Published online Oct 15, 2018. doi: 10.4251/wjgo.v10.i10.282
Peer-review started: July 19, 2018
First decision: August 2, 2018
Revised: August 7, 2018
Accepted: August 12, 2018
Article in press: August 13, 2018
Published online: October 15, 2018
Processing time: 88 Days and 19.5 Hours
Peritoneal carcinomatosis (PC) from gastric cancer has traditionally been considered a terminal progression of the disease and is associated with poor survival outcomes. Positive peritoneal cytology similarly worsens the survival of patients with gastric cancer and treatment options for these patients have been limited. Recent advances in multimodality treatment regimens have led to innovative ways to care for and treat patients with this disease burden. One of these advances has been to use neoadjuvant therapy to try and convert patients with positive cytology or low-volume PC to negative cytology with no evidence of active peritoneal disease. These strategies include the use of neoadjuvant systemic chemotherapy alone, using neoadjuvant laparoscopic heated intraperitoneal chemotherapy (NLHIPEC) after systemic chemotherapy, or using neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) in a bidirectional manner. For patients with higher volume PC, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been mainstays of treatment. When used together, CRS and HIPEC can improve overall outcomes in properly selected patients, but overall survival outcomes remain unacceptably low. The extent of peritoneal disease, commonly measured by the peritoneal carcinomatosis index (PCI), and the completeness of cytoreduction, has been shown to greatly impact outcomes in patients undergoing CRS and HIPEC. The uses of NLHIPEC and NLHIPEC plus NIPS have both been shown to decrease the PCI and thus increase the opportunity for complete cytoreduction. Newer therapies like pressurized intraperitoneal aerosol chemotherapy and immunotherapy, such as catumaxomab, along with improved systemic chemotherapeutic regimens, are being explored with great interest. There is exciting progress being made in the management of PC from gastric cancer and its’ treatment is no longer futile.
Core tip: Peritoneal carcinomatosis (PC) from gastric cancer, along with positive peritoneal cytology, are associated with poor overall outcomes. The treatment of patients with this disease burden has greatly improved and new multimodality treatment regimens have been introduced. Some of these include neoadjuvant laparoscopic heated intraperitoneal chemotherapy and bidirectional therapies like neoadjuvant intraperitoneal and systemic therapy. Appropriate patient selection remains crucial for optimal outcomes but we can be optimistic about the prospects for carefully selected patients with PC from gastric cancer.
- Citation: Leiting JL, Grotz TE. Optimizing outcomes for patients with gastric cancer peritoneal carcinomatosis. World J Gastrointest Oncol 2018; 10(10): 282-289
- URL: https://www.wjgnet.com/1948-5204/full/v10/i10/282.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i10.282
Gastric cancer, more than any other malignancy, has a particular predilection for peritoneal dissemination. The incidence of peritoneal carcinomatosis (PC) at diagnosis ranges anywhere from 5%-30% depending on the staging modality used[1,2]. Furthermore, PC is the most common form of relapse after undergoing curative resection as 30% of all recurrences are in the peritoneum and up to 60% of patients have PC at their time of death[3,4]. Imaging is inadequate with computed tomography (CT) scans having a sensitivity of only 33% and specificity of 99% for detecting PC and 2-[18F]-Fluoro-2-Deoxy-D-Glucose ([18F]FDG) and positron emission tomography (PET) scans having a senstivity of 28% and specificity of 97%[5]. Therefore, diagnostic laparoscopy and peritoneal cytology is indicated for clinical stage T1b or higher gastric cancer as a vital step to detect radiologically occult PC in nearly 40% of patients[6,7]. The presence of microscopic cancer cells within the peritoneal cavity can be identified in up to 6% of patients with no other evidence of metastatic disease[8]. Patients without visible peritoneal metastases but with positive cytology are considered to have stage M1 disease according to the most recent American Joint Committee on Cancer (AJCC) staging as the outcomes are more similar to patients with gross peritoneal metastasis than those with local disease only[9-11].
PC from gastric cancer has generally been considered a terminal progression of disease and has worse outcomes than PC from other malignancies such as ovarian cancer or appendiceal cancer[9,10,12]. Survival for patients with PC is limited but varies based on the burden of disease. A recent series from MD Anderson of patients treated with modern systemic chemotherapy reported 1 year survivals of 24%, 57% and 84% for patients with radiographic PC, PC identified on diagnostic laparoscopy only and positive cytology only, respectively[13]. A similar report from Memorial Sloan-Kettering confirmed a poor overall survival (OS) for patients with gastric cancer and peritoneal cytology with a median OS of 1.3 years compared to 0.8 years for patients with radiographic evidence of peritoneal disease[7].
The management of patients with positive peritoneal cytology is an evolving field. The role for gastrectomy in patients with limited primary disease and positive cytology without any other peritoneal disease has been debated. Some small studies have shown a survival benefit with a gastrectomy in this subset of patients[14,15]. However, gastrectomy in the setting of untreated positive peritoneal cytology invariably leads to recurrence. National Comprehensive Cancer Network (NCCN) guidelines recommend peritoneal cytology be managed similar to other patients with metastatic gastric cancer with systemic chemotherapy and no surgery[16].
The need to overcome this seemingly small volume and yet unfavorable disease burden has led investigators to seek ways to convert patients with positive cytology to negative cytology so they can proceed to a curative intent gastrectomy (Table 1). The use of neoadjuvant chemotherapy is one of these methods. Aizawa et al[17] found that 23 of 47 patients (48.9%) with positive cytology converted to negative cytology after neoadjuvant systemic chemotherapy. R0 resections were able to be performed on all patients. The patients who had a conversion to negative cytology and underwent salvage gastrectomy had a survival benefit of 30.4 mo vs 15.0 mo (P = 0.03) when compared to those who had persistently positive cytology treated with gastrectomy[17]. Similarly, a study from Memorial Sloan-Kettering demonstrated that 21 of 48 (44%) patients with initially positive peritoneal cytology treated with systemic chemotherapy achieved negative cytology on repeat laparoscopy[7]. Unfortunately, the Aizawa et al[17] study reported that 19% of patients progressed on systemic chemotherapy and the MSKCC study reported that 56% had disease progression while receiving systemic chemotherapy. Therefore, better induction treatments are needed[7,17].
| Ref. | Patient No. | Treatment group(s) | Intraperitoneal regimen | Systemic regimen | Outcomes | |
| Aizawa et al[17], 2015 | 47 | NA systemic chemo | -- | Variable | 48.9% converted to negative cytology | |
| Negative cytology Median OS: 30.4 mo | Positive cytology Median OS: 15.0 mo | |||||
| Badgwell et al[18], 2017 | 19 | NA systemic chemo, then NLHIPEC, then gastrectomy if peritoneal disease cleared | MMC and cisplatin | Variable | 36.8% converted to negative cytology or had clearance of PC Entire cohort median OS: 30.2 mo | |
| Fujiwara et al[19], 2011 | 25 | NA systemic and IP chemo → gastrectomy if peritoneal disease cleared | MMC and cisplatin | IV docetaxel, 5-fu, cisplatin | 56% converted to negative cytology or had clearance of PC | |
| Negative Median OS: 27.1 mo | Positive Median OS: 9.6 mo | |||||
| Ishigami et al[20], 2009 | 40 | NA systemic and IP chemo | Paclitaxel | IV paclitaxel and oral S-1 | Median OS: 22.5 mo | |
One potential induction treatment is neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy (NLHIPEC). In a small phase 2 study, Badgwell et al[18] found that 7 of 19 patients (36.8%) with positive peritoneal cytology or low volume PC had resolution in their peritoneal disease and 5 were able to proceed to gastrectomy. Of note, all patients had undergone systemic chemotherapy before being enrolled in the study. Median OS from the time of diagnosis for the entire cohort was 30.2 mo and median OS for the patients who proceeded to gastrectomy was 29 mo from the time of their resection[18]. This approach utilized systemic chemotherapy first, followed by direct intraperitoneal therapy, with encouraging results. Unfortunately, 63.2% of patients had persistently positive cytology or residual PC and did not go onto salvage gastrectomy.
Neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) is another method that utilizes systemic chemotherapy and intraperitoneal chemotherapy, but performs this at the same time in a bidirectional design. Fujiwara et al[19] reported 14 of 25 patients (56%) had resolution of their peritoneal disease with either negative cytology or complete regression of PC. Median OS rate for the group with resolution of peritoneal disease was 27.1 mo vs 9.6 mo (P < 0.0001) in patients with persistently positive cytology or residual PC[19]. Ishigami et al[20] looked at the safety and efficacy of bidirectional treatment for patients with positive cytology or PC. They showed a median OS of 22.5 mo and 1-year survival rates of 78%.
The role of gastrectomy in patients with peritoneal disease was addressed in the REGATTA trial[21]. This phase 3 trial enrolled 175 patients with a single incurable factor and randomized them to systemic chemotherapy alone or gastrectomy plus systemic chemotherapy. PC was the incurable factor in three-quarters of the patients enrolled. The authors reported no survival benefit to patients undergoing gastrectomy in addition to systemic chemotherapy[21]. This confirmed that removing the primary tumor without addressing the metastases is not beneficial to the patient.
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) attempt to address both the primary and the peritoneal metastases simultaneously (Table 2). This aggressive approach has been investigated for gastric cancer since the late 1980’s[22-24]. It includes resection of all visible tumor from the peritoneal cavity, followed by the instillation of HIPEC[22]. For the past 30 years, CRS combined with HIPEC has remained the only potentially curative treatment for this advanced stage of gastric cancer[25,26]. A recent meta-analysis that included 11 randomized controlled trials and 21 high quality prospective studies demonstrated an increased median survival of 4 mo in patients with gastric cancer PC treated with HIPEC[27], however, the HIPEC group did experience a higher risk of severe complications. Similarly, CRS and HIPEC have shown a significant improvement in survival for patients with PC from other primaries like appendiceal and ovarian cancer[28,29].
| Ref. | Patient No. | Treatment group(s) | Intraperitoneal regimen | Systemic regimen | Outcomes | ||
| Bonnot et al[30], 2018 | 277 | CRS alone vs CRS + HIPEC | 1 | 1 | CRS Alone Median OS: 12.1 mo | CRS + HIPEC Median OS: 12.1 mo | |
| Yang et al[31], 2011 | 68 | CRS alone vs CRS + HIPEC | Cisplatin and MMC | - | CRS Alone Median OS: 6.5 mo | CRS + HIPEC Median OS: 11.0 mo | |
| Glehen et al[32], 2010 | 159 | CRS with PIC (HIPEC or EPIC) | Variable | - | Median OS: 9.2 mo | ||
| Rudloff et al[33], 2014 | 16 | CRS/HIPEC/SC vs SC alone | Oxaliplatin | FOLFOXIRI | SC Alone 4.3 mo | CRS/HIPEC/SC Median OS: 11.3 mo | |
| Canbay et al[34], 2014 | 194 | NA systemic and IP chemo, then CRS and HIPEC if responsive | Docetaxel and cisplatin | Oral S-1 | 78.3% had negative cytology and underwent CRS and HIPEC | ||
| No response (no CRS or HIPEC) Median OS: 7.5 mo | Response (CRS with HIPEC) Median OS: 15.8 mo | ||||||
| Yonemura et al[38], 2017 | 105 | NLHIPEC → CRS or NLHIPEC → NIPS → CRS | Docetaxel and cisplatin | Oral S-1, IV docetaxel and cisplatin | NLHIPEC + CRS Median OS: 14.1 mo PCI: 14.2 → 11.8 | NLHIPEC + NIPS + CRS Median OS: 19.2 mo PCI: 14.8 → 9.9 | |
Furthermore, the recent CYTO-CHIP study investigated whether CRS alone was beneficial compared to CRS with HIPEC[30]. They found a significantly improved OS in the CRS with HIPEC group (18.8 mo vs 12.1 mo), suggesting that it is the combination of CRS and HIPEC that improves survival[30]. Yang et al[31] reported similar results with improved survival for CRS and HIPEC when compared to CRS alone. Median OS for patients undergoing CRS and HIPEC was 11.0 mo compared to 6.5 mo (P = 0.046) for CRS alone. Lastly, in a large retrospective study, Glehen et al[32] reported a 9.2 mo median OS for 159 patients undergoing CRS with HIPEC or EPIC, with improvement to 15 mo if the cytoreduction was complete.
The benefit of CRS and HIPEC over systemic chemotherapy alone was shown by Rudloff et al[33]. In a small cohort of 16 patients, those that underwent CRS, HIPEC, and systemic chemotherapy had an overall median survival rate of 11.3 mo compared to 4.3 mo in the systemic chemotherapy alone group[33].
Unfortunately, although these studies all demonstrated a modest benefit to CRS and HIPEC, OSs remain unacceptably low. It appears that not all patients benefit from CRS and HIPEC and that appropriate patient selection is vital in to order to optimize outcomes. The two most commonly found prognostic factors for survival are consistently the extent of disease, most commonly measured by the peritoneal carcinomatosis index (PCI), and the completeness of cytoreduction. Glehen et al[32] showed that the PCI was the only independent prognostic factor in patients with a complete cytoreduction. No patient survived more than 3 years if their PCI was > 12[32]. A meta-analysis confirmed this with no patients being alive after 3 years if their PCI was > 12[4]. A lower threshold of PCI ≤ 6 was an independent prognostic factor for patients undergoing CRS and HIPEC after bi-directional chemotherapy (HR 2.16, 95%CI: 1.17-3.98, P = 0.013) in a recent Japanese study[34]. Similarly, Chia et al[35] found that a PCI of < 7 was a significant predictor of survival. Those with PCI < 7 had a median OS of 26.4 mo compared to 10.9 mo in those who had a PCI ≥ 7 (HR 2.67, 95%CI: 1.54-4.64, P < 0.001). All the patients who were considered cured as defined by being disease-free at 5 years had a PCI < 7. This same PCI cut-off was seen in a study by Yonemura et al[36] who found that a PCI < 7 was associated with improved survival (median survival 2.8 years vs 1.1 years, P = 0.0001).
With a lower volume of disease, there is a higher probabilty of being able to completely remove all the metastatic disease. This is the only population that can be expected to have a chance at long-term survival. A meta-analysis showed that cytoreductive scores of 0 or 1 significantly improved survivals in patients with gastric PC[4]. Glehen et al[37] showed that patients undergoing a complete cytoreduction with a CC score of 0 or 1 achieved a median OS of 21.3 mo compared to only 6 mo for those with an incomplete cytoreduction. The 5-year OS was 29.4% for those who attained a complete cytoreduction with no survivors in the incomplete cytoreduction group[37]. Canbay et al[34] used bidirectional therapy (neoadjuvant systemic and intraperitoneal therapy) to reduce the volume of disease before CRS and HIPEC for patients that responded to treatment. They found better OS in patients who responded to the neoadjuvant treatment and were able to undergo CRS and HIPEC (15.8 mo vs 7.5 mo)[34].
There is substantial interest in novel and innovative ways to reduce the PCI prior to cytoreduction. This is crucial because PCI is a determinant in achieving a complete cytoreduction and only patients with a low volume of disease who undergo a complete cytoreduction have a long-term survival benefit from the procedure. Yonemura et al[38] used NLHIPEC and NLHIPEC plus NIPS to try and reduce PCI levels before CRS. They found that while NLHIPEC alone reduced PCI levels (14.2 ± 10.7 to 11.8 ± 11.0, P = 0.023), NLHIPC plus NIPS doubled the PCI reduction (14.8 ± 11.4 to 9.9 ± 11.3, P < 0.0001). This may provide more patients with the opportunity for a complete cytoreduction when this would have otherwise not been possible due to a high PCI.
Even with all the advances in therapy for patients with PC from gastric cancer, there are still a large number of patients who are not eligible for these therapies given their high tumor burden or conditional status. Palliative treatment for these patients includes chemotherapy, chemoradiation, or supportive care. None of these regimens treat the peritoneal disease burden and patients generally have very limited survivals.
A new experimental therapy that has emerged to treat these patients is pressurized intraperitoneal aerosol chemotherapy, or PIPAC[39]. This method delivers aerosolized chemotherapy to the peritoneum. The benefit of this method is that the pressure allows for greater lesion penetration as well as allowing for diffuse and even coverage throughout the abdomen[40]. This deeper penetration is likely more critical in these patients with advanced bulky peritoneal metastases. Nadiradze et al[39] recently published data on 24 patients with end stage gastric cancer with PC. These patients underwent 1 or more rounds of PIPAC with doxorubicin and cisplatin. The median OS for these patients was 15.4 mo with 52% alive at one year[39]. A multi-center study of PIPAC for advanced PC from a variety of histologies including gastric cancer demonstrated that 63.5% of patients achieved resolution of symptoms[41]. This therapy may prove to be beneficial for more than just end stage gastric cancer patients but additional research is needed.
Innovative discoveries and continued efforts to optimize treatment for patients with PC from gastric cancer are needed. This includes improved systemic chemotherapy options such as FLOT, which has been demonstrated to be effective in patients with limited metastatic disease[42]. The AIO-FLOT3 trial reported a median OS of 31.3 mo and a 60% radiographic response rate for patients who were treated with perioperative FLOT systemic chemotherapy and surgical resection of all metastatic disease[42].
Another innovative approach is the use of immunotherapy, like catumaxomab, as an intraperitoneal treatment (Table 3). Catumaxomab is an antibody that binds to both epithelial cells through epithelial cell adhesion molecule (EpCAM) and T-cells through CD3[43]. Gastric cancer expresses high levels of EpCAM so the intraperitoneal administration of EpCAM provides targeted therapy to peritoneal implants[44]. In patients with malignant ascites from PC of gastric origin, it was found to significantly prolong OS from 44 to 71 d[45]. Bokemeyer et al[46] conducted a phase 2 study where patients underwent intra- and post-operative intraperitoneal catumaxomab administration after undergoing neoadjuvant chemotherapy and resection. These patients had four-year disease-free survival rates of 38% and four-year OS rates as high as 50%. Though catumaxomab is no longer available, the use of intraperitoneal immunotherapy remains promising and is under continued investigation[47].
| Ref. | Patient No. | Treatment group(s) | Intraperitoneal regimen | Systemic regimen | Outcomes | |
| Heiss et al[45], 2010 | 66 | Paracentesis + catumaxomab vs Paracentesis alone | Catumaxomab | - | Paracentesis Alone Median OS: 44 d | Paracentesis + Catumaxomab Median OS: 71 d |
| Bokemeyer et al[46], 2015 | 54 | NA chemotherapy, surgery, intra- and post-op catumaxomab | Catumaxomab | Variable | 4 yr DFS: 38% 4 yr OS: 50% | |
There remain many areas related to the management of PC from gastric cancer that can be improved. Better detection of early occult peritoneal metastases would allow the clinician to select more appropriate patients for these multidisciplinary treatments. This may be in the form of improved imaging modalities like fluorescence and antibody-labelled imaging[48] or the use of RT-PCR with cytology to improve the sensitivity of detecting cancer cells in peritoneal washings[49]. The optimal chemotherapeutic agent, or agents, to use is unclear, both systemically and in the peritoneal cavity. Many of the studies discussed here used different treatment regimens with some varying even within the same study, so it is difficult to compare outcomes from one study to the next. Also, the ideal sequence, route, and duration of treatment for these patients that will deliver the greatest long-term benefit with manageable side-effects is unknown, though there are many promising options.
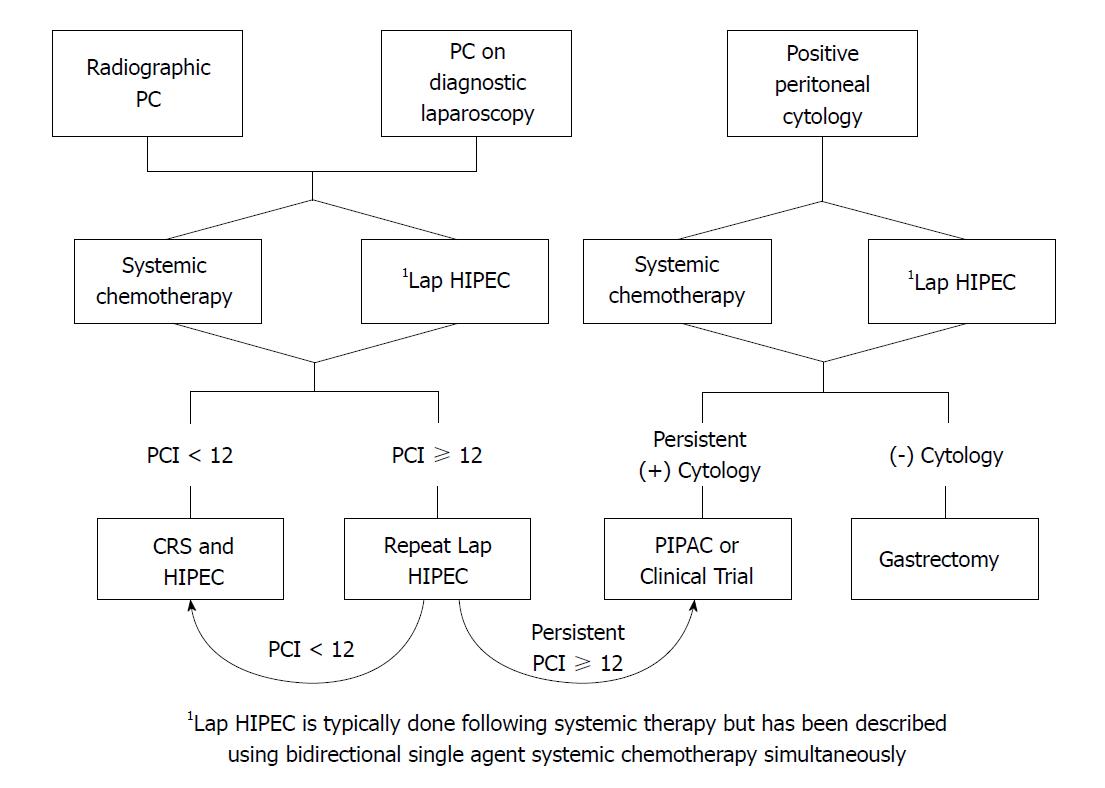
Appropriate patient selection remains crucial for optimal outcomes in patients with gastric cancer, but patients with PC or positive cytology should no longer be immediately excluded from potentially curative multimodality treatment regimens. There are treatment options that can be offered to suitable patients with PC from gastric cancer that have the possibility of extended survival (Figure 1). We are finally seeing progress in the management of a disease that has traditionally been thought of as terminal and it is time to change our approach. We are not yet at a point where we can offer these patients a cure, but the treatment of PC from gastric cancer is no longer a futile endeavor and can be approached with careful optimism.
| 1. | Yonemura Y, Canbay E, Li Y, Coccolini F, Glehen O, Sugarbaker PH, Morris D, Moran B, Gonzaletz-Moreno S, Deraco M. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Goéré D, Gras-Chaput N, Aupérin A, Flament C, Mariette C, Glehen O, Zitvogel L, Elias D. Treatment of gastric peritoneal carcinomatosis by combining complete surgical resection of lesions and intraperitoneal immunotherapy using catumaxomab. BMC Cancer. 2014;14:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 494] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Montori G, Ansaloni L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol. 2011;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol. 2013;68:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173-3180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | National Comprehensive Cancer Network. Gastric Cancer. Version 2. 2018; Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. |
| 10. | Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Rowsell C, Coburn NG. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S27-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D; French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608-5618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Shiozaki H, Elimova E, Slack RS, Chen HC, Staerkel GA, Sneige N, Shimodaira Y, Sagebiel T, Lee JH, Bhutani MS. Prognosis of gastric adenocarcinoma patients with various burdens of peritoneal metastases. J Surg Oncol. 2016;113:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Nakagohri T, Yoneyama Y, Kinoshita T, Konishi M, Inoue K, Takahashi S. Prognostic significance of peritoneal washing cytology in patients with potentially resectable gastric cancer. Hepatogastroenterology. 2008;55:1913-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Suzuki O, Fukuchi M, Mochiki E, Ishiguro T, Sobajima J, Onozawa H, Imaizumi H, Kumagai Y, Baba H, Kumamoto K. Prognostic role of gastrectomy in patients with gastric cancer with positive peritoneal cytology. Int Surg. 2014;99:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Pak LM, Coit DG, Eaton AA, Allen PJ, D’Angelica MI, DeMatteo RP, Jarnagin WR, Strong VE, Kingham TP. Percutaneous Peritoneal Lavage for the Rapid Staging of Gastric and Pancreatic Cancer. Ann Surg Oncol. 2017;24:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Aizawa M, Nashimoto A, Yabusaki H, Nakagawa S, Matsuki A, Homma K, Kawasaki T. The clinical significance of potentially curative resection for gastric cancer following the clearance of free cancer cells in the peritoneal cavity by induction chemotherapy. Surg Today. 2015;45:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Badgwell B, Blum M, Das P, Estrella J, Wang X, Ho L, Fournier K, Royal R, Mansfield P, Ajani J. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann Surg Oncol. 2017;24:3338-3344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Okada K, Mori M, Doki Y. Neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal dissemination. Ann Surg Oncol. 2011;18:3726-3731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 530] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 22. | Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer. 1988;61:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Fujimoto S, Shrestha RD, Kokubun M, Kobayashi K, Kiuchi S, Konno C, Ohta M, Takahashi M, Kitsukawa Y, Mizutani M. Positive results of combined therapy of surgery and intraperitoneal hyperthermic perfusion for far-advanced gastric cancer. Ann Surg. 1990;212:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Glehen O, Gilly FN, Cotte E. Hyperthermic intraperitoneal chemotherapy in advanced gastric cancer: the end of skepticism? Ann Surg Oncol. 2011;18:1524-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, Parisi A, Woo Y. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1543] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 29. | Shaib WL, Martin LK, Choi M, Chen Z, Krishna K, Kim S, Brutcher E, Staley C 3rd, Maithel SK, Philip P, Abdel-Misih S, Bekaii-Saab TS, El-Rayes BF. Hyperthermic Intraperitoneal Chemotherapy Following Cytoreductive Surgery Improves Outcome in Patients With Primary Appendiceal Mucinous Adenocarcinoma: A Pooled Analysis From Three Tertiary Care Centers. Oncologist. 2015;20:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Bonnot PE, Piessen G, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S. CYTO-CHIP: Cytoreductive surgery versus cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: A propensity-score analysis from BIG RENAPE and FREGAT working groups. J Clin Oncol. 2018;36:8. |
| 31. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 508] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 32. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 33. | Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, Webb CC, Walker M, Toomey MA, Schrump D. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 34. | Canbay E, Mizumoto A, Ichinose M, Ishibashi H, Sako S, Hirano M, Takao N, Yonemura Y. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol. 2014;21:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, Bereder JM, Arvieux C, Boschetti G, Glehen O; BIG RENAPE Group. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann Surg Oncol. 2016;23:1971-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, Ichinose M, Miura M, Li Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (8)] |
| 37. | Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, François Y, Vignal J, Gilly FN. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, Takao N, Ichinose M, Liu Y, Li Y. Effects of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy and Neoadjuvant Intraperitoneal/Systemic Chemotherapy on Peritoneal Metastases from Gastric Cancer. Ann Surg Oncol. 2017;24:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J Gastrointest Surg. 2016;20:367-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, Zieren J, Schwab M, Reymond MA. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 41. | Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D, Quenet F, Glehen O, Bakrin N, Passot G. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43:2178-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 43. | Atanackovic D, Reinhard H, Meyer S, Spöck S, Grob T, Luetkens T, Yousef S, Cao Y, Hildebrandt Y, Templin J. The trifunctional antibody catumaxomab amplifies and shapes tumor-specific immunity when applied to gastric cancer patients in the adjuvant setting. Hum Vaccin Immunother. 2013;9:2533-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Warneke VS, Behrens HM, Haag J, Krüger S, Simon E, Mathiak M, Ebert MP, Röcken C. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br J Cancer. 2013;109:2217-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 46. | Bokemeyer C, Stein A, Ridwelski K, Atanackovic D, Arnold D, Wöll E, Ulrich A, Fischer R, Krüger C, Schuhmacher C. A phase II study of catumaxomab administered intra- and postoperatively as part of a multimodal approach in primarily resectable gastric cancer. Gastric Cancer. 2015;18:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Seidl C, Zöckler C, Beck R, Quintanilla-Martinez L, Bruchertseifer F, Senekowitsch-Schmidtke R. 177Lu-immunotherapy of experimental peritoneal carcinomatosis shows comparable effectiveness to 213Bi-immunotherapy, but causes toxicity not observed with 213Bi. Eur J Nucl Med Mol Imaging. 2011;38:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Ito A, Ito Y, Matsushima S, Tsuchida D, Ogasawara M, Hasegawa J, Misawa K, Kondo E, Kaneda N, Nakanishi H. New whole-body multimodality imaging of gastric cancer peritoneal metastasis combining fluorescence imaging with ICG-labeled antibody and MRI in mice. Gastric Cancer. 2014;17:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | To EM, Chan WY, Chow C, Ng EK, Chung SC. Gastric cancer cell detection in peritoneal washing: cytology versus RT-PCR for CEA transcripts. Diagn Mol Pathol. 2003;12:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fiorentini G, Jeong KY, Mohamed SY, Saglam S, Shu X S- Editor: Ji FF L- Editor: A E- Editor: Tan WW