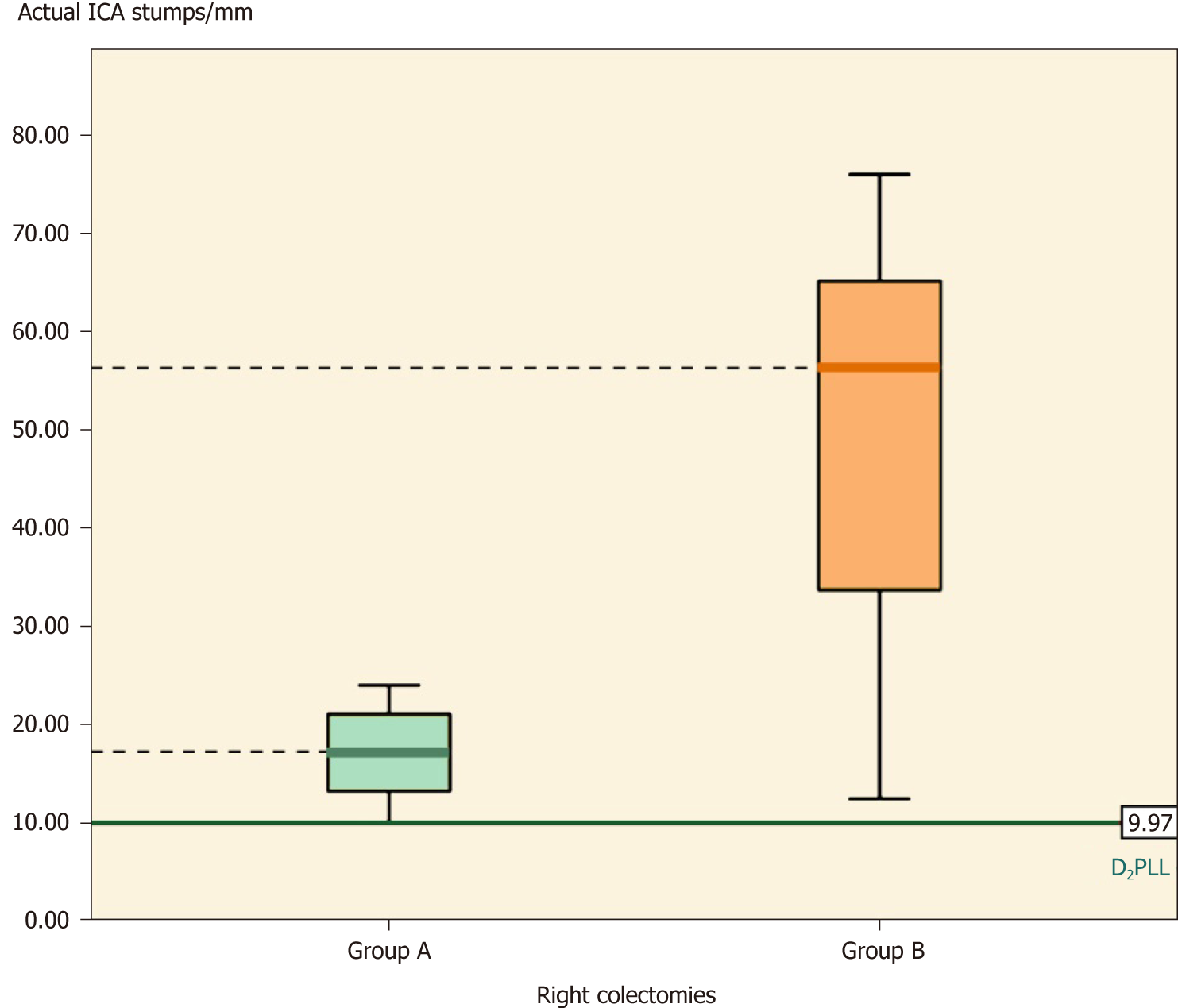
Overall, there were a total of 22 ICA stumps, with the D2PLL median length of 9.97 mm (IQR: 7.85-12.70), and a total of 36 IMA stumps, with the D2PLL mean ± SD length of 37.87 ± 8.22 (95%CI: 35.09-40.65). The level of inter-observer agreement assessed for the three observers with the Kruskal-Wallis test revealed no significant difference between the sets (Table 2, P = 0.999) (Figure 3).
| Observer | n | Median length, mm | IQR, mm | χ2 | 1P-value |
| Livadaru C | 58 | 35.70 | 21.38-60.30 | 0.01 | 0.999 |
| 1st Radiologist | 58 | 34.00 | 21.50-61.63 | ||
| 2nd Radiologist | 58 | 35.00 | 20.98-62.50 |
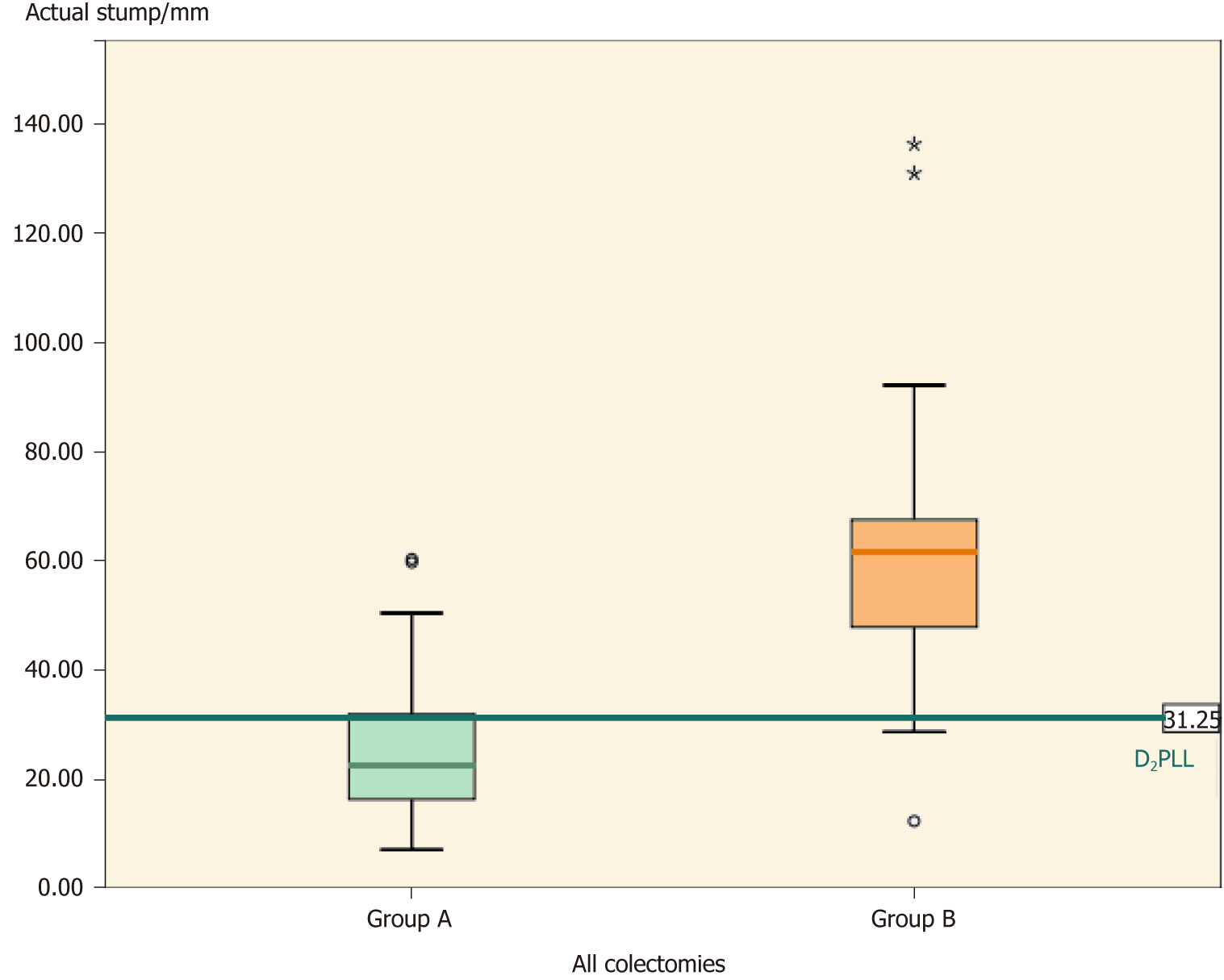
Overall, the groups differed significantly (Table 2, P < 0.001) in actual stump length (-35.66 ± 30.83 mm; P < 0.001) and in D2 improvement potential (-31.88 ± 26.52 mm; P < 0.001). The D2PLL was similar between the two groups (-3.79 ± 13.98 mm; P = 0.353), both overall (Table 3, Figure 4) and when stratified for type of colectomy (Tables 4, 5 and 6). Levene’s test showed that the variances of actual stump lengths between the groups (204.5 vs 732.2) reached the level of statistical significance (P = 0.04).
| Group A, median (mean) in mm, (IQR) | Group B, median (mean) in mm, (IQR) | A – B difference, mean in mm ± SD, (95%CI) | Statistics for A – B difference1 | P-value | |
| All colectomies, n | 29 | 29 | - | - | - |
| Actual stump | 22.63 (25.60), (20.16-31.04) | 61.80 (61.27), (45.07-69.87) | -35.66 ±30.83, (21.24-62.26) | χ2 = 27.38, η² = 0.47 | < 0.0012 |
| D2PLL | 26.00 (25.34), (20.15-30.53) | 34.00 (29.13), (11.00-41.10) | -3.79 ±13.98, (-11.90–4.32) | t = -0.936, df = 56 | 0.353 |
| D2IP, mean ± SD | 0.26 ± 12.18 | 32.14 ± 26.15 | -31.88 ± 26.52, (-41.96-21.79) | χ2 = 22.13, η² = 0.38 | < 0.0012 |
| Statistics for D2IP, χ2 (df), P | 0.005 (1), 0.944 | 21.77 (1), < 0.001 | - | - | - |
| Group A, mean in mm ± SD, (95%CI) | Group B, mean in mm ± SD, (95%CI) | A – B difference, mean in mm ± SE, (95%CI) | Statistics for A – B difference, t (df)1 | P-value | |
| Right colectomies, n | 12 | 10 | - | - | - |
| Actual stump | 16.97 ± 4.77, (13.94-19.96) | 49.93 ± 20.29, (35.40-64.44) | -32.96 ± 6.53, [-47.62-(-18.30)] | -5.02 (9.83) | 0.0012 |
| D2PLL | 11.12 ± 2.97, (9.23-13.00) | 7.80 ± 6.53, (3.13-12.47) | 3.32 ± 2.10, (-1.06-7.69) | 1.58 (20) | 0.130 |
| D2IP | 5.85 ± 4.71, (2.85-8.84) | 42.13 ± 21.50, (26.75-57.50) | -35.78 ± 6.74, [-50.83-(-20.72)] | -5.31 (9.77) | < 0.0012 |
| Statistics for D2IP, t (df), P | 4.30 (11), 0.0012 | 6.20 (9), < 0.0012 | - | - | - |
| Group A, mean in mm ± SD, (95%CI) | Group B, mean in mm ± SD, (95%CI) | A – B difference, mean in mm ± SE, (95%CI) | Statistics for A – B difference, t (df) | P-value | |
| Sigmoidectomies, n | 13 | 16 | - | - | - |
| Actual stump | 35.42 ± 1 5.62, (25.98-44.86) | 69.92 ± 30.29, (53.78-86.06) | -34.50 ± 9.28, [-53.55-(-15.45)] | -3.72 (27) | < 0.0011 |
| D2PLL | 35.15 ± 8.85, (29.80-40.50) | 40.08 ± 8.36, (35.62-44.53) | -4.93 ± 3.20, (-11.50-1.65) | -1.54 (27) | 0.136 |
| D2IP | 0.27 ± 12.90, [-7.53-(-8.06)] | 29.84 ± 29.67, (14.56-45.12) | -29.57 ± 8.01, [-46.20-(-12.94)] | -3.69 (21.72) | 0.0011 |
| D3IP | 25.42 ± 15.62, (15.98-34.86) | 59.92 ± 30.29, (43.78-76.06) | -34.50 ± 9.28, [-53.55-(-15.45)] | -3.72 (27) | < 0.0011 |
| Statistics for D2IP, t (df), P | 0.07 (12), 0.9421 | 4.16 (15), < 0.011 | - | - | - |
| Statistics for D3IP, t (df), P | 5.87 (12), 0.0011 | 7.91 (15), < 0.011 | - | - | - |
| Group A, mean in mm ± SD, (95%CI) | Group B, mean in mm ± SD, (95%CI) | A – B difference, mean in mm ± SE, (95%CI) | Statistics for A – B difference, t (df) | P-value | |
| Left colectomy, n | 4 | 3 | - | - | - |
| Actual stump | 19.60 ± 9.22, (4.94-34.27) | 52.93 ± 13.15, (20.27-85.60) | -33.33 ± 8.37, [-54.85-(-11.81)] | -3.98 (5) | 0.0111 |
| D2PLL | 36.12 ± 3.52, (30.52-41.73) | 40.17 ± 8.25, (19.67-60.66) | -4.04 ±4.50, (-15.60-7.52) | -0.90 (5) | 0.410 |
| D2IP | -16.52 ± 11.71, (-35.16-2.12) | 12.77 ± 19.79, [-36.40-(-61.93)] | -29.29 ± 11.8, [-59.64-(-1.06)] | -2.48 (5) | 0.056 |
| D3IP | 9.60 ± 9.21, (-5.06-24.27) | 42.93 ± 13.15, (10.27-75.60) | -33.33 ± 8.37, [-54.85-(-11.81)] | -3.98 (5) | 0.0111 |
| Statistics for D2IP, t (df), P | -2.82 (3), 0.0671 | 1.12 (2), 0.38 | - | - | - |
| Statistics for D3IP, t (df), P | 2.08 (3), 0.1291 | 5.66 (2), 0.031 | - | - | - |
When we compared the actual ICA stumps between the two groups, the t-test showed that group A had significantly shorter stumps than group B, the difference being -32.96 ± 6.56 mm (mean ± SE) (Table 4, P = 0.001). Furthermore, the D2IP was still significantly longer in group B, by almost 4 cm (Table 4, P < 0.001) (Figure 5).
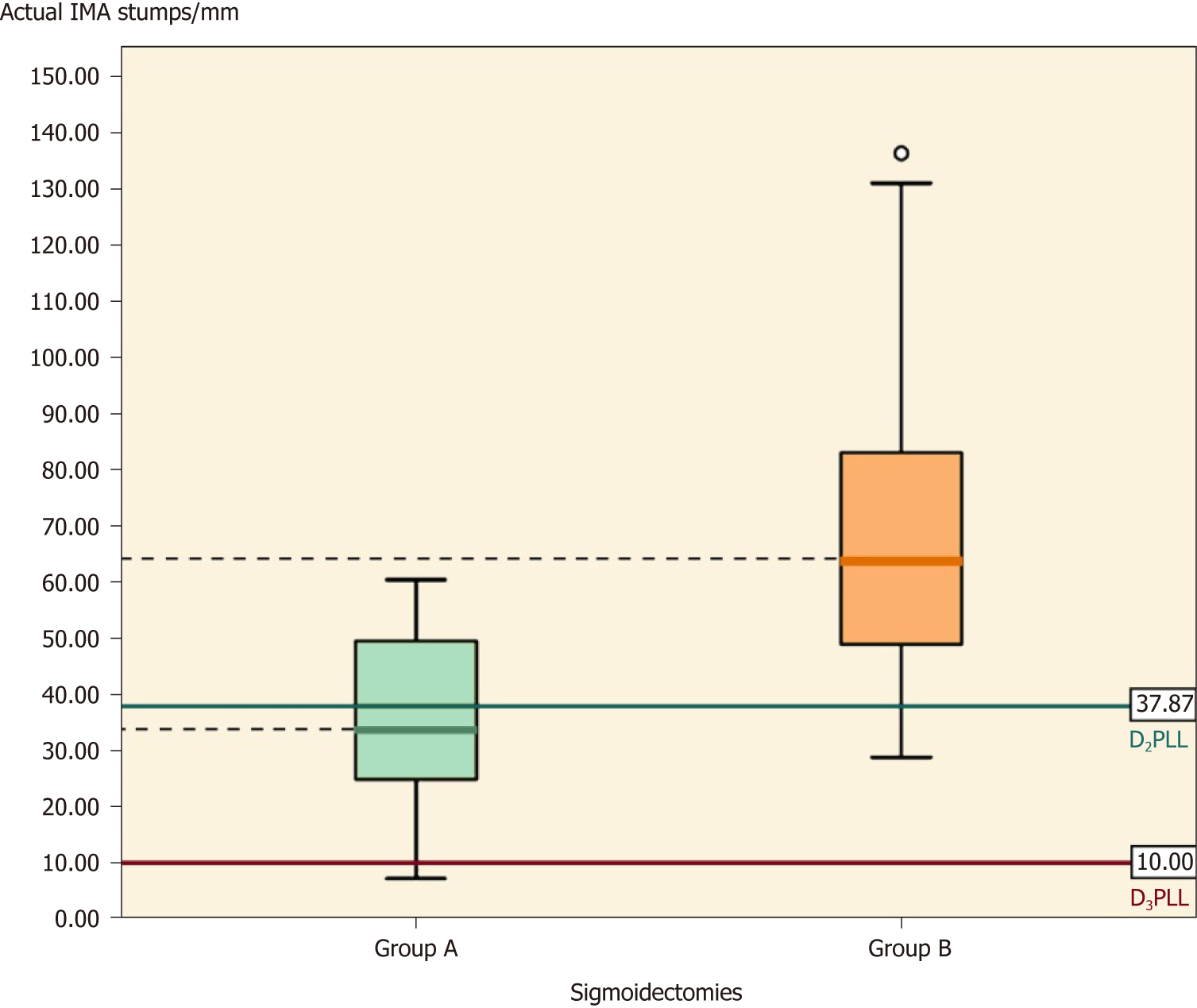
In Table 5, the comparison of IMA stumps between the two groups shows that, apart from D2PLL, all the parameters from group A were significantly shorter, as shown by the independent t-test. The actual difference (mean ± SE) was -34.50 ± 9.28 mm (Table 5, P < 0.001), and the D2IP difference was -29.57 ± 8.01 mm (Table 5, P = 0.001) while the D3IP difference was -34.50 ± 9.28 mm (Table 5, P < 0.001) (Figure 6).
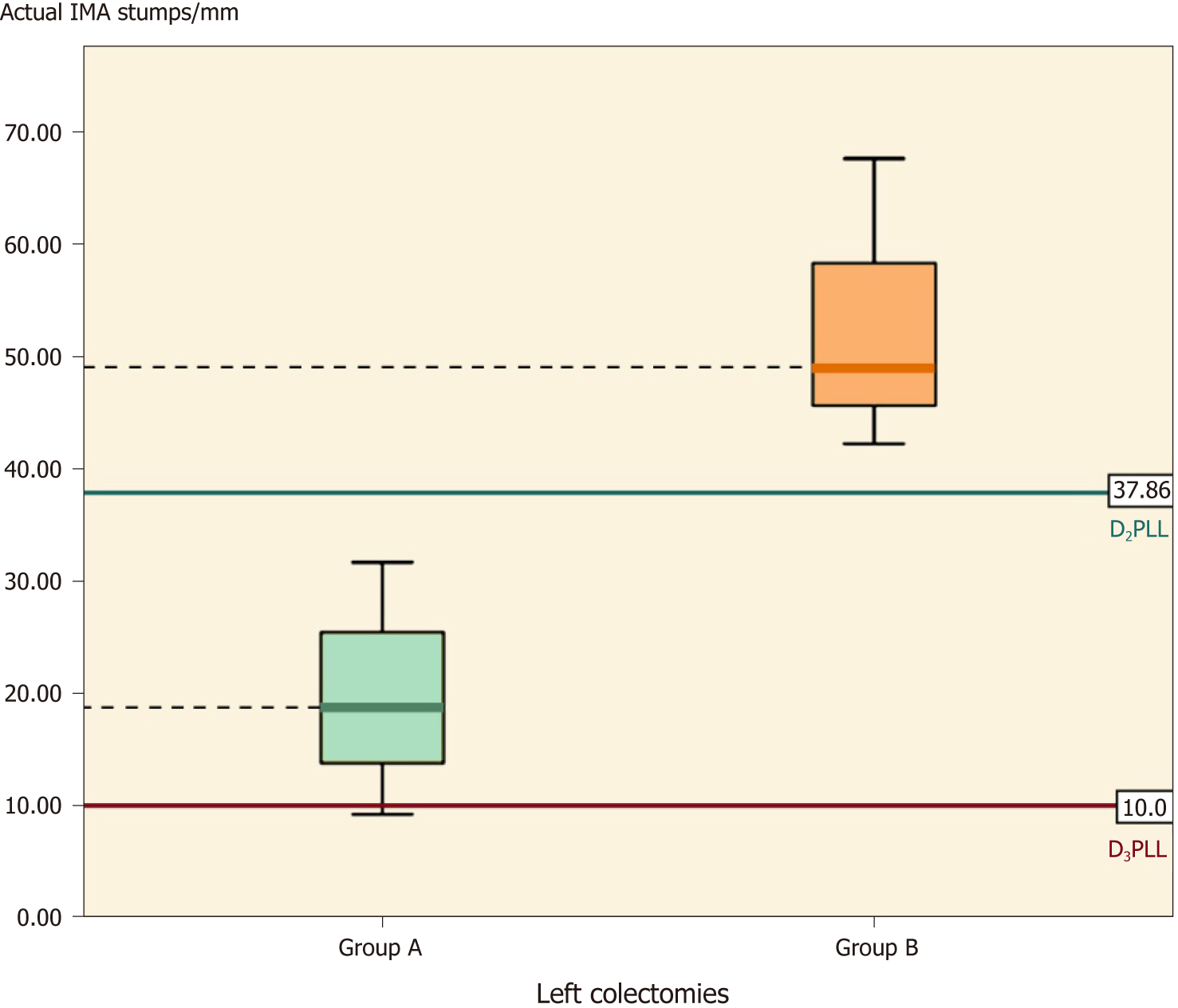
This subgroup followed the same trend of significance, with shorter actual stumps in group A (by 33.33 ± 8.37 mm) (Table 6, P = 0.011). The D2IP appeared similar between the groups (P = 0.056), but group A significantly surpassed the D2 level and achieved D3 as intended, with a D3IP difference of -33.33 ± 8.37 mm (Table 6, P = 0.011) (Figure 7).
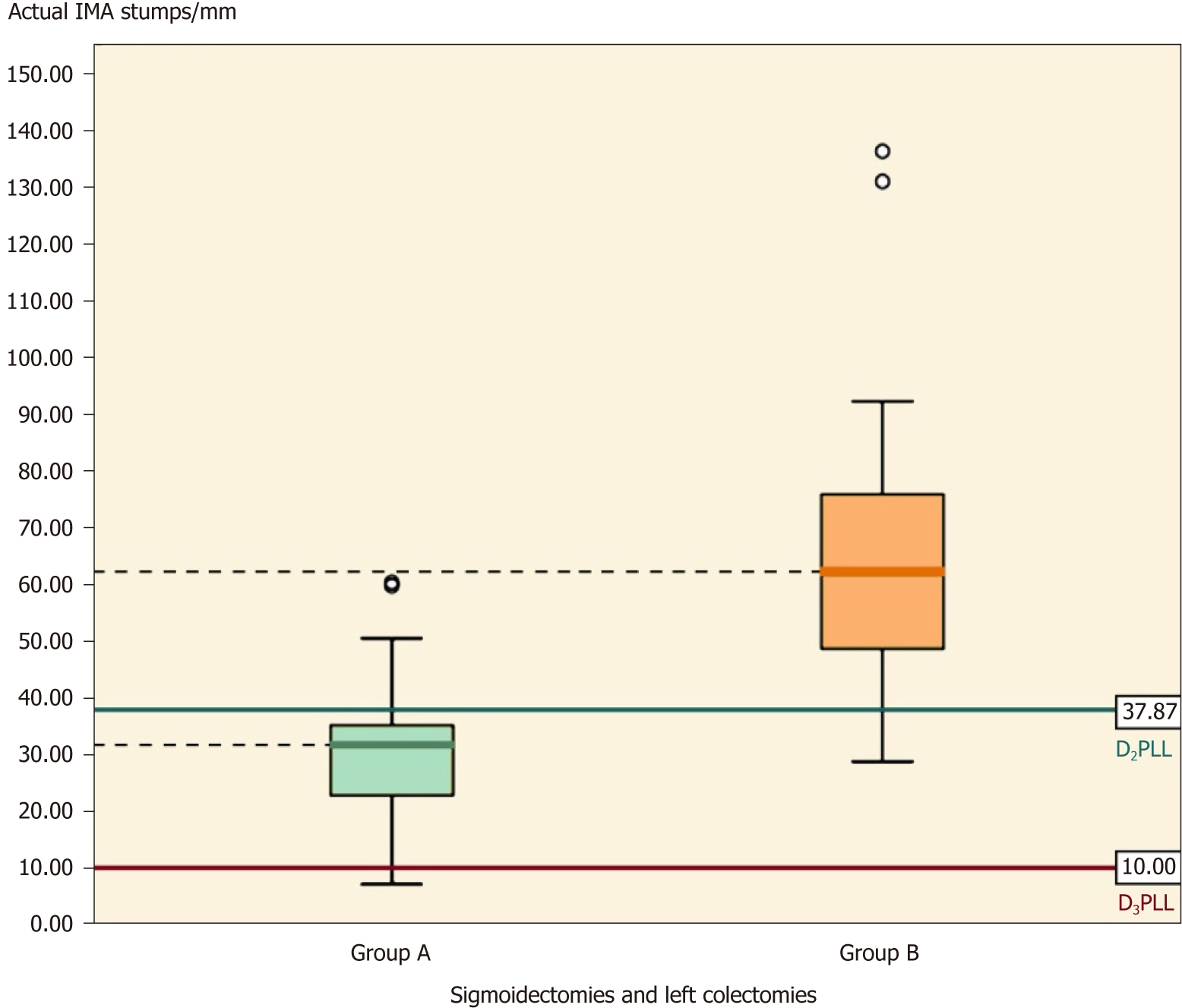
Table 7 presents a summary of all the IMA stumps from this study, by taking sigmoidectomies and left colectomies together. Except for the D2PLL, all the parameters in group A are significantly shorter then group B (Table 7, P < 0.001) (Figure 8).
| Group A, mean in mm ± SD, (95%CI) | Group B, mean in mm ± SD, (95%CI) | A – B difference, mean in mm ± SE, (95%CI) | Statistics for A – B difference, t (df) | P-value | |
| Left colectomy, n | 17 | 19 | - | - | - |
| Actual stump | 31.70 ± 15.71, (23.62-39.77) | 67.24 ± 28.71, (53.40-81.07) | -35.54 ± 7.85, [-51.48-(-19.59)] | -4.53 (34) | < 0.001 |
| D2PLL | 35.38 ± 7.83, (31.36-39.41) | 40.09 ± 8.11, (36.18-44.00) | -4.71 ± 2.66, (-10.13-0.70) | -1.77 (34) | 0.086 |
| D2IP | -3.68 ± 14.30, (-11.04-3.67) | 27.14 ± 27.74, (13.77-40.51) | -30.83 ± 7.25, [-45.68-(-15.97)] | -4.25 (27.5) | < 0.001 |
| D3IP | 21.70 ± 15.71, (13.62-29.78) | 57.24 ± 28.71, (43.40-71.07) | -35.54 ± 7.85, [-51.48-(-19.59)] | -4.53 (34) | < 0.001 |
| Statistics for D2IP, t (df), P | -1.06 (16), l0.304 | 4.26 (18), < 0.011 | - | - | - |
| Statistics for D3IP, t (df), P | 5.69 (16), < 0.001 | 8.68 (18), < 0.001 | - | - | - |
For the 29 actual stumps in group A, the median length was 22.63 mm and the Kruskal-Wallis test showed no statistically significant D2 improvement potential (mean ± SD: 0.26 ± 12.18 mm; χ2 = 0.005, P = 0.944) (Table 3).
In right colectomies, the actual ICA length (mean ± SD) was 16.97 ± 4.77 mm. The mean difference between actual stump lengths and D2PLL was statistically significant, as determined by the paired t-test, revealing a D2 improvement potential of 5.85 ± 4.71 mm (Table 4, P = 0.001).
On the left side, there were a total of 17 residual IMAs examined; the mean length was 31.70 ± 15.71 mm for which the ligation had been done correctly at the D2 level in all colectomies, without any D2IP remaining (-3.68 ± 14.30 mm; P < 0.304), as shown in Table 7.
When stratified for type of colectomy, the paired t-test revealed no D2IP for sigmoidectomies (0.27 ± 12.90 mm) (Table 5, P = 0.942) and left colectomies (-16.52 ± 11.71 mm) (Table 6, P = 0.067). When we tested for the D3 ligation, only left colectomies reached this level (9.60 ± 9.21 mm) (Table 6, P = 0.129), whereas sigmoidectomies fell short of this objective (by 25.42 ± 15.62 mm) (Table 5, P = 0.001).
For the 29 actual stumps in group B, the median length was 61.27 mm, while the mean potential for improvement of 32.14 ± 26.15 mm was statistically significant (χ2 = 21.77, P < 0.001) (Table 3).
In right colectomies, the actual ICA length (mean ± SD) was 49.93 ± 20.29 mm. The mean difference between actual stump lengths and D2PLL was statistically significant by the paired t-test, revealing a D2IP of 42.13 ± 21.50 mm (Table 4, P < 0.001).
The mean IMA stump length from 19 left-sided colectomies (sigmoidectomies and left colectomies) was 67.24 ± 28.71 mm. The paired t-test showed a statistically significant difference between actual and presumed lengths, showing that in group B the ligation had been made lower than the D2 level, with a mean D2IP of 27.14 ± 27.74 mm (Table 7, P < 0.01).
When stratified for type of colectomy, the 16 sigmoidectomies had mean IMA stumps of 69.92 ± 30.29 mm and statistically significant D2IP (29.84 ± 28.67 mm) (Table 5, P < 0.01) and D3IP (59.92 ± 30.29 mm) (Table 5, P < 0.01). The subgroup of left colectomies was the only one to output no statistically significant D2IP (P = 0.38), but significant D3IP (42.93 ± 13.15 mm) (Table 6, P = 0.03).
In group A, the mean ± SD number of LNs harvested with the CME technique was 34.83 ± 16.75 and Spearman’s test showed moderate (rs = -0.40) inverse correlation with the stump lengths (Table 8, P = 0.032). Similarly, the significance persisted for the specimen length with moderate correlation (rs = -0.44); as such, the greater the specimen length was, the shorter the stump length (Table 8, P = 0.016).
In group B, LNs were available in the Oncology outpatient registries only for 18 out of the 29 patients. The median number of LNs was 20 (IQR: 10.75-26.50). Spearman’s correlation with the stump length was negligible (rs = -0.112) and without significance (P = 0.659).
Out of the 22 ileocolic stumps, the SMV was crossed posteriorly by the ICA in 68% of cases. In group A, surgeons preserved the LCA in 77% of sigmoidectomies, demonstrating a D2 ligation intent, whereas none of the left colectomies had a preserved LCA, showing D3 ligation intent. In group B, the LCA was present in 81% of sigmoidectomies and in all left colectomies, suggesting a D2 intent for sigmoidectomies and a questionable D2 intent for left colectomies.
As far as our research into the literature has shown, this is the first study to radiologically compare post-resectional stumps between two groups, of which one provides standard resection quality and the second study, after that of Munkedal et al[22], assessed both ileocolic and IMA stumps after CME. The retrospective setting of our study ensured that the surgical team operated based on their regular standard, without making efforts for achieving shorter stumps. In this sense, there is the advantage of reflecting our surgical practice in a more trustworthy manner.
The tight distribution of arterial stump lengths from our series (group A) is proof of a uniform and disciplined surgical procedure (Figures 4, 5 and 8). The argument behind this statement is the 0% locoregional recurrence at a minimum of 2 years for the 88 colectomies from which group A was extracted[27]. By constantly performing the D2 ligation, rigorous harvesting of LNs with their corresponding mesocolons was achieved and, thus, contributed to a zero local recurrence rate in the larger cohort[27]. The fact that group B did not use a standardized technique was also revealed by the significantly larger variation in stump size, as compared to group A (P = 0.04). This probably reflects an incomplete mesocolic excision because, theoretically, the mesocolic fascia leads up to the origin of the primary feeding vessels and would result in a short stump if completely excised. This presumption will be verified in the future, but a 60-mm stump is certainly representative of an incomplete resection. Other similar studies[20,26] showed that the wider the variation in stump length, the larger the variation in fascial plane of dissection. Hence, when we have the prerequisites for a large variance in technique (i.e. non-standardized surgery), there is a higher risk of having low quality surgery, which impacts correct staging, local recurrence, and overall survival[19,31-34].
Although our results are comparable with the null loco-regional recurrence from the prospectively performed CME surgeries of Galizia et al[35] and with the 3.6% from Hohenberger and colleague’s[1] series of 1329 CME colectomies with D3 CVL, the clear extra benefit of D3 vs D2 ligation is controversial when compared to the benefit of pristine embryologic plane dissection[3,4,36,37]. We believe that the foremost important factor for minimizing local recurrence is respecting the mesocolic plane, in order to prevent spillage of cancerous cells. Performing the central ligation in right colectomy at the D3 rather than the D2 level is not supported by any randomized trial yet[5], increasing peri-operative risks without major benefits for the general population.
All the arterial stumps were shorter in group A than group B, not only overall but also when stratified for type of colectomy. We wanted to assess if this was caused by the variability in the ICA-SMV crossing lengths, on the right side, or the LCA emerging lower down from the IMA, on the left side. Hence, we also measured the personalized presumed lengths for true D2 ligation, in each group. By subtracting the actual stumps from the presumed stumps, the D2 potential for improvement was calculated as a personal quality measurement for the surgical team. These were significantly shorter in group A, proving that across group B colectomies, the standardized D2 level of ligation was systematically not reached (Tables 4, 5, 6 and 7). The only exception was the subgroup of left colectomies that showed statistical similarity at the D2 level, even though this did not reflect similar ligation intents. Group A surgeons intended and significantly reached D3 (P = 0.129), whereas in group B, the D3 potential was not reached (P = 0.03) (Table 6).
The right colectomy subgroup from group A had slightly longer stumps than expected for D2 (by 5.80 mm), but they were still the shortest CT-evaluated post-colectomy stumps in the literature[22,25,26] (Table 9). Moreover, by calculating the potentials for improvement for left-sided colectomies performed in our department (0.27 mm), we determined that they are correctly realised at the D2 level (P = 0.94). In contrast to other similar studies[21], our experience showed no excess IMA stumps compared to pre-determined vascular and anatomical boundaries for the D2 ligation (Table 10). The left colectomy subgroup was the only one in which the D3 level had been achieved in all cases (9.60 mm; P = 0.129).
| Study, year | n | Actual ileocolic stump in mm | Presumed D2 ileocolic stump in mm |
| Group A - our study, 2018, mean ± SD (95%CI) | 12 | 17.69 ± 4.77 (13.94-19.96) | 11.12 ± 2.97 (9.23-13.00) |
| Munkedal et al[22] 2017, mean (95%CI) | 32 | 31.0 (25-37) | 10.01 |
| Kaye et al[26] 2015, mean, (range) | 128 | 28.1 (2.5–74.3) | 14.4 (6.4) |
| Spasojevic et al[25] 2011, mean ± SD | 11 | 28.0 ± 9.3 | 18.1 ± 4.0 |
The LN yield is considered to be a very important independent prognostic factor for colon cancer survival[32-34,38]. Using our D2 CME technique, we harvested a mean number of 34.8 LNs, which proved to be superior to the number of LNs reported through the employment of CME with D3 CVL from Erlangen: a median of 32 LNs reported in the first series[1] and of 30 LNs reported in a subsequent series[20]. The length between the origin of the IMA and the departure of the LCA was 37.87 ± 8.22 mm (mean ± SD) in our study, and this segment of artery alone was previously shown to harbour up to 10 LNs[39]. Rosi et al[40] demonstrated that up to 22% of patients with sigmoid tumours had positive LNs at a distance of 10 to 30 mm from the origin of the IMA, thus showing that the ligation height impacted survival. Since we showed that LNs inversely correlate to the feeding stump length, their prognostic value could be transferred to some extent onto the stump length. These results aid the arguments for considering the measurement of post-colectomy arterial stumps as an additional marker for surgical quality evaluation, with the potential to impact survival.
One similar study[22] that reported longer stumps found no correlations between node count and stump length. In that sense it supports the argument that, a more central ligation provides a more realistic correlation with harvested LNs, assuming that a dedicated pathology department counted the LNs[27]. This impacts a more accurate staging[41] and could theoretically transfer an under-staged patient from stage II to III, in order to provide benefit not just from the adjuvant chemotherapy but also from the additional prognostic factor. Additionally, the advantage of a surgical technique that comprehensively retrieves the mesocolon ensures that significantly more of the newly described tumour deposits are being included in the specimen, when comparing CME specimens to classic colectomy specimens[35]. The prognostic capability of tumour deposits in the mesocolon is analogous to that of positive LNs, with node-negative patients in stages I or II requiring adjuvant chemotherapy[38].
A limit of our study could be the small population size, but we used strict inclusion and exclusion criteria and analysed a single surgical team with unchanging practice of surgical technique to determine the accuracy of stump measurement. Seven scans originated in different radiology departments; hence, for these, the acquisition protocols varied slightly in terms of slice reconstruction interval, but we do not believe this produced a sizeable difference. The presence of metal clips on the stump would have accelerated the identification process, but from our radiologists’ viewpoint, they tend to add measuring artefacts. The first author (Livadaru C), who measured and interpreted the quantitative and qualitative parameters, is not a radiologist, but all the data were validated by two independent radiologists (Paiu-Spiridon EF and Sava F) and the inter-observer agreement test showed no statistically significant difference between the measurements of the actual stump lengths. This strong correlation between observers indicates that there is high feasibility of performing the arterial stump evaluation on follow-up contrast CTs by non-radiological observers, regardless of metal clips. Pre-operative CT scans paired with the follow-up scans would have aided the stump identification but we do not consider pre-operative scans essential, as a member of the surgical team would be able to clearly identify the desired artery based on familiarity with anatomical landmarks from the operating field.
Regarding the time interval from surgery to the post-operative CT, other authors have already shown that the stumps are clearly visible more than 2 years after surgery[26]. Even if the residual arteries could be affected by post-resectional retraction over this time, the two groups in our study had statistically similar time intervals from operation to CT scan (P = 0.227), precluding it from having a major influence on our comparison.
The CME consensus study group advises a 10 mm safety stump when performing the CVL[2]. However, a recent systematic review and meta-analysis on mesenteric vessels variations determined the mean distance from the origin of the ICA to the right side of the SMV to be 15.2 mm[9]; our study also showed a median of 9.97 mm. Although these values correspond to a D2 ligation, they are comparable with the safety margin of 10 mm proposed for the central ligation. Thus, it would not be wrong to state that a CME with D2 ligation could be equivalent to a CME with CVL in the cases when the crossing distance is less or equal to 10 mm, thus sparing the patient from unnecessary haemorrhagic risks that dissection under the SMV would entail. The original work of Spasojevic et al[13] shows a benefit of five to six LNs when the D2 ligation properly includes the vertical compartment situated lateral to the SMV. Furthermore, as they showed the LN distribution is dependent to anterior or posterior ICA crossing patterns, they emphasised tailoring the central ligation with respect to these anatomical variants. Hence, a correct D3 lymphadenectomy could be performed without dissecting behind the SMV in 42.6% of the reported cases, where the ICA ran in front of the SMV (calculated from a recent pooled analysis of 6090 ileocolic arteries[9]) and the retrieved nodal compartment would additionally yield a mean of three LNs[13]. Whether these three LNs would make a substantial difference in the outcomes remains unknown.
Most importantly, we showed both significantly longer stumps and greater spread of results upon comparing non–standardized colectomies with CME. We believe that measuring the arterial stump after colectomies is a simple and reproducible tool for controlling surgical quality and does not require a specialist radiologist. The fact that it quantifies the potential for improving the ligation allows for the surgical technique to be monitored and improved accordingly. In order to ensure the best survival outcomes for each patient, this instrument should be associated with the pathologist’s evaluation of fascial plane stratification[19]. With these two instruments of standardization, standardized interventions can be promoted amongst surgeons and the learning curve for D2 CME could grow in non-tertiary surgical centres.
We put forward now the feasibility of performing accurate arterial stump evaluation by non-radiologist interpreters. The tight distribution of arterial stumps reflects the constancy and quality of a disciplined surgery. The retrospective setting offers insurance that stumps had not been ligated shorter for the sake of comparison, as their length reflects only strong adherence to the oncological principles of CME. As the mesocolic root inserts high on the mesenteric vessels, longer stumps would inherently be surrounded by residual mesocolic tissue which allows risk of local recurrence. Measuring arterial stumps could be an additional tool for a surgical team’s personal quality measurement. As we showed it to significantly correlate with a major prognostic factor (LN yield), this straightforward and reproducible tool may similar potential to predict outcomes. Considering that no serious risks are added when attempting a D2 vascular ligation, it is recommendable that all surgeons apply D2-CME as standardized technique for colon cancer, in order to maximize benefits for their patients.
Surgery has witnessed a paradigm shift in colon cancer management ever since prominent researchers have proposed complete mesocolic excision (CME) as the optimal surgical technique. CME specimens have been demonstrated as superior to standard resections, and patient outcomes have significantly improved. Despite this, adoption among surgeons is still not clear because quality criteria are not well defined. On this basis, researchers have proposed various quality markers - lymph node yield, mesocolon area, distance from central vascular ligation (CVL) to colon margin, etc.
Because quality criteria in colon surgery are not yet defined, CME adoption is still behind total mesorectal excision. A consensus is needed to group already-proven pathological quality markers with newly-advocated radiological markers and to establish standards in colon surgery. The value of measuring arterial stumps on post-operative computed tomography (CT) scans has been previously analysed, but never in a comparative study between CME and standard specimens.
In our advent to better define quality criteria for colon resections, we sought to analyse the value of measuring arterial stumps on post-operative CT scans in a comparative setting between CME and non-CME specimens, for the first time. By testing our hypothesis that arterial stumps are shorter in the CME group and are correlated with prognosis, we aimed to establish arterial stumps as tools to assess CME surgery.
This study was designed as a retrospective analysis and conducted on a prospectively maintained database. Two groups of adult patients were included in consecutive order. All underwent surgery with curative intent for colon cancer (stages I-III UICC 7th edition) and had at least one post-operative good quality contrast-enhanced CT scan that was available for re-evaluation. Group A were operated based on standard CME principles whereas group B underwent conventional colectomy. Measurements of arterial stumps were done by three observers. Shapiro-Wilk test was used to verify normal distribution of data. Kruskal-Wallis test confirmed inter-observer correlation. Stump measurements were analysed comparatively using Student’s t-test. Paired and independent t-test was used to quantify potential for improvement of the ligation height and to compare potentials for improvement between the two groups. Non-normal distribution and non-parametric data was analysed using Kruskal-Wallis test.
From 193 consecutive patients, 58 patients were selected after applying the inclusion and exclusion criteria (29 in CME group, 29 in non-CME group). After comparatively analyzing stump length in both groups, Shorter lengths were obtained in group A, by a mean difference of 35.66 mm (χ2= 27.38, P < 0.001), which was significant for all types of colectomies. Ligations from group A significantly reached their potential height (0.26 ± 12.18 mm from D2PLL; χ2= 0.005, P = 0.944) in comparison with group B were the overwhelming majority failed to reach D2PLL, by a mean difference of 32.14 ± 26.15 mm (χ2 = 21.77, P < 0.001). Moreover, improvement potentials were far shorter in group A than group B (χ2= 22.13, P < 0.001). Significant more variability was found in resections of group B (P = 0.004). No significant difference was found when measurements of three different observers were analysed (P = 0.866). Stump length was statistically correlated with specimen length and lymph node yield (P = 0.018 and P = 0.008 respectively).
Measuring arterial stumps is a simple and standard tool for defining surgical quality of colon resections. It may be, as well, a straightforward prognostic factor given its correlation with lymph node yield.
Our study is a step forward in refining quality criteria for colon surgery. Further research is needed on larger cohorts to compare the value of stump measurement to specimen measurements such as CVL distance or mesocolon surface area. The threshold for CVL should be further analysed, as D3 dissection may not aid in significantly better surgical specimens and outcomes.
We would like to express our gratitude to Mrs. Oana Frunza for illustrating the statistical data into graphical forms.
| 1. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 2. | Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D'Hoore A, Kennedy RH, West NP, Kim SH, Heald R, Storli KE, Nesbakken A, Moran B. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Quirke P, West N. Quality of surgery: has the time come for colon cancer? Lancet Oncol. 2015;16:121-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Madoff RD. Defining quality in colon cancer surgery. J Clin Oncol. 2012;30:1738-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ong ML, Schofield JB. Assessment of lymph node involvement in colorectal cancer. World J Gastrointest Surg. 2016;8:179-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 6. | Bertelsen CA. Complete mesocolic excision an assessment of feasibility and outcome. Dan Med J. 2017;64. [PubMed] |
| 7. | Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gögenur I; Copenhagen Complete Mesocolic Excision Study (COMES); Danish Colorectal Cancer Group (DCCG). Short-term outcomes after complete mesocolic excision compared with 'conventional' colonic cancer surgery. Br J Surg. 2016;103:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017;19:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 9. | Negoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. Surgical Anatomy of the Superior Mesenteric Vessels Related to Colon and Pancreatic Surgery: A Systematic Review and Meta-Analysis. Sci Rep. 2018;8:4184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Dobrowolski S, Hać S, Kobiela J, Sledziński Z. Should we preserve the inferior mesenteric artery during sigmoid colectomy? Neurogastroenterol Motil. 2009;21:1288-e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Liang JT, Huang KC, Lai HS, Lee PH, Sun CT. Oncologic results of laparoscopic D3 lymphadenectomy for male sigmoid and upper rectal cancer with clinically positive lymph nodes. Ann Surg Oncol. 2007;14:1980-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Ignjatovic D, Sund S, Stimec B, Bergamaschi R. Vascular relationships in right colectomy for cancer: clinical implications. Tech Coloproctol. 2007;11:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Spasojevic M, Stimec BV, Dyrbekk AP, Tepavcevic Z, Edwin B, Bakka A, Ignjatovic D. Lymph node distribution in the d3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Dis Colon Rectum. 2013;56:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Slim K, Blay JY, Brouquet A, Chatelain D, Comy M, Delpero JR, Denet C, Elias D, Fléjou JF, Fourquier P, Fuks D, Glehen O, Karoui M, Kohneh-Shahri N, Lesurtel M, Mariette C, Mauvais F, Nicolet J, Perniceni T, Piessen G, Regimbeau JM, Rouanet P, sauvanet A, Schmitt G, Vons C, Lasser P, Belghiti J, Berdah S, Champault G, Chiche L, Chipponi J, Chollet P, De Baère T, Déchelotte P, Garcier JM, Gayet B, Gouillat C, Kianmanesh R, Laurent C, Meyer C, Millat B, Msika S, Nordlinger B, Paraf F, Partensky C, Peschaud F, Pocard M, Sastre B, Scoazec JY, Scotté M, Triboulet JP, Trillaud H, Valleur P. [Digestive oncology: surgical practices]. J Chir (Paris). 2009;146 Suppl 2:S11-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Otchy D, Hyman NH, Simmang C, Anthony T, Buie WD, Cataldo P, Church J, Cohen J, Dentsman F, Ellis CN, Kilkenny JW, Ko C, Moore R, Orsay C, Place R, Rafferty J, Rakinic J, Savoca P, Tjandra J, Whiteford M; Standards Practice Task Force; American Society of Colon and Rectal Surgeons. Practice parameters for colon cancer. Dis Colon Rectum. 2004;47:1269-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Chang GJ, Kaiser AM, Mills S, Rafferty JF, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of colon cancer. Dis Colon Rectum. 2012;55:831-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 617] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 18. | Helsedirektoratet. National action plan with guidelines for diagnosis, treatment and follow up of cancer in the colon and rectum. Oslo: Helsedirektoratet 2017; . |
| 19. | West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 20. | West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 21. | West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012;30:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 22. | Munkedal DLE, Rosenkilde M, Nielsen DT, Sommer T, West NP, Laurberg S. Radiological and pathological evaluation of the level of arterial division after colon cancer surgery. Colorectal Dis. 2017;19:O238-O245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Solon JG, Cahalane A, Burke JP, Gibbons D, McCann JW, Martin ST, Sheahan K, Winter DC. A radiological and pathological assessment of ileocolic pedicle length as a predictor of lymph node retrieval following right hemicolectomy for caecal cancer. Tech Coloproctol. 2016;20:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Hohenberger P, Schlag P, Kretzschmar U, Herfarth C. Regional mesenteric recurrence of colorectal cancer after anterior resection or left hemicolectomy: inadequate primary resection demonstrated by angiography of the remaining arterial supply. Int J Colorectal Dis. 1991;6:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Spasojevic M, Stimec BV, Gronvold LB, Nesgaard JM, Edwin B, Ignjatovic D. The anatomical and surgical consequences of right colectomy for cancer. Dis Colon Rectum. 2011;54:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Kaye TL, West NP, Jayne DG, Tolan DJ. CT assessment of right colonic arterial anatomy pre and post cancer resection - a potential marker for quality and extent of surgery? Acta Radiol. 2016;57:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Morarasu S, Frunza T, Bilavschi K, Patrascu AM, Lunca S, Dimofte G. Histopathology report on colon cancer specimens; measuring surgical quality, an increasing stress for surgeons. J Mind Med Sci. 2018;5:75-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Spasojevic M, Stimec BV, Fasel JF, Terraz S, Ignjatovic D. 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surg Endosc. 2011;25:1883-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Vandamme JP, Van der Schuren G. Re-evaluation of the colic irrigation from the superior mesenteric artery. Acta Anat (Basel). 1976;95:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Panagouli E, Lolis E, Venieratos D. A morphometric study concerning the branching points of the main arteries in humans: relationships and correlations. Ann Anat. 2011;193:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gögenur I; Danish Colorectal Cancer Group. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (2)] |
| 32. | Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 858] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 33. | Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 34. | Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Galizia G, Lieto E, De Vita F, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, Castellano P, Napolitano V, Orditura M. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int J Colorectal Dis. 2014;29:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Hogan AM, Winter DC. Complete mesocolic excision--a marker of surgical quality? J Gastrointest Surg. 2009;13:1889-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Dimitriou N, Griniatsos J. Complete mesocolic excision: Techniques and outcomes. World J Gastrointest Oncol. 2015;7:383-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Jessup JM, Goldberg RM, Asare EA, Benson III AB, Brierley JD, Chang GJ, Chen V, Compton CC, De Nardi P, Goodman KA, Gress D, Guinney J, Gunderson LL, Hamilton SR, Hanna NN, Kakar S, Kosinski LA, Negoita S, Ogino S, Overman MJ, Quirke P, Rohren E, Sargent DJ, Schumacher-Penberthy LT, Shibata D, Sinicrope FA, Steele SR, Stojadinovic A, Tejpar S, Weiser MR, Welton ML, Washington MK, Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE. Colon and Rectum. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE. AJCC Cancer Staging Manual. New York: Springer 2017; 251-274. |
| 39. | Grinnell RS. Results Of Ligation Of Inferior Mesenteric Artery At The Aorta In Resections Of Carcinoma Of The Descending And Sigmoid Colon And Rectum. Surg Gynecol Obstet. 1965;120:1031-1036. [PubMed] [DOI] [Full Text] |
| 40. | Rosi PA, Cahill WJ, Carey J. A ten year study of hemicolectomy in the treatment of carcinoma of the left half of the colon. Surg Gynecol Obstet. 1962;114:15-24. [PubMed] |
| 41. | Titu LV, Tweedle E, Rooney PS. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: a systematic review. Dig Surg. 2008;25:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Prevot F, Sabbagh C, Deguines JB, Potier A, Cosse C, Yzet T, Regimbeau JM. Are there any surgical and radiological correlations to the level of ligation of the inferior mesenteric artery after sigmoidectomy for cancer? Ann Anat. 2013;195:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao ZF, Chen XZ, Hori T, Jeong KY S- Editor: Ma YJ L- Editor: A E- Editor: Song H