Published online Nov 15, 2019. doi: 10.4251/wjgo.v11.i11.1031
Peer-review started: June 6, 2019
First decision: August 23, 2019
Revised: September 7, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: November 15, 2019
Processing time: 164 Days and 14.9 Hours
Cetuximab in combination with oral fluoropyrimidine (FP) remains controversial in metastatic colorectal cancer (mCRC). In view of the regional variation in the tolerability of FP, we conducted a retrospective analysis to compare oral FP with infusional FP in combination with cetuximab in Chinese population.
To compare the efficacy and safety profile of cetuximab in combination with oral FP and infusional FP in Chinese population in the real-world setting.
A retrospective cohort study was done to analyse consecutive patients with Kras wild-type mCRC who received first-line treatment with cetuximab and FP-based chemotherapy in our unit from January 2010 to December 2015. Ninety-five eligible patients were included. The median follow-up of our cohort was 65.0 mo.
The median progression-free survival (mPFS) and median overall survival (mOS) of the entire cohort were 9.66 mo (95%CI: 7.72–12.5) and 25.8 mo (95%CI: 18.7–35.6), respectively. Between oral FP and infusional FP, there was no statistical significant difference in the mPFS [9.79 mo (95%CI: 7.49–12.7) vs 9.63 mo (95%CI: 6.34–13.4); P = 0.72] and mOS [25.8 mo (95%CI: 15.2–35.6) vs 26.3 mo (95%CI: 18.7–41.2); P = 0.63]. Grade 3 or above adverse events were reported in 28.4% of patients, being similar with oral and infusional FP, and included 10.5% of neutropenia and 2.1% of diarrhoea events.
The current analysis demonstrates comparable efficacy and safety profiles of cetuximab in combination with oral and infusional FP in Chinese population. The results expand treatment options for Chinese patients and invite revision of existing treatment guidelines to incorporate oral FP-based chemotherapy plus cetuximab.
Core tip: Cetuximab in combination with oral fluoropyrimidine (FP) remains controversial in metastatic colorectal cancer. A retrospective cohort analysis was performed to compare oral FP with infusional FP in combination with cetuximab in Chinese population in the real-world setting. Our analysis demonstrated comparable efficacy and safety profiles of cetuximab in combination with oral and infusional FP. Thus, oral FP-based chemotherapy should be considered as a valid option in Chinese patients receiving cetuximab.
- Citation: Lam KO, Fu MC, Lau KS, Lam KM, Choi CW, Chiu WH, Yuen CM, Kwok LH, Tam FK, Chan WL, Chan SY, Ho PY, Leung TW, Lee HF. Revisiting oral fluoropyrimidine with cetuximab in metastatic colorectal cancer: Real-world data in Chinese population. World J Gastrointest Oncol 2019; 11(11): 1031-1042
- URL: https://www.wjgnet.com/1948-5204/full/v11/i11/1031.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i11.1031
Addition of cetuximab, an anti-epidermal growth factor receptor monoclonal antibody (anti-EGFR mAb), to the standard chemotherapy regimens has been shown to improve outcomes for RAS wild-type metastatic colorectal cancer (mCRC)[1-3] and this has been the standard of care recommendation in various regional and international guidelines[4,5].
At present, no chemotherapy backbone was clearly known to be superior to one another when combined with cetuximab. However, the efficacy of cetuximab in combination with oral fluoropyrimidine (FP)-based chemotherapy is controversial[6-8]. In the subgroup analysis of the phase III MRC COIN trial, survival benefits of cetuximab were only demonstrated when combined with infusional FP but not oral FP. One of the postulations was that the dose intensity of the chemotherapy regimen was more likely to be compromised in patients receiving both oral FP-based chemotherapy and cetuximab due to overlapping treatment toxicities, notably diarrhoea. The average duration of treatment for patients treated with oral FP-based chemotherapy plus cetuximab was also shorter. On the contrary, this phenomenon was not observed among patients who received infusional FP[6]. These findings are also consistent with subsequent meta-analyses of multiple randomized controlled trials[7,8]. Therefore, the use of oral FP-based chemotherapy in combination with anti-EGFR mAb is not recommended in various regional and international guidelines[4,5].
Yet, it has been shown that there exist regional differences for the tolerability profile of FP and East Asians appear to tolerate FP the best[9]. With this in mind, the lack of efficacy of cetuximab when combining with capecitabine-based chemotherapy observed in the MRC COIN trial, in which most participants were Caucasians, may not be applicable to East Asians. This concurs with the findings of the FLEET study, a non-randomized multicenter phase II study conducted in Japan, which demonstrated similar safety and efficacy of XELOX or FOLFOX plus cetuximab as the first-line therapy in mCRC[10,11].
The demonstration of non-inferiority of oral FP versus infusional FP in combination with cetuximab carries important implications as oral FP offers potential advantages over infusional FP. First, oral FP is more cost effective with lower direct cost[12,13]. Second, studies also showed higher patient preference for oral FP as it is more convenient and reduces the need of hospital admission[13,14].
In view of the above, we conducted a retrospective cohort analysis to compare the efficacy and safety profile of cetuximab in combination with oral FP and infusional FP in Chinese population in the real-world setting.
This study was a single institutional retrospective cohort analysis. The treatment records of all patients with mCRC who were treated between January 2010 to December 2015 at the Department of Clinical Oncology, Queen Mary Hospital, Hong Kong were screened. Patients were eligible for further analysis if they fulfilled the following criteria: Histologically confirmed colorectal adenocarcinoma; known wild-type KRAS (NRAS has to be wild-type if the status was known); received first-line treatment with cetuximab-based therapy; FP-based chemotherapy backbone; and complete clinical and follow-up data available. Patients with co-existing active malignancy were excluded. This study was approved by the institutional review board (HKU/HA HKW IRB UW 15-315) and the research was reported according to the STROBE statement.
Eligible patients were divided into two groups according to chemotherapy backbone, namely, an oral FP group and an infusional FP group (Table 1). Baseline characteristics of the two groups of patients including age, gender, and disease characteristics such as site of primary tumor were recorded. Treatment characteristics including number of treatment cycles and treatment modification were also recorded.
| Oral fluoropyrimidine-based regimen1,3 |
| Cetuximab + CAPOX every 3 wk |
| Cetuximab: 500 mg/m2 on day 1 and 250 mg/m2 on day 15 |
| Oxaliplatin: 130 mg/m2 on day 1 |
| Capecitabine: 1000 mg/m2 from day 1 to day 14 |
| Cetuximab + CAPIRI every 3 wk |
| Cetuximab: 500 mg/m2 on day 1 and 250 mg/m2 on day 15 |
| Irinotecan: 200 mg/m2 on day 1 |
| Capecitabine: 800 mg/m2 from day 1 to day 14 |
| Infusional fluoropyrimidine-based regimen2,3 |
| Cetuximab + FOLFOX every 2 wk |
| Cetuximab: 500 mg/m2 on day 1 |
| Oxaliplatin: 85 mg/ m2 on day 1 |
| Leucovorin: 200 mg/m2 over 2 h on day 1 and 2 |
| 5-Fluorouracil: 400 mg/m2 bolus on day 1 and 2 |
| 5-Fluorouracil: 600 mg/m2 over 20 h on day 1 and 22 h on day 2 |
| Cetuximab + FOLFIRI every 2 wk |
| Cetuximab: 500 mg/m2 on day 1 |
| Irinotecan: 180 mg/ m2 on day 1 |
| Leucovorin: 200 mg/m2 over 2 h on day 1 and 2 |
| 5-Fluorouracil: 400 mg/m2 bolus on day 1 and 2 |
| 5-Fluorouracil: 600 mg/m2 over 20 h on day 1 and 22 h on day 2 |
The primary outcome measures of this study were the median progression-free survival (mPFS) and median overall survival (mOS). Progression-free survival (PFS) was defined as the duration between treatment initiation and tumor progression or death from any cause, whichever was earlier, while overall survival (OS) was defined as the duration between treatment initiation to death from any cause. Patients known to be alive or lost to follow-up at the time of analysis were censored at their last follow-up. The secondary outcome measures of this study were safety profiles of cetuximab plus different chemotherapy backbones. The adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Our study also included exploratory analysis to investigate the effects of different predictors of outcomes and their interaction with the chemotherapy backbones.
Categorical outcomes were compared using Fisher’s exact test or chi-squared test, while continuous outcomes were assessed by two-sample unpaired t-test. OS and PFS were calculated by Kaplan-Meier analysis and the survival curves were compared by log-rank test. Cox regression analysis was used for both univariable and multivariable analysis for factors associated with survival. All P-values less than 0.05 were considered statistically significant. Statistical analyses were carried out using R version 3.3.2.
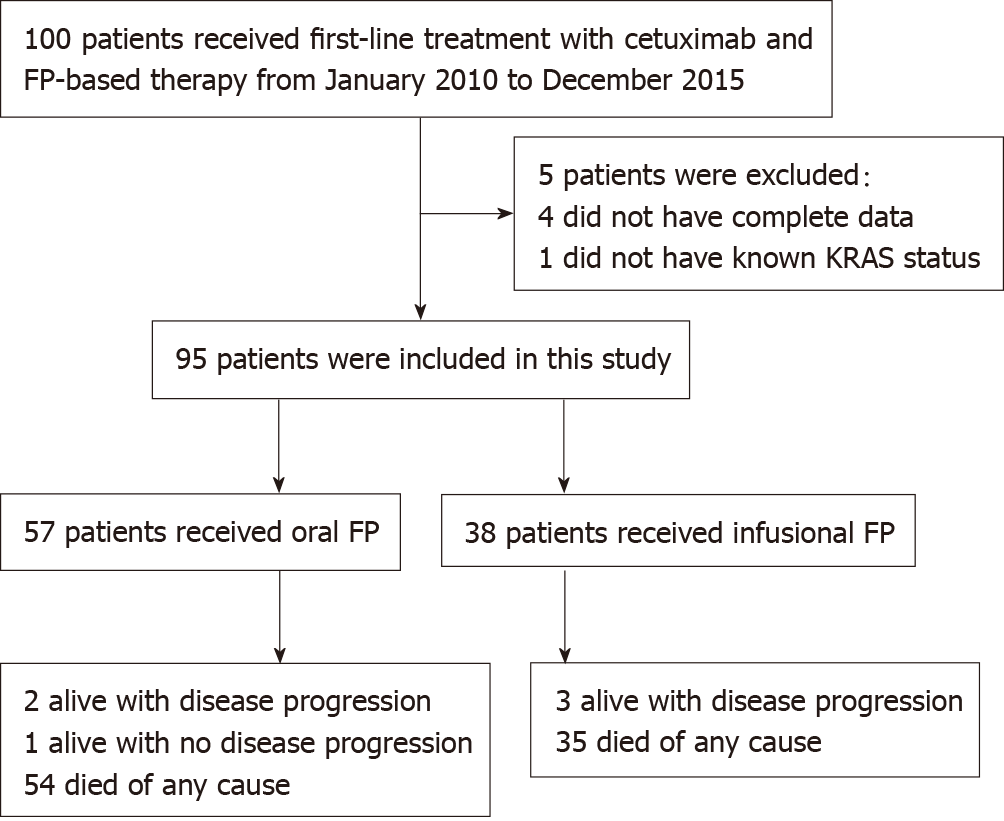
Figure 1 illustrates the number of patients at different stages of study enrollment. One hundred patients with mCRC received first-line treatment with cetuximab and FP-based therapy between January 2010 to December 2015 at the Department of Clinical Oncology, Queen Mary Hospital. A total of 95 patients were eligible for analysis in this study, and the median follow-up period of the surviving patients was 65.0 mo [Interquartile range (IQR): 55.3–86.1]. Ninety-four patients progressed after the first-line treatment and eighty-nine patients have passed away at the time of analysis.
The baseline characteristics of eligible patients are shown in Table 2. The median age at the start of the first-line chemotherapy of all eligible patients was 61.0 years (IQR: 53.5–68.0 years). No significant differences were recognized in the distribution of the baseline patient characteristics between the oral and infusional FP groups. With regard to the clinical characteristics and extent of the disease, the two groups of patients were comparable in terms of site of primary tumour, resection of primary tumour, timing of the metastasis, and extent of metastasis.
| Total (n = 95) | Oral (n = 57) | Infusional (n = 38) | P value | |
| Gender (%) | ||||
| Male | 52 (54.7) | 32 (56.1) | 20 (52.6) | 0.900 |
| Age (yr) | ||||
| Median | 61.0 | 61.0 | 61.0 | 0.622 |
| Age ≥ 70 (%) | 21 (22.1) | 12 (21.1) | 9 (23.7) | 0.960 |
| ECOG performance status | ||||
| 0 | 7 (7.4) | 4 (7.0) | 3 (7.9) | 0.383 |
| 1 | 80 (84.2) | 50 (87.7) | 30 (78.9) | |
| 2 | 8 (8.4) | 3 (5.3) | 5 (13.2) | |
| 3 | 0 (0) | 0 (0) | 0 (0) | |
| 4 | 0 (0) | 0 (0) | 0 (0) | |
| Site of primary tumor (%) | ||||
| Right-sided colon | 20 (21.1) | 15 (26.3) | 5 (13.2) | 0.114 |
| Left-sided colon | 49 (51.6) | 25 (43.9) | 24 (63.2) | |
| Rectum | 22 (23.2) | 13 (22.8) | 9 (23.7) | |
| Multiple | 4 (4.2) | 4 (7.0) | 0 (0.0) | |
| Timing of metastasis (%) | ||||
| Synchronous | 72 (75.8) | 40 (70.2) | 32 (84.2) | 0.187 |
| Metachronous | 23 (24.2) | 17 (29.8) | 6 (15.8) | |
| Extent of the disease (%) | ||||
| Primary resected1 | 68 (71.6) | 42 (73.7) | 26 (68.4) | 0.745 |
| Liver-only metastatsis | 44 (46.3) | 26 (45.6) | 18 (47.4) | 1.000 |
| > 1 site of metastasis | 40 (42.1) | 24 (42.1) | 16 (42.1) | 1.000 |
| Site of metastasis (%) | ||||
| Liver | 67 (70.5) | 39 (68.4) | 28 (73.7) | |
| Lymph Nodes | 28 (29.5) | 17 (29.8) | 11 (28.9) | |
| Peritoneum | 21 (22.1) | 11 (19.3) | 10 (26.3) | |
| Lung | 17 (17.9) | 11 (19.3) | 6 (15.8) | |
| Bone | 3 (3.2) | 2 (3.5) | 1 (2.6) | |
| Others | 8 (8.4) | 6 (10.5) | 2 (5.3) |
Table 3 summarizes the first-line chemotherapy regimens received by eligible patients. Fifty-seven (60.0%) patients received capecitabine, an oral FP, while thirty-eight (40.0%) received intravenous infusion of 5-fluorouracil (infusional FP). Oxaliplatin (76.7%) was more commonly used as the partner of fluoropyrimidine in our cohort, compared to irinotecan (23.3%). There was no statistically significant difference in the proportions of patients receiving treatment modification of the first-line treatment, metastasectomy of curative intent, subsequent systemic therapy, and the mean treatment durations in the two treatment groups.
| Total (n = 95) | Oral (n = 57) | Infusional (n = 38) | P value | |
| Chemotherapy backbone (%)1 | ||||
| FOLFOX | 26 (27.4) | 0 (0) | 26 (68.4) | |
| CAPOX | 43 (45.3) | 43 (75.4) | 0 (0) | |
| FOLFIRI | 12 (12.6) | 0 (0) | 12 (31.6) | |
| CAPIRI | 9 (9.5) | 9 (15.8) | 0 (0) | |
| Capecitabine | 5 (5.3) | 5 (8.8) | 0 (0) | |
| Type of fluoropyrimidine (%) | ||||
| Oral | 57 (60.0) | 57 (100) | 0 (0) | |
| Infusional | 38 (40.0) | 0 (0) | 38 (100) | |
| Chemotherapy partner (%) | ||||
| Oxaliplatin | 69 (76.7) | 43 (75.4) | 26 (68.4) | 0.184 |
| Irinotecan | 21 (23.3) | 9 (15.8) | 12 (31.6) | |
| Mean treatment duration | ||||
| Oral/Infusional FP (wk)2 | 18.5 | 19.4 | 17.2 | 0.147 |
| Any treatment modification (%) | ||||
| Yes | 75 (78.9) | 43 (75.4) | 32 (84.2) | 0.441 |
| Due to age | 21 (22.1) | 12 (21.1) | 9 (23.7) | |
| Due to adverse effects | 54 (56.8) | 31 (54.4) | 23 (60.5) | |
| Subsequent systemic therapy (%) | ||||
| Yes | 65 (68.4) | 40 (70.2) | 25 (65.8) | 0.822 |
| Metastasectomy of curative intent (%) | ||||
| Yes | 16 (16.8) | 6 (10.5) | 10 (26.3) | 0.083 |
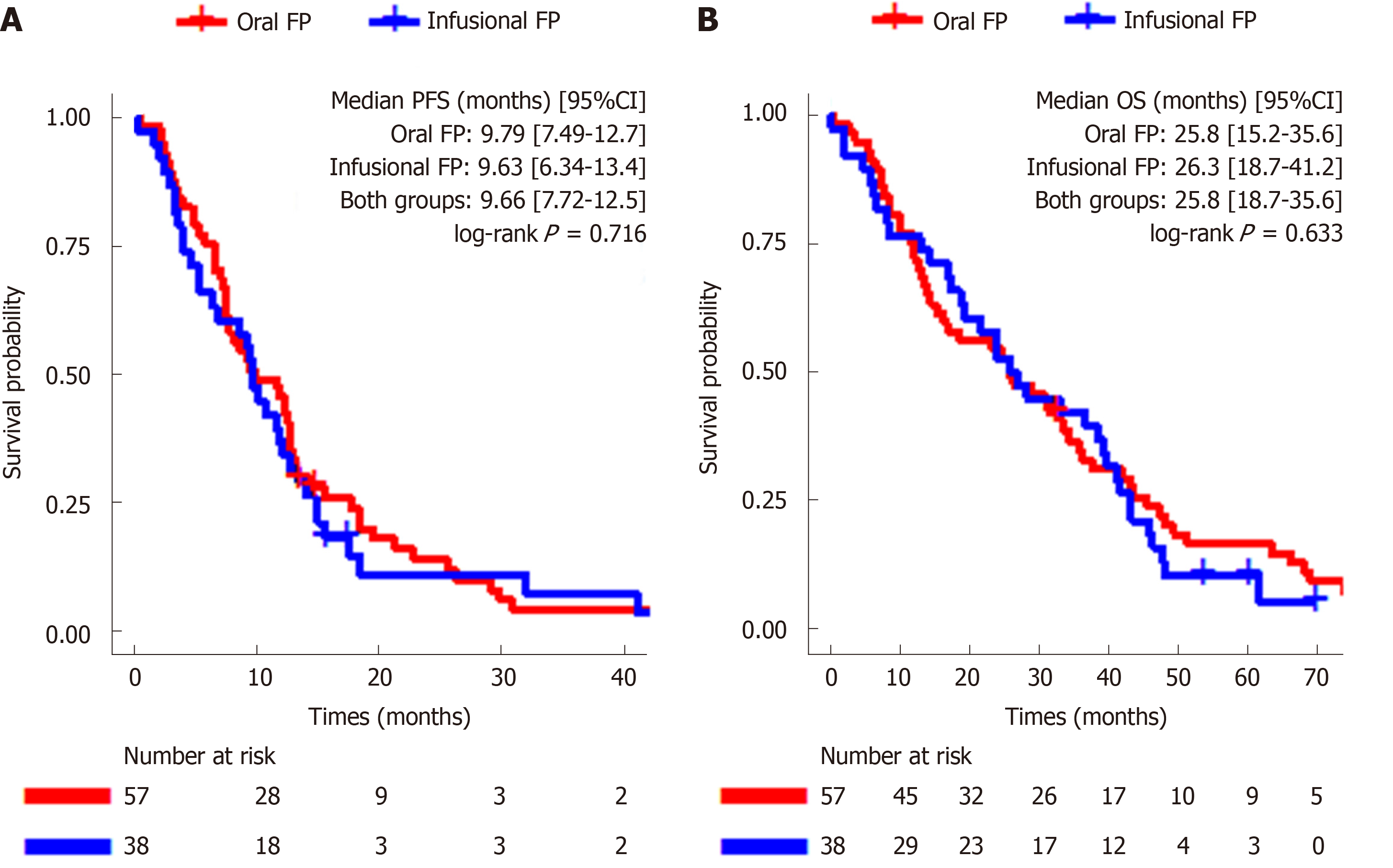
Kaplan-Meier analysis of the PFS and OS of all eligible patients and the two treatment groups is presented in Figure 2. The mPFS of the entire cohort was 9.66 mo (95%CI: 7.72–12.5). There was no statistical difference in the mPFS between the oral FP group and infusional FP group (9.79 mo [95%CI: 7.49–12.7] vs 9.63 mo [95%CI: 6.34–13.4]; log-rank P = 0.72; Figure 2A). The mOS of the entire cohort was 25.8 mo (95%CI: 18.7–35.6) and there was also no statistical difference between the oral FP group and infusional FP group [25.8 mo (95%CI: 15.2–35.6) vs 26.3 mo (95%CI: 18.7-41.2), log-rank P = 0.63, Figure 2B]. Among the twelve 5-year survivors, all of them had resection of primary tumor and ten (83.3%) of them had left-sided tumor and synchronous metastasis, respectively. Eight (66.7%) patients had metastasis limited to the liver only and four (33.3%) of them had resection of liver metastasis with curative intent.
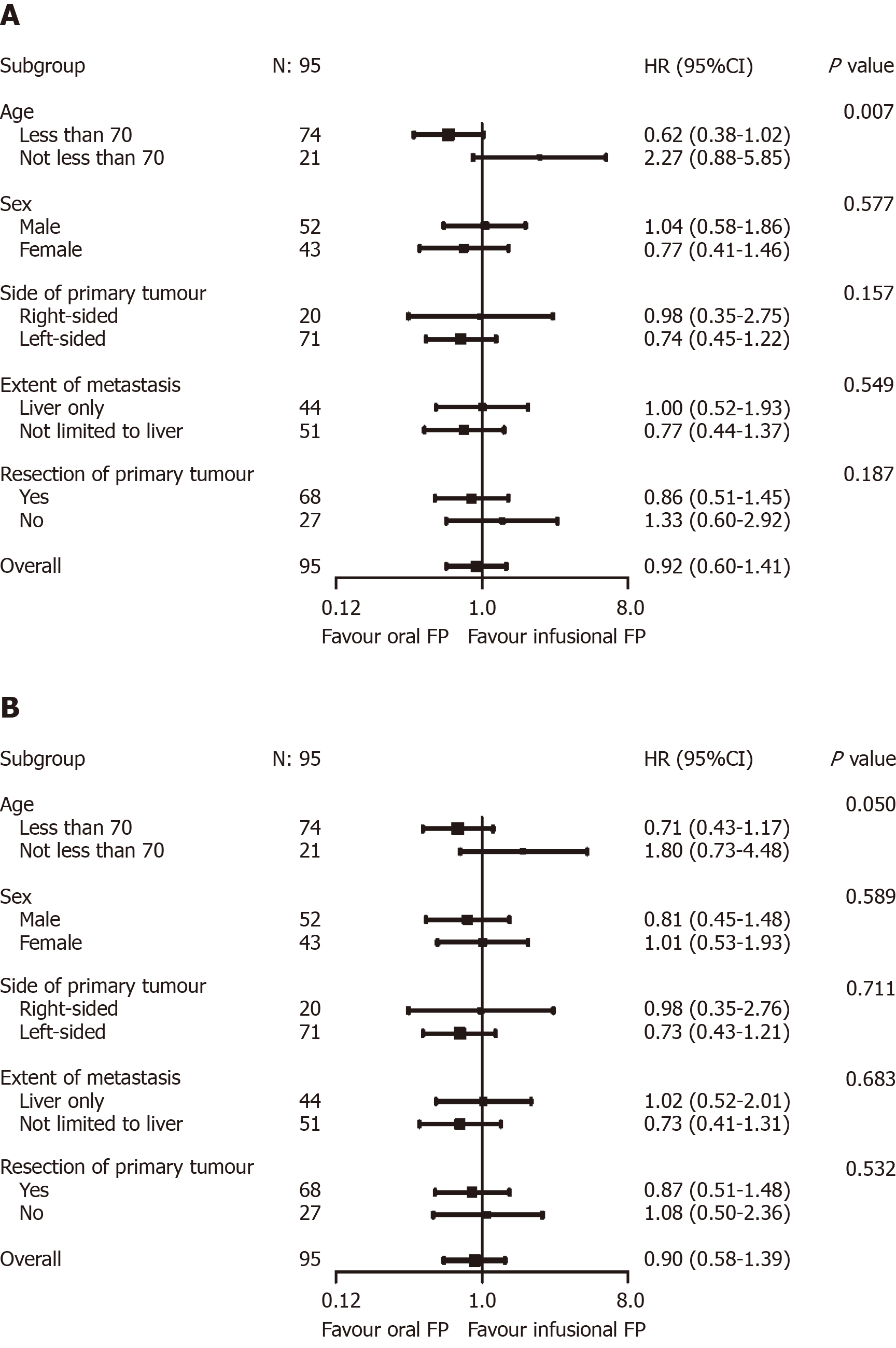
Cox regression analysis was performed to evaluate the impact of various baseline and treatment characteristics on PFS and OS of eligible patients. The crude and adjusted hazard ratios (HR) of different predictors of PFS are presented in Table 4, while the crude and adjusted HR of different predictors of OS are presented in Table 5. It was shown that right-sided primary tumor confered poorer PFS (adjusted HR = 1.87, 95%CI: 1.08–3.25, P = 0.03) and OS (adjusted HR = 1.92, 95%CI: 1.10–3.36, P = 0.02), compared to left-sided primary tumor. On the contrary, primary tumor resection improved the outcomes of patients in terms of both PFS (adjusted HR = 0.35, 95%CI: 0.21–0.59, P < 0.01) and OS (adjusted HR = 0.37, 95%CI: 0.22–0.62, P < 0.01). As for the chemotherapy backbone, oral FP-based chemotherapy did not appear to impact on the PFS (adjusted HR = 1.00, 95%CI: 0.63–1.57, P = 0.99) and OS (adjusted HR = 0.87, 95%CI: 0.56–1.37, P = 0.56) of patients receiving cetuximab as compared to infusional FP-based chemotherapy. Other predictors in the model did not have significant effects on the PFS and OS. The results of the subgroup analysis are shown in Figure 3. In the subgroup analysis, age group was shown to modify the effects of chemotherapy backbone on PFS (P = 0.007, Figure 3A) and a similar trend was observed in OS (P = 0.050, Figure 3B). However, no particular subgroup was identified to have more benefit with either oral or infusional FP regimen.
| Factor | Crude HR (95%CI) | Adjusted HR (95%CI) | P value1 |
| Age | 1.00 (0.98-1.02) | 1.00 (0.98-1.02) | 0.909 |
| Male | 0.91 (0.60-1.38) | 1.00 (0.64-1.54) | 0.985 |
| Right sided primary | 1.63 (0.97-2.74) | 1.87 (1.08-3.25) | a0.026 |
| Primary resected | 0.39 (0.24-0.63) | 0.35 (0.21-0.59) | b<0.001 |
| Liver only disease | 0.56 (0.37-0.86) | 0.57 (0.36-0.91) | c0.019 |
| Infusional FP2 | 0.92 (0.60-1.42) | 1.00 (0.63-1.57) | 0.992 |
| Factor | Crude HR (95%CI) | Adjusted HR (95%CI) | P value1 |
| Age | 1.01 (0.99-1.03) | 1.01 (0.99-1.03) | 0.394 |
| Male | 0.86 (0.56-1.31) | 1.04 (0.67-1.63) | 0.848 |
| Right sided primary | 1.96 (1.16-3.31) | 1.92 (1.10-3.36) | a0.023 |
| Primary resected | 0.34 (0.21-0.55) | 0.37 (0.22-0.62) | b<0.001 |
| Liver only disease | 0.54 (0.35-0.83) | 0.58 (0.36-0.95) | c0.029 |
| Infusional FP2 | 0.90 (0.58-1.39) | 0.87 (0.56-1.37) | 0.558 |
The frequency of adverse events in different treatment groups is shown in Table 6. Twenty-seven (28.4%) patients experienced adverse events of grade 3 or above with the two commonest adverse events being neutropenia (10.5%) and thrombocytopenia (5.3%). No significant difference was noted in the toxicity profiles of the two treatment groups.
| Total (n = 95) | Oral (n = 57) | Infusional (n = 38) | P value | |
| Any adverse events (%) | 95 (100) | 57 (100) | 38 (100) | 1.000 |
| G3 or above adverse events (%)1 | 27 (28.4) | 19 (33.3) | 8 (21.1) | 0.286 |
| Hematologic (%) | ||||
| Anaemia | 64 (67.4) | 36 (63.2) | 28 (73.7) | 0.396 |
| G3 or above | 4 (4.2) | 4 (7.0) | 0 (0) | 0.147 |
| Leucopenia | 44 (46.3) | 25 (43.9) | 19 (50.0) | 0.705 |
| G3 or above | 3 (3.2) | 1 (1.8) | 2 (5.3) | 0.562 |
| Neutropenia | 49 (51.6) | 26 (45.6) | 23 (60.5) | 0.224 |
| G3 or above | 10 (10.5) | 5 (8.8) | 5 (13.2) | 0.514 |
| Thrombocytopenia | 53 (55.8) | 37 (64.9) | 16 (42.1) | 0.047 a |
| G3 or above | 5 (5.3) | 5 (8.8) | 0 (0) | 0.081 |
| Biochemistry (%) | ||||
| Raised AST | 68 (71.6) | 47 (82.5) | 21 (55.3) | 0.008 b |
| G3 or above | 2 (2.1) | 1 (1.8) | 1 (2.6) | 1.000 |
| Raised bilirubin | 21 (22.1) | 14 (24.6) | 7 (18.4) | 0.650 |
| G3 or above | 2 (2.1) | 1 (1.8) | 1 (2.6) | 1.000 |
| Non-hematologic (%) | ||||
| Acneiform rash | 58 (61.1) | 36 (63.2) | 22 (57.9) | 0.764 |
| G3 or above | 3 (3.2) | 1 (1.8) | 2 (5.3) | 0.562 |
| Diarrhoea | 25 (26.3) | 17 (29.8) | 8 (21.1) | 0.476 |
| G3 or above | 2 (2.1) | 2 (3.5) | 0 (0) | 0.515 |
| Hand-foot syndrome | 2 (2.1) | 2 (3.5) | 0 (0) | 0.515 |
| G3 or above | 2 (2.1) | 2 (3.5) | 0 (0) | 0.515 |
The results of the current study demonstrated the real-world efficacy and safety of oral FP-based chemotherapy in combination with cetuximab in Chinese population, compared with infusional FP-based chemotherapy. The mPFS and mOS of the patients in our study, in which both infusional and oral FP were used, were 9.66 mo (95%CI: 7.72–12.5) and 25.8 mo (95%CI: 18.7–35.6), respectively. These findings are comparable to those reported in other landmark trials using infusional FP plus cetuximab in KRAS wild-type mCRC[1,2]. More importantly, cetuximab plus oral FP-based chemotherapy achieved similar outcomes to infusional FP-based chemotherapy. Hence, in contrast to Caucasian patients, oral FP-based chemotherapy should still be considered as a valid option in Chinese patients receiving cetuximab. Albeit the slight difference in dosing schedule, this is consistent with the findings of the FLEET and FLEET2 studies conducted in Japan which demonstrated the validity of combining cetuximab with XELOX[10,11]. In our study, we also performed a subgroup analysis for the effects of oral FP versus infusional FP on PFS and OS. Although no subgroup was identified to have more benefit with either regimen, age group may potentially modify the effects on PFS (P = 0.007). The interaction was still significant after adjusting P-values by Bonferroni correction for multiple testing. The lack of significant effects of different FP-based chemotherapy in the two age groups may be accounted by inadequate power of the subgroup analysis due to limited sample size in each subgroup (74 patients aged below 70 vs 21 patients aged 70 or above). Moreover, the significant interaction may be related to the upfront dose reduction of the chemotherapy regimen. As the subgroup analysis was exploratory in nature, this interaction effect still need verification by future studies.
It has been increasingly recognized that left- and right-sided colorectal cancers have distinct clinical and molecular features which translates into different clinical outcomes[15,16]. In our study, patients with right-sided primary tumor had worse PFS (adjusted HR = 1.87, 95%CI: 1.08–3.25, P = 0.03) and OS (adjusted HR = 1.92, 95%CI: 1.10–3.36, P = 0.02), adjusted for factors such as age and presence of liver-only diseases. The results were in line with the findings of the existing literature[17]. Apart from the poorer prognosis of the right-sided cancer, a retrospective pooled analysis of six randomized trials found out that the significant benefit for chemotherapy plus anti-EGFR antibody in RAS wild-type colorectal cancer could only be observed in left-sided tumors[18]. This also explained the worse prognosis of right-sided tumors in our patients receiving cetuximab as the first-line systemic therapy.
Another significant finding of the current study is that primary tumor resection appears to improve both the PFS (adjusted HR = 0.35, 95%CI: 0.21–0.59, P < 0.01) and OS (adjusted HR = 0.37, 95%CI: 0.22–0.62, P < 0.01). This was in accord with the existing evidence that palliative primary tumor resection was associated with improved survival[19-22]. Anyway, palliative primary tumor resection is still not a standard recommendation for asymptomatic mCRC as the improvement in survival has never been validated by a high-quality randomized trial yet[4,5]. Apart from resection of primary tumor, liver-only disease was also shown to improve the PFS (adjusted HR = 0.57, 95%CI = 0.36–0.91, P = 0.02) and OS (adjusted HR = 0.58, 95%CI: 0.36–0.95, P = 0.03) significantly, which was also consistent with existing knowledge. Despite this was a Chinese-only cohort of patients, the sharing of known prognostic factors as in the reported literature supported similar tumor biology observed in our patients and consolidated the external validity of our findings that ethnicity may explain the differences in outcomes with regard to the combination of cetuximab and oral FP.
The adverse event profile of FP-based chemotherapy in combination with cetuximab was another main focus of our study. The frequency of adverse events of grade 3 or above in the cohort was 28.4% (Table 6). This is far less than the reported figures in various clinical trials such as CRYSTAL (79.3%) and FIRE-3 (71.0%)[2,23]. Haematological adverse events remained one of the commonest with this combination regimen. Non-haematological side effects of grade 3 or above were uncommon in our cohort. The frequency of grade 3 or above diarrhoea was 2.1%, which was also far lower than that observed in randomized controlled trials such as the MRC COIN trial (20% for fluorouracil-based therapy; 26% for capecitabine-based therapy) while it is similar to the findings in the Japanese FLEET study (5.4%)[6,10,11]. The toxicity patterns of both hematological and non-hematological adverse events did not differ between oral FP and infusional FP. These findings highlighted the regional differences in the tolerability profiles of FP. Haller et al[9] analyzed the safety data of three phase III studies that compared oral FP with infusional FP and suggested that East Asians had a better tolerability profile than Caucasians. This underlies the success of oral FP-based chemotherapy in combination with cetuximab in our cohort and in the FLEET study as well as the negative overall outcomes of the MRC COIN trial[6]. However, the apparently better tolerability may also be explained partly by the fact that patients in our cohort were younger (median age = 61 years) and upfront dose reduction was allowed for selected patients (75% dose for patients older than 70 years and/or creatinine clearance < 50 mL/min).
Our study shared the limitations intrinsic to all retrospective analysis and the results should be considered hypothesis-generating rather than confirmatory. Due to the retrospective nature, we could not answer the underlying mechnism linking ethnicity and FP tolerability. We did not report the response rate in our patients as the assessment schedule and response evaluation would not be as stringent as in prospective clinical trial settings. Instead, we reported the more robust outcomes of PFS and OS which were less biased by assessment schedule and physicians’ decision in the real-world setting. Selection bias is criticized for retrospective analysis but the results of our overall population was comparable to the reported literature, and that both treatment groups of oral FP and infusional FP served as internal control with balanced baseline characteristics. Adverse events were prone to under-reporting in the real-world setting but the effects should be the same in both oral and infusion FP groups and thus our conclusions should be considered as valid. The sample size of 95 in this study was small and did not have adequate power to detect subtle differences in outcomes. Nevertheless, the current cohort represented one of the largest that asked similar questions on this unmet clinical needs where there will unlikely be any further phase III randomized controlled trials.
The finding that combination of oral FP-based chemotherapy and cetuximab is a valid alternative to infusional FP in Chinese patients is important. It has been shown that oral fluoropyrimidine is more cost-effective and more preferred among patients[12-14]. In addition, infusional FP requires more hospital stay and venous access. Hence, the use of oral FP in combination with cetuximab may be beneficial to both patients and health services.
When combined with cetuximab, oral FP-based chemotherapy had a comparable safety and efficacy profile with infusional FP-based chemotherapy in Chinese population. The findings support oral FP as a valid option to combine with anti-EGFR mAb and call for reposition of such regimens for Asian patients in both regional and international guidelines.
Although cetuximab is shown to provide survival benefits in patients with RAS wild-type metastatic colorectal cancer, little is known about the optimal chemotherapy backbone for cetuximab except that oral fluoropyrimidine appeared to be inferior to infusional fluoropyrimidine and is not recommended in various international guidelines.
Before the evidence of the inferiority of oral fluoropyrimidine combining with cetuximab, it was commonly used due to convenience and cost effectiveness. The inferiority of oral fluoropyrimidine was not readily observed in our previous experience. In addition, research has shown that there might be a regional difference in the tolerability of oral fluopyrimidine. The inferiority of oral fluopyrimidine demonstrated in previous studies might not be applicable to our locality with Chinese population.
This study would like to compare oral fluoropyrimidine with infusional fluoropyrimidine in combination with cetuximab in Chinese population in terms of progression-free survival and overall survival.
A retrospective cohort study was employed to compare and contrast the survival and adverse effect profile of oral fluoropyrimidine (FP) and infusional FP in combination with cetuximab in Chinese population in the real-world setting.
There was no significant difference in median progression-free survival and median overall survival between the two groups. The incidence of various grade 3 or above adverse effects was similar in both groups.
Oral and infusional fluoropyrimidine has comparable efficacy and safety profiles when used with cetuximab.
Oral fluoropyrimidine may be a good alternative to infusional fluoropyrimidine when in combination with cetuximab.
| 1. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 439] [Article Influence: 36.6] [Reference Citation Analysis (7)] |
| 2. | Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH, Ciardiello F. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 641] [Article Influence: 58.3] [Reference Citation Analysis (3)] |
| 3. | Stintzing S, Miller-Phillips L, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Moehler M, Jagenburg A, Kirchner T, Jung A, Heinemann V; FIRE-3 Investigators. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2540] [Article Influence: 254.0] [Reference Citation Analysis (41)] |
| 5. | National Comprehensive Cancer Network. Colon Cancer (version 1. 2019- Mar 15, 2019). Cited 15 May 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 6. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 768] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 7. | Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, Parmar MK, Meade AM. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Chan DL, Pavlakis N, Shapiro J, Price TJ, Karapetis CS, Tebbutt NC, Segelov E. Does the Chemotherapy Backbone Impact on the Efficacy of Targeted Agents in Metastatic Colorectal Cancer? A Systematic Review and Meta-Analysis of the Literature. PLoS One. 2015;10:e0135599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Haller DG, Cassidy J, Clarke SJ, Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB, Schmoll HJ, Allegra C, Bertino JR, Douillard JY, Gustavsson BG, Milano G, O'Connell M, Rustum Y, Tabernero J, Gilberg F, Sirzén F, Twelves C. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Soda H, Maeda H, Hasegawa J, Takahashi T, Hazama S, Fukunaga M, Kono E, Kotaka M, Sakamoto J, Nagata N, Oba K, Mishima H. Multicenter Phase II study of FOLFOX or biweekly XELOX and Erbitux (cetuximab) as first-line therapy in patients with wild-type KRAS/BRAF metastatic colorectal cancer: The FLEET study. BMC Cancer. 2015;15:695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Hazama S, Maeda H, Iwamoto S, Kim HM, Takemoto H, Kobayashi K, Sakamoto J, Nagata N, Oba K, Mishima H. A Phase II Study of XELOX and Cetuximab as First-Line Therapy in Patients With KRAS Wild Type Metastatic Colorectal Cancer (FLEET2 Study). Clin Colorectal Cancer. 2016;15:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | De Portu S, Mantovani LG, Ravaioli A, Tamburini E, Bollina R, Cozzi C, Grimaldi AM, Testa TE, Bianchessi C, Carteni G. Cost analysis of capecitabine vs 5-fluorouracil-based treatment for metastatic colorectal cancer patients. J Chemother. 2010;22:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Best JH, Garrison LP. Economic evaluation of capecitabine as adjuvant or metastatic therapy in colorectal cancer. Expert Rev Pharmacoecon Outcomes Res. 2010;10:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, van der Born K, Wanders J, de Boer RF, Martin C, Fumoleau P. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 16. | Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59:A4444. [PubMed] |
| 17. | Aarts F, de Hingh I, de Wilt JH, Lemmens VEP, Verhoeven RHA. Differences in outcome between right- and left-sided colon cancer: A population based study. J Clin Oncol. 2013;31:493–493. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 650] [Article Influence: 81.3] [Reference Citation Analysis (5)] |
| 19. | Nitsche U, Stöß C, Stecher L, Wilhelm D, Friess H, Ceyhan GO. Meta-analysis of outcomes following resection of the primary tumour in patients presenting with metastatic colorectal cancer. Br J Surg. 2018;105:784-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Faron M, Pignon JP, Malka D, Bourredjem A, Douillard JY, Adenis A, Elias D, Bouché O, Ducreux M. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer. 2015;51:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | van Rooijen KL, Shi Q, Goey KKH, Meyers J, Heinemann V, Diaz-Rubio E, Aranda E, Falcone A, Green E, de Gramont A, Sargent DJ, Punt CJA, Koopman M. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: Individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. 2018;91:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, Güller U. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann Surg. 2015;262:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1371] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Budai B S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Li X