Published online Jan 15, 2019. doi: 10.4251/wjgo.v11.i1.1
Peer-review started: October 7, 2018
First decision: October 26, 2018
Revised: November 23, 2018
Accepted: December 12, 2018
Article in press: December 13, 2018
Published online: January 15, 2019
Processing time: 100 Days and 19.2 Hours
Hepatocellular carcinoma is one of the most common malignant tumors worldwide. Currently, the most accurate diagnosis imaging modality for hepatocellular carcinoma is enhanced magnetic resonance imaging. However, it is still difficult to distinguish cirrhosis lesions, and novel diagnosis modalities are still needed.
To investigate the feasibility of hyperspectral analysis for discrimination of rabbit liver VX2 tumor.
In this study, a rabbit liver VX2 tumor model was established. After laparotomy, under direct view, VX2 tumor tissue and normal liver tissue were subjected to hyperspectral analysis.
The spectral signature of the liver tumor was clearly distinguishable from that of the normal tissue, simply from the original spectral curves. Specifically, two absorption peaks at 600-900 nm wavelength in normal tissue disappeared but a new reflection peak appeared in the tumor. The average optical reflection at the whole waveband of 400-1800 nm in liver tumor was higher than that of the normal tissue.
Hyperspectral analysis can differentiate rabbit VX2 tumors. Further research will continue to perform hyperspectral imaging to obtain more information for differentiation of liver cancer from normal tissue.
Core tip: Hepatocellular carcinoma is one of the most common malignant tumors worldwide. Currently, the most accurate diagnosis imaging modality for HCC is magnetic resonance imaging; however, it is still difficult to distinguish cirrhosis lesions. Thus, hyperspectral imaging might be a novel modality as an early/fast diagnosis. In this study, a rabbit liver VX2 tumor model was established. After laparotomy and under direct view, VX2 tumor tissue and normal liver tissue were subjected to hyperspectral analysis. The result of this study demonstrated the feasibility of hyperspectral analysis for discrimination of rabbit VX2 liver tumor.
- Citation: Duan F, Yuan J, Liu X, Cui L, Bai YH, Li XH, Xu HR, Liu CY, Yu WX. Feasibility of hyperspectral analysis for discrimination of rabbit liver VX2 tumor. World J Gastrointest Oncol 2019; 11(1): 1-8
- URL: https://www.wjgnet.com/1948-5204/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i1.1
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide[1,2]. The diagnosis of HCC mainly depends on imaging studies, including ultrasonography, computed tomography, magnetic resonance imaging, etc[3]. Although there are many diagnostic imaging modalities for HCC, early diagnosis remains a challenge[4].
Hyperspectral analysis has emerged as a promising diagnostic modality in recent years[5,6]. The principle of hyperspectral imaging is to acquire 2D spatial images in hundreds of contiguous bands of high-spectral resolution, including the ultraviolet, visible, and near-infrared bands. Therefore, hyperspectral imaging can yield more diagnostic information, not only in the visible wavelength region, but also in the ultraviolet and near-infrared wavelength regions. This feature makes it possible to discriminate normal tissue from tumor tissue. Until now, hyperspectral analysis has been applied to the diagnoses of skin cancer and prostate carcinoma[7,8]. To the best of our knowledge, there are few reports about hyperspectral analysis for HCC. In this study, we evaluated the feasibility of hyperspectral analysis for the discrimination of rabbit VX2 liver tumor from normal liver tissue.
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of the Chinese PLA General Hospital, Beijing, China, and was conducted in accordance with the animal experimental guidelines set by Chinese PLA Medical College. A total of six 12-mo-old male New Zealand white rabbits, weighing approximately 2.5 kg, were purchased from the Experimental Animal Center of the Hospital.
Frozen VX2 tumor cells were provided by the Orthopedic Laboratory of the Hospital. The cells were resuscitated by general cell culture technique[9,10] and centrifuged for 5 min (800 r/min; g = 9.80665 m/s2). After removing the supernatant, phosphate buffered saline was added and cells were centrifuged again for 5 min. The supernatant was discarded and phosphate buffered saline was added. The suspension was stained with trypan blue and the dead and live cells were counted. The suspension was modulated to 106 viable cells/mL. The suspension was drawn into 2-mL syringes with 19-gauge needles for a 2-mL volume per injection.
One rabbit was used as a VX2 carrier and 2 mL cell suspension was injected into the hind leg. After 21 d, two tumors with a diameter of 3 cm were palpated. Tumors were excised from the VX2 carrier rabbit, minced into small fragments with a diameter of 1-2 mm, and stored in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, United States).
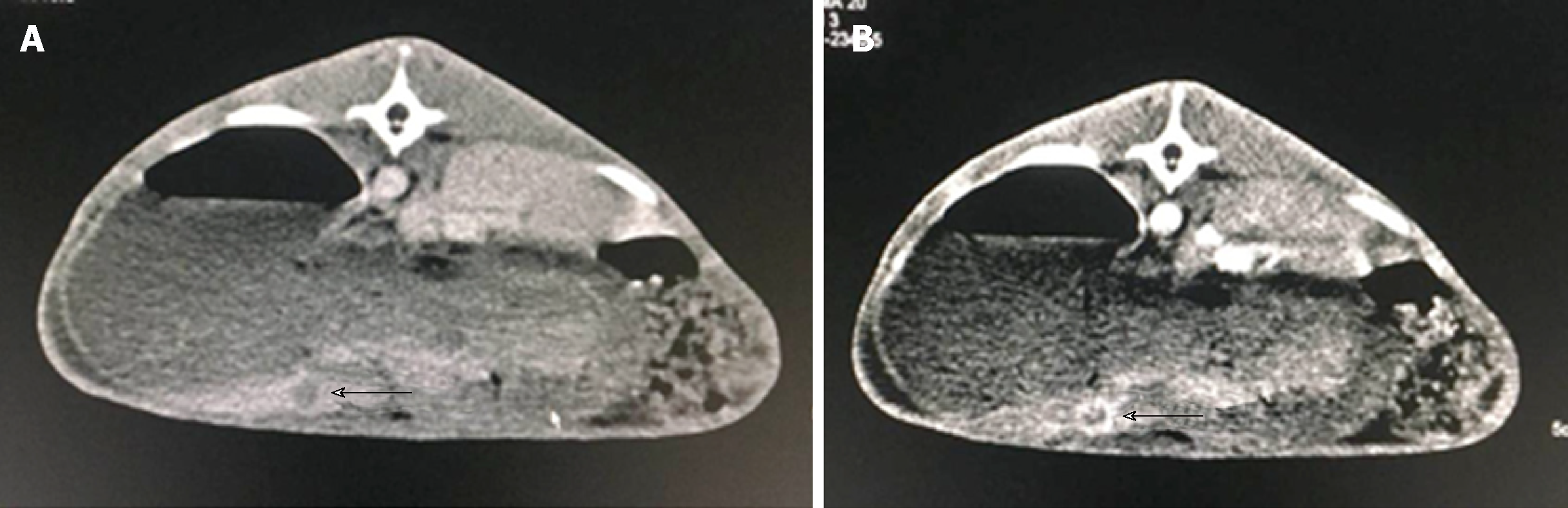
The other five rabbits were not permitted any food 12 h prior to surgery. Following general anesthesia by pentobarbital sodium (30 mg/kg) administered via the marginal ear vein, a 1-cm long surgical incision was made in the upper abdominal wall aseptically. Subsequently, the left lobe of the liver was exposed and a 5-mm deep tunnel was formed on the surface. The prepared tumor fragment was implanted into the tunnel, followed by blocking of the tunnel with Gelfoam (Xiang’en Medical Technology Development Co., LTD). After tumor implantation, the abdominal wall was closed by suture. Rabbits were administered penicillin G (100000 U; Lu Kang Medical Corp., China) twice daily for 3 d after tumor implantation. Enhanced computed tomography was performed 2 wk after implantation to confirm successful establishment of the model (Figure 1).
VX2 tumor model was successfully established in all five rabbits, and then hyperspectral analysis was performed to identify tumor tissue and normal liver tissue. After laparotomy and under direct view, VX2 tumor tissue and normal liver tissue were subjected to hyperspectral analysis. The spectrometer used was a commercially available high-resolution spectrometer, Fieldspec 4 model (Analytical Spectral Devices, Boulder, CO, United States). The working waveband of this spectrometer was 350–2500 nm with a high spectral resolution of 3 nm at 700 nm. A halogen light with a power of 5 W was used as the illuminating light source. The output light was collimated with an optical collimating lens to achieve a relative uniform circle light spot with a diameter of approximate 5 mm.
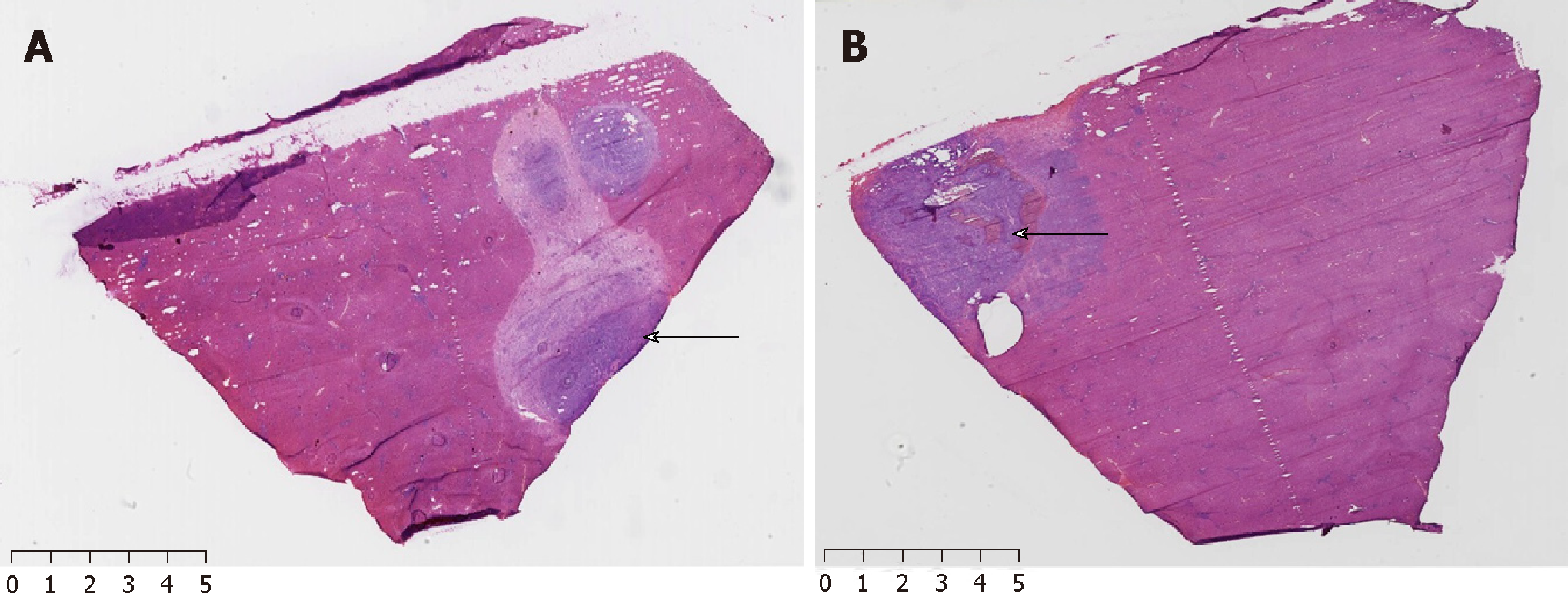
The optical spectrum testing of the samples was conducted by following the steps below. First, a white board that is a perfectly diffused surface was used to calibrate the maximum reflectance within the span of the working waveband (350-2500 nm). The reflectance of the rabbit liver had to be referred to this maximum reflection. In this step, the integration time of the detector was also optimized and the dark current was also tested. After white reference calibration, the light was illuminated onto the liver tissue of the rabbit to capture its reflective spectrum. Because the tumor tissue was > 5 mm in diameter, the collimated light spot fell onto the tumor area, which ensured that a pure spectrum of the tumor could be obtained. Figure 2 shows the tested spectra of the normal and tumor tissues of rabbits, as well as the image of the liver tumor captured by a digital camera. Rabbits were sacrificed following hyperspectral analysis, and VX2 liver tumors were harvested for pathology examination as a gold standard for comparison (Figure 3).
Figure 2 shows a comparison of the reflective spectra of the normal and tumor tissues of the rabbit livers. There were 15 spectral lines for normal and tumor issues each. The average amplitudes of the spectral curves were different, which was caused by two reasons: (1) the tissue distribution was not uniform, especially for tumor tissues. Because the light spot falling onto the tissue surface might not have been at the same place for each test at different times, the total reflectance might have changed; and (2) there could have been blood leaking from the tissue during the test, and as a result the light absorbance from the tissue could have been different. This would have led to the change in average amplitude of the reflectance. However, these variables can be eliminated if the experimental conditions can be controlled carefully. Although there were different amplitudes between the spectral curves, the whole profiles for either normal or tumor tissue were consistent and the spectral signatures were distinct for both cases. In general, the spectral profiles for the two cases were different at the wavelength of 600-900 nm, but were similar at 1000-1800 nm and showed no obvious difference in spectral signature.
Specifically, two absorption peaks at the wavelength of 600-900 nm in normal tissue disappeared but a new reflection peak appeared in the tumor tissue (Figure 4). The first absorption peak was at 650 nm for normal tissues, while for tumor tissues there was no obvious absorption peak at this wavelength, and the spectral curve showed a smoothly rising trend. The second absorption peak for normal tissues was at 757 nm, while the tumor tissues showed no absorption peak but a smooth and continuous drop around this wavelength. The most significant signature for tumor tissue was that there was a reflection peak at 700 nm, while the normal tissue did not have this signature but showed a roughly linear rising trend. These distinct spectral signatures can be used to discriminate tumor tissues from normal tissues.
Currently, pathology is the gold standard for tumor diagnosis; however, tissue samples are obtained after biopsy or surgical removal, and histopathology results for the final diagnosis of a suspected tumor may take several days[11]. Hyperspectral analysis can capture both the spatial and spectral information of tissue in one snapshot, which could distinguish between tumor and normal tissue[12]. Hyperspectral analysis may be a powerful tool for tissue analysis with many advantages, including rapidity and no ionizing radiation and contrast agents[13].
In this study, we used a VX2 carcinoma model to perform hyperspectral analysis for two reasons. First, the main blood supply from the hepatic artery and the rapid growth demonstrated by the hepatic VX2 carcinoma model are similar to the characteristics of human HCC[14]. Second, the VX2 tumor is homogeneous and its spectral analysis is simple[15]. The results of this study confirm that hyperspectral analysis can accurately distinguish VX2 tumor and normal tissues.
Because hyperspectral analysis is not penetrable, previous research on diagnosis has mainly focused on superficial tissue and establishment of the tumor margin during surgery[16,17]. The development of interventional medicine provides another potential application for hyperspectral analysis. We can reach deeper organs with minimal invasion by percutaneous approach, and a millimeter-level optical fiber can be introduced into the deep tissue, such as the liver, to obtain reflecting hyperspectral signals or images. Compared with the hyperspectral features of tumors obtained in previous studies, we could judge the characteristics of the region of interest. With the accumulation of different hyperspectral features of different tumors[18], real-time diagnosis of tumors can be achieved in the future.
Hyperspectral imaging is possible for cell level analysis[19], which provides a new direction for our analysis of tumor heterogeneity. Tumor heterogeneity is important for treatment choice and prognostic analysis. However, the methods for analysis of heterogeneity currently used in the clinical cannot be used for large-scale heterogeneity analysis because of the limitations of the sample. By hyperspectral analysis, many tissue areas can be evaluated simultaneously[20], which is beneficial to the analysis of tumor heterogeneity. For deep tumors, we can use the means described earlier to establish a channel through percutaneous puncture of the tumor to detect the tumor cell heterogeneity in the channel and to provide a reference for the choice of treatment and assessment of prognosis.
The results of this study have demonstrated that hyperspectral analysis could discriminate rabbit VX2 liver tumor from normal tissue. The limitations of this study include the small number of animals used, and we did not perform hyperspectral imaging. Further research will continue to obtain the maximum useful information that differentiates liver cancer from normal tissue.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Currently, the most accurate diagnosis imaging modality for HCC is magnetic resonance imaging. However, it is difficult to distinguish cirrhosis lesions. Hyperspectral imaging may be a novel modality as an early/fast diagnosis. To the best of our knowledge, there are few reports about hyperspectral analysis for HCC.
Because hyperspectral analysis is not penetrable, previous research on diagnosis has mainly focused on superficial tissue and establishment of the tumor margin during surgery. The development of interventional medicine provides another potential application for hyperspectral analysis. We can reach deeper organs with minimal invasion by a percutaneous approach, and a millimeter-level optical fiber can be introduced into the deep tissue to obtain reflecting hyperspectral signals or images. With the combination of interventional techniques and hyperspectral analysis, we want to provide a new complementary diagnostic tool for HCC.
In this study, we evaluated the feasibility of hyperspectral analysis for the discrimination of rabbit VX2 liver tumor from normal liver tissue, and to identify if hyperspectral imaging can distinguish HCC from normal tissue.
A rabbit liver VX2 tumor model was established. After laparotomy and under direct view, VX2 tumor tissue and normal liver tissue were subjected to hyperspectral analysis.
The spectral signature of the liver tumor was clearly distinguishable from that of the normal tissue, simply from the original spectral curves. Specifically, two absorption peaks at 600–900 nm wavelength in normal tissue disappeared in tumor tissue, but a new reflection peak appeared in the tumor tissue. The average optical reflection at the whole waveband of 400-1800 nm in liver tumor was higher than that of the normal tissue.
Hyperspectral analysis can differentiate rabbit VX2 tumors from normal tissue. Further research will focus on performing hyperspectral imaging to obtain more information for differentiation of liver cancer from normal tissue.
With the combination of interventional techniques and hyperspectral analysis, it is expected to bring us a novel complementary diagnostic tool for HCC. Hyperspectral analysis may be a powerful tool for HCC analysis, with many advantages, including rapidity and no ionizing radiation or contrast agents.
We would like to extend our gratitude the departmental staff members for their support in this study.
| 1. | Hemming AW, Berumen J, Mekeel K. Hepatitis B and Hepatocellular Carcinoma. Clin Liver Dis. 2016;20:703-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Seyda Seydel G, Kucukoglu O, Altinbasv A, Demir OO, Yilmaz S, Akkiz H, Otan E, Sowa JP, Canbay A. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. 2016;15:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (1)] |
| 3. | Ghanaati H, Alavian SM, Jafarian A, Ebrahimi Daryani N, Nassiri-Toosi M, Jalali AH, Shakiba M. Imaging and Imaging-Guided Interventions in the Diagnosis and Management of Hepatocellular Carcinoma (HCC)-Review of Evidence. Iran J Radiol. 2012;9:167-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (4)] |
| 5. | Akbari H, Halig LV, Zhang H, Wang D, Chen ZG, Fei B. Detection of Cancer Metastasis Using a Novel Macroscopic Hyperspectral Method. Proc SPIE Int Soc Opt Eng. 2012;8317:831711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Lu G, Qin X, Wang D, Chen ZG, Fei B. Estimation of Tissue Optical Parameters with Hyperspectral Imaging and Spectral Unmixing. Proc SPIE Int Soc Opt Eng. 2015;9417:pii: 94170Q. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Martin ME, Wabuyele MB, Chen K, Kasili P, Panjehpour M, Phan M, Overholt B, Cunningham G, Wilson D, Denovo RC, Vo-Dinh T. Development of an advanced hyperspectral imaging (HSI) system with applications for cancer detection. Ann Biomed Eng. 2006;34:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Akbari H, Halig LV, Schuster DM, Osunkoya A, Master V, Nieh PT, Chen GZ, Fei B. Hyperspectral imaging and quantitative analysis for prostate cancer detection. J Biomed Opt. 2012;17:076005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Tong H, Duan LG, Zhou HY, Feng S. Modification of the method to establish a hepatic VX2 carcinoma model in rabbits. Oncol Lett. 2018;15:5333-5338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lee KH, Liapi E, Buijs M, Vossen JA, Prieto-Ventura V, Syed LH, Geschwind JF. Percutaneous US-guided implantation of Vx-2 carcinoma into rabbit liver: A comparison with open surgical method. J Surg Res. 2009;155:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Nguyen A, Moore J, Zuccon G, Lawley M, Colquist S. Classification of pathology reports for cancer registry notifications. Stud Health Technol Inform. 2012;178:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Akbari H, Kosugi Y, Kojima K, Tanaka N. Detection and analysis of the intestinal ischemia using visible and invisible hyperspectral imaging. IEEE Trans Biomed Eng. 2010;57:2011-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Yan S, Cui S, Ke K, Zhao B, Liu X, Yue S, Wang P. Hyperspectral Stimulated Raman Scattering Microscopy Unravels Aberrant Accumulation of Saturated Fat in Human Liver Cancer. Anal Chem. 2018;90:6362-6366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Crow P, Molckovsky A, Stone N, Uff J, Wilson B, WongKeeSong LM. Assessment of fiberoptic near-infrared raman spectroscopy for diagnosis of bladder and prostate cancer. Urology. 2005;65:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | van Lienden KP, Hoekstra LT, van Trigt JD, Roelofs JJ, van Delden OM, van Gulik TM. Effect of hepatic artery embolization on liver hypertrophy response in a rabbit liver VX2 tumor model. Hepatobiliary Pancreat Dis Int. 2013;12:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Uhr JW, Huebschman ML, Frenkel EP, Lane NL, Ashfaq R, Liu H, Rana DR, Cheng L, Lin AT, Hughes GA, Zhang XJ, Garner HR. Molecular profiling of individual tumor cells by hyperspectral microscopic imaging. Transl Res. 2012;159:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Daeschlein G, Langner I, Wild T, von Podewils S, Sicher C, Kiefer T, Jünger M. Hyperspectral imaging as a novel diagnostic tool in microcirculation of wounds. Clin Hemorheol Microcirc. 2017;67:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Nakariyakul S, Casasent D. Fusion algorithm for poultry skin tumor detection using hyperspectral data. Appl Opt. 2007;46:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Grégori G, Patsekin V, Rajwa B, Jones J, Ragheb K, Holdman C, Robinson JP. Hyperspectral cytometry at the single-cell level using a 32-channel photodetector. Cytometry A. 2012;81:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Smith RT, Post R, Johri A, Lee MD, Ablonczy Z, Curcio CA, Ach T, Sajda P. Simultaneous decomposition of multiple hyperspectral data sets: signal recovery of unknown fluorophores in the retinal pigment epithelium. Biomed Opt Express. 2014;5:4171-4185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Emi M, Hanaoka N, Park SJ S- Editor: Ji FF L- Editor: Filipodia E- Editor: Wu YXJ