©The Author(s) 2025.
World J Gastrointest Oncol. Jul 15, 2025; 17(7): 106723
Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.106723
Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.106723
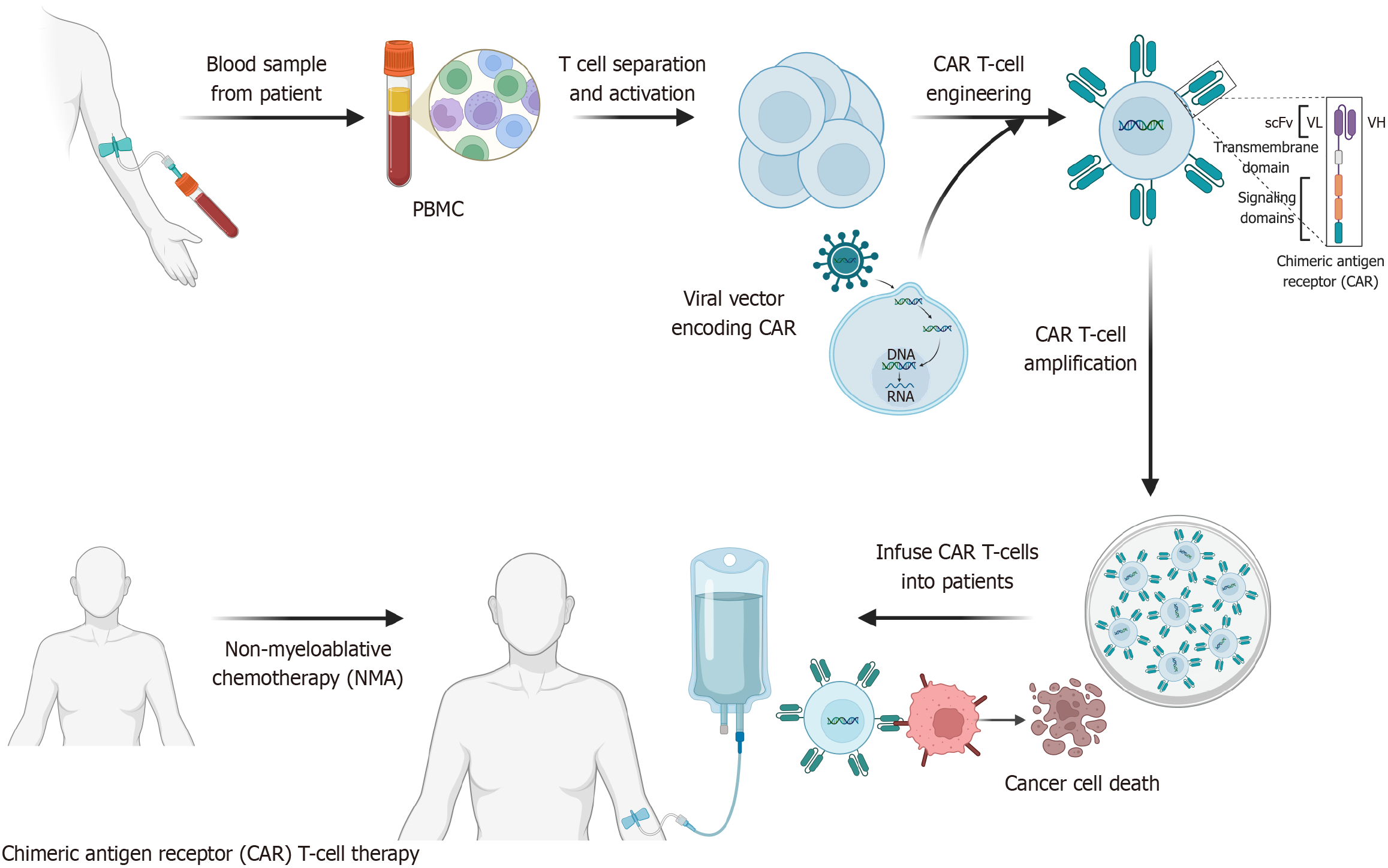
Figure 1 Schematic diagram of chimeric antigen receptor-T cell construction and preparation.
Chimeric antigen receptor (CAR)-T therapy involves isolating T cells from the patient’s blood, genetically engineering them to express CARs, and expanding the modified cells in vitro. After lymphodepleting therapy, the engineered CAR-T cells are infused back into the patient, where they recognize and eliminate tumor cells. PBMC: Peripheral blood mononuclear cell; CAR: Chimeric antigen receptor; scFv: Single-chain variable fragments; VL: Variable region of light chain; VH: Variable region of heavy chain. (Created with BioRender.com).
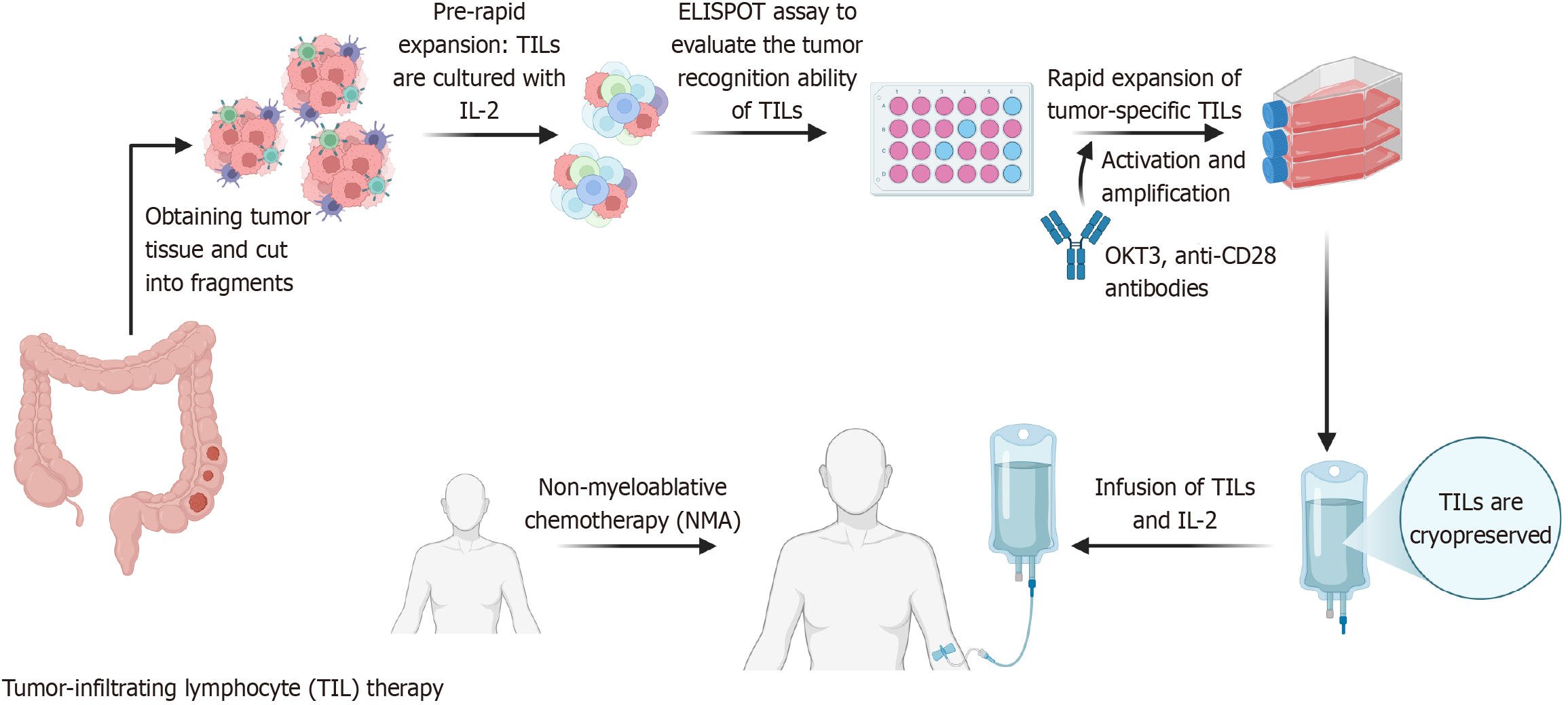
Figure 2 Schematic diagram of tumor-infiltrating lymphocytes construction and preparation.
The process includes tumor digestion and tumor-infiltrating lymphocyte (TIL) isolation, interleukin (IL)-2-mediated culture and expansion, tumor antigen recognition assessment via enzyme linked immune spot assay, and rapid expansion using activating antibodies or magnetic beads. Expanded TILs are cryopreserved and transferred to treatment centers. Before infusion, patients receive lymphodepleting chemotherapy, followed by IL-2 administration to stimulate TIL proliferation and activation. TIL: Tumor-infiltrating lymphocyte; IL: Interleukin; ELISPOT: Enzyme-linked immune spot assay; CD: Cluster of differentiation. (Created with BioRender.com).
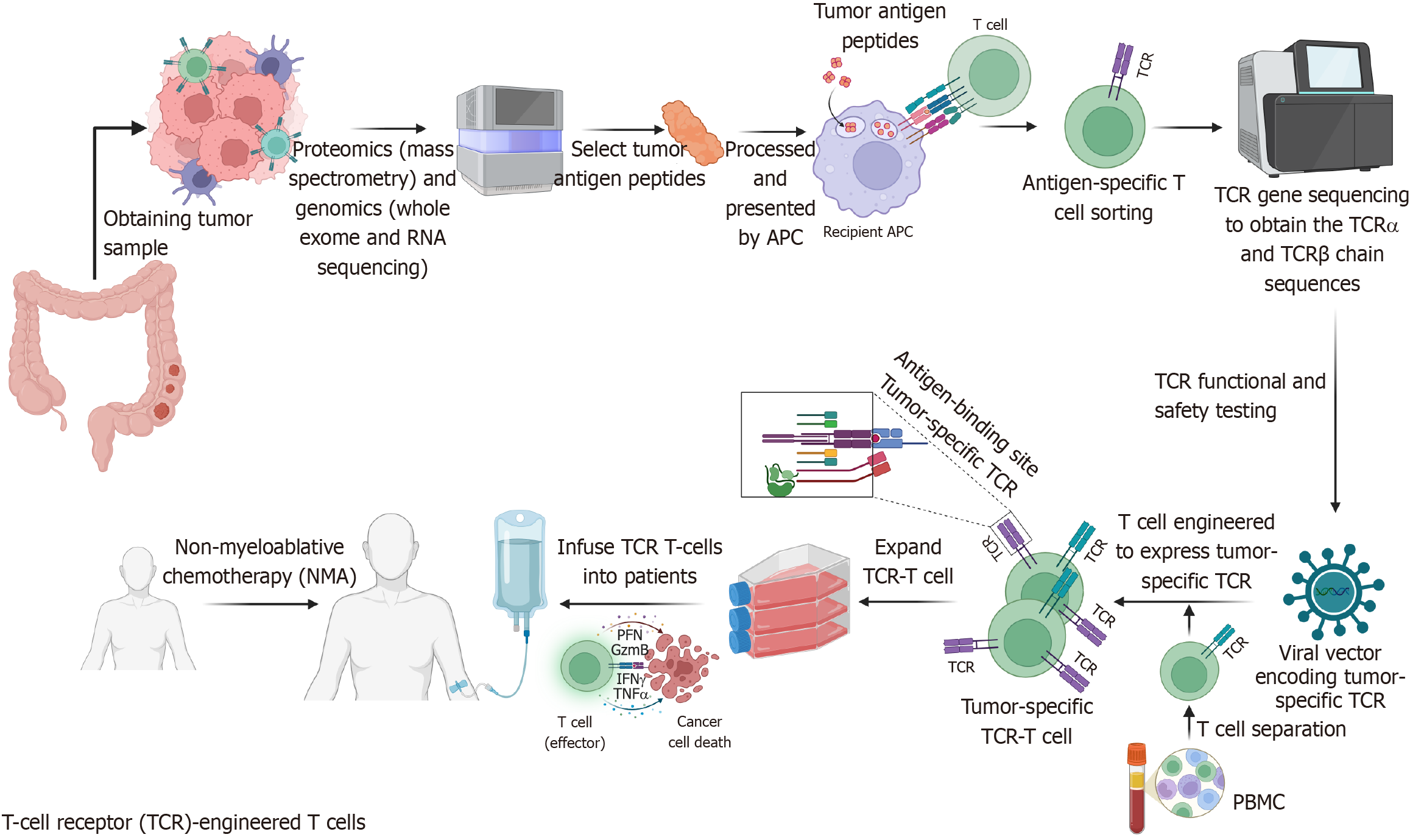
Figure 3 Schematic diagram of T-cell receptor-T cell construction and preparation.
T-cell receptor (TCR)-T cell therapy involves genetically modifying T cells to express tumor-specific TCRs, enhancing immune recognition of tumor antigens presented by human leukocyte antigen (HLA) molecules. The process includes tumor and blood sample collection, antigen identification via proteomics and sequencing, T cell stimulation and selection using HLA-I tetramer staining, TCR gene sequencing and cloning, and lentiviral transfection to generate engineered TCR-T cells. These cells are expanded and reinfused into the patient to specifically target and eliminate tumor cells. TCR: T-cell receptor; IFN: Interferon; TNF: Tumor necrosis factor; APC: Antigen-presenting cell; PBMC: Peripheral blood mononuclear cell. (Created with BioRender.com).
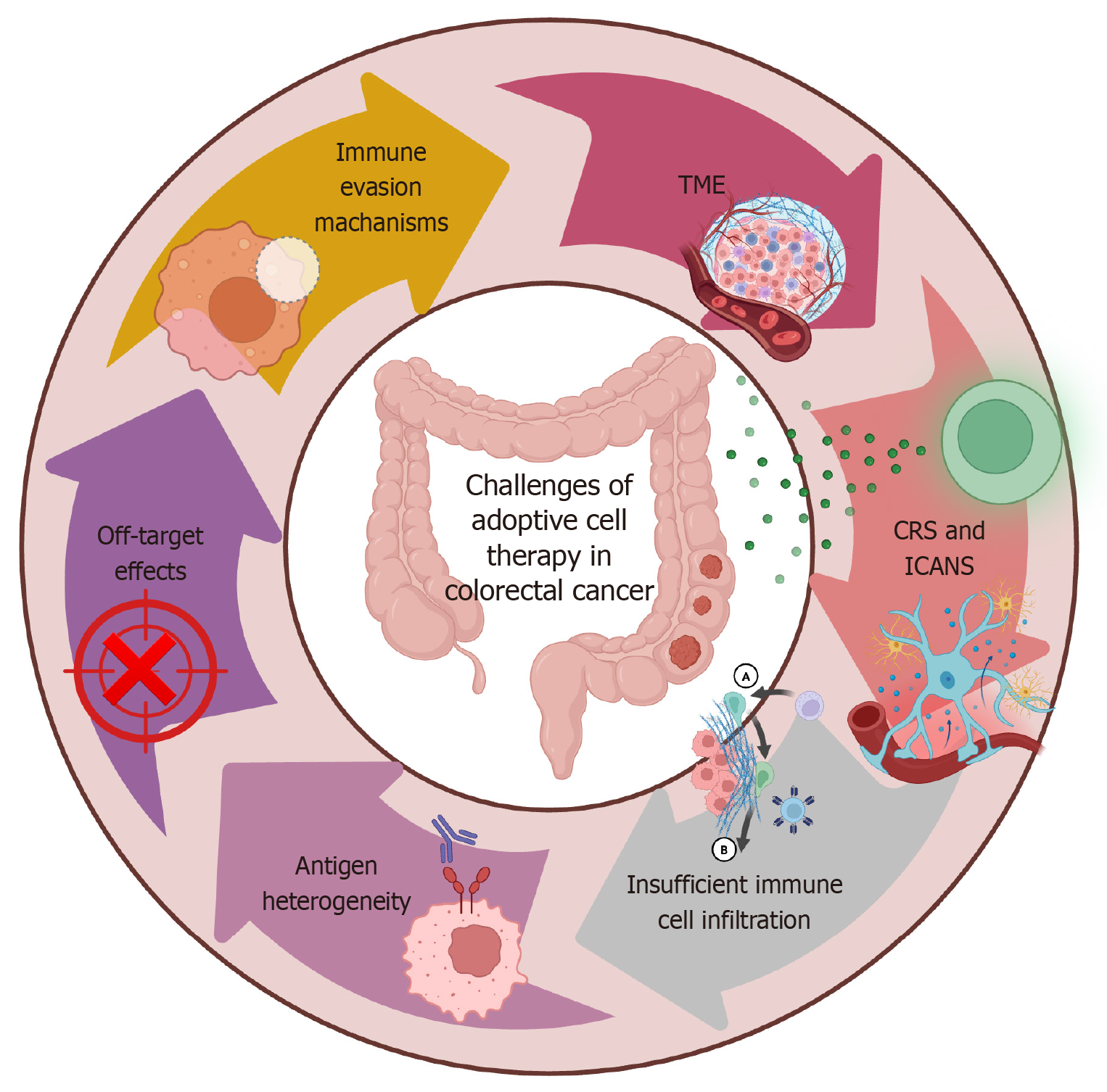
Figure 4 Challenges in adoptive cell therapy for colorectal cancer.
Schematic diagram of the challenges in adoptive cell therapy for colorectal cancer, including immune evasion, immunosuppressive tumor microenvironment, insufficient immune cell infiltration, antigen heterogeneity, off-target effects, cytokine release syndrome, and immune effector cell-associated neurotoxicity syndrome. TME: Tumor microenvironment; CRS: Cytokine release syndrome; ICANS: Immune effector cell-associated neurotoxicity syndrome. (Created with BioRender.com).
- Citation: Chen MY, Wang C, Wang YG, Shi M. Adoptive cell therapy in colorectal cancer: Advances in chimeric antigen receptor T cells. World J Gastrointest Oncol 2025; 17(7): 106723
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/106723.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.106723