©The Author(s) 2018.
World J Gastrointest Oncol. Sep 15, 2018; 10(9): 244-259
Published online Sep 15, 2018. doi: 10.4251/wjgo.v10.i9.244
Published online Sep 15, 2018. doi: 10.4251/wjgo.v10.i9.244
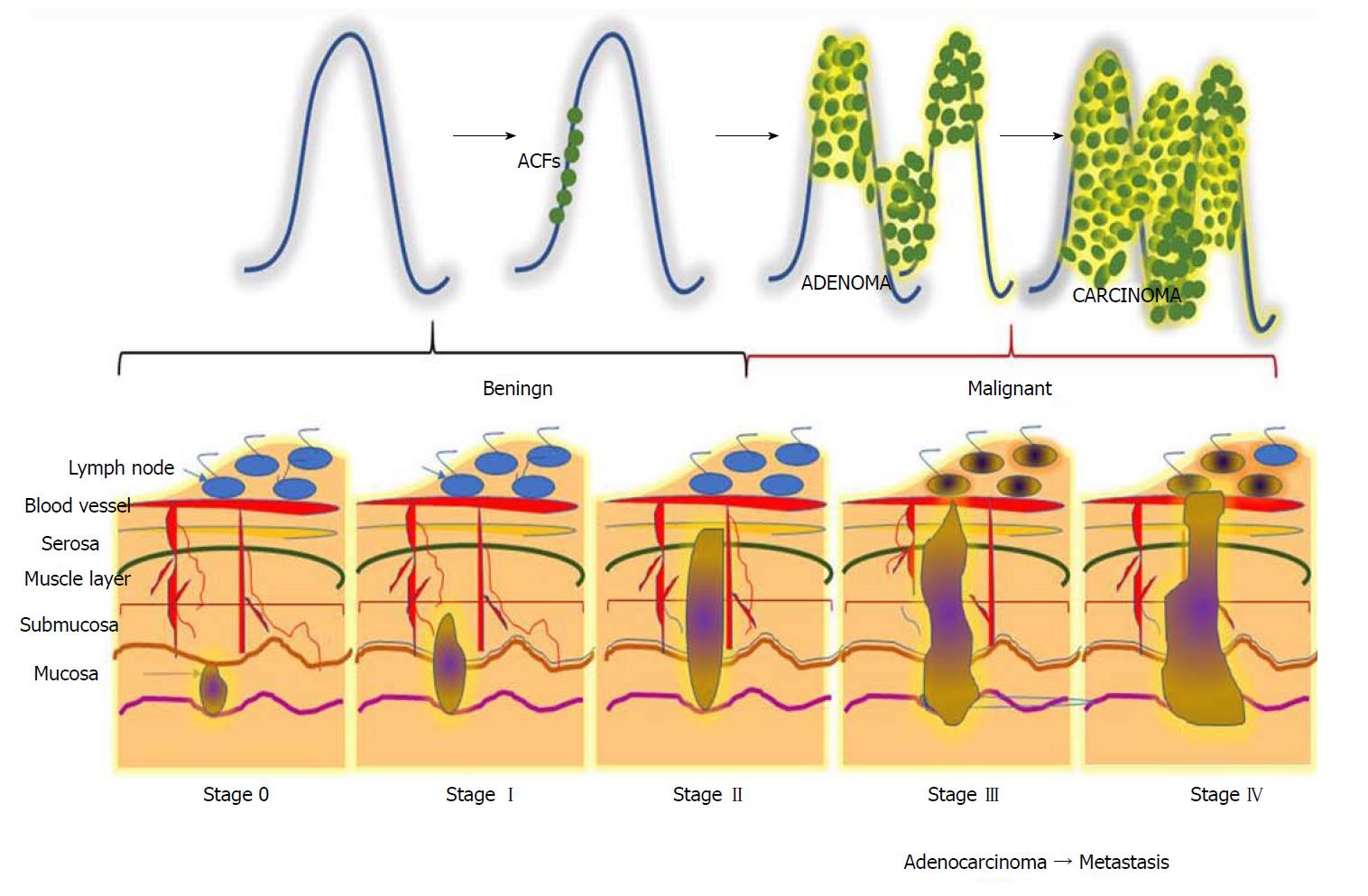
Figure 1 Different stages during the progression of colorectal carcinogenesis.
Stage 0: The cancerous cells grow within the inner lining of the mucosa; Stage I: The cancerous cells grow throughout the mucosa and submucosa. The cancerous growth invades into the muscular layer of the colon; Stage II: The cancerous growth penetrates through the wall of the colon without spreading to neighbouring tissues or lymph nodes; Stage III: The cancer penetrates through layers of muscle into the serosa, the layer of visceral peritoneum. The cancer begins to spread to the lymph nodes; Stage IV: A tumour nodule forms in the tissue surrounding the colon, cancer cells appears within the lymph nodes, and the cancer begins to metastasize.
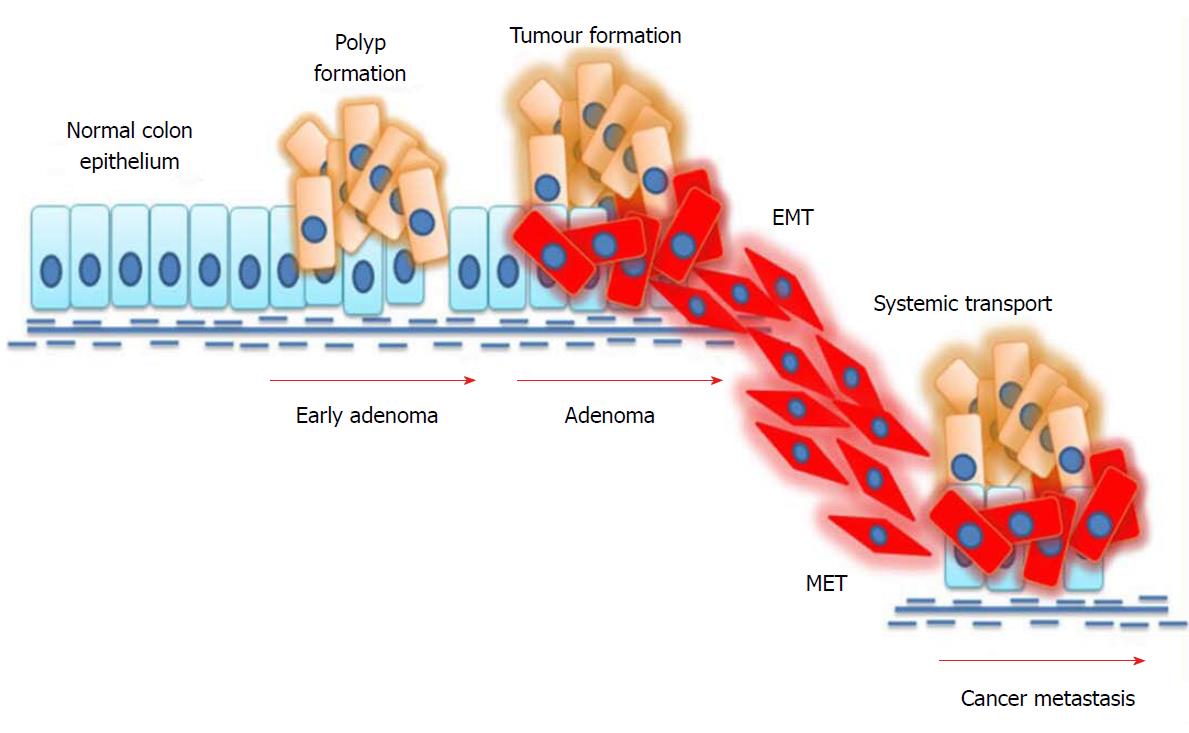
Figure 2 Mechanism of epithelial-to-mesenchymal transition in colorectal cancer.
External stimuli or mutations in cancer cells induce epithelial-to-mesenchymal transition (EMT), where epithelial cells undergo phenotypic changes and transit into an invasive mesenchymal cell state. Mesenchymal cells invade the systemic circulation and undergo a mesenchymal-to-epithelial transition (MET) in distant organs, thus facilitating metastasis.
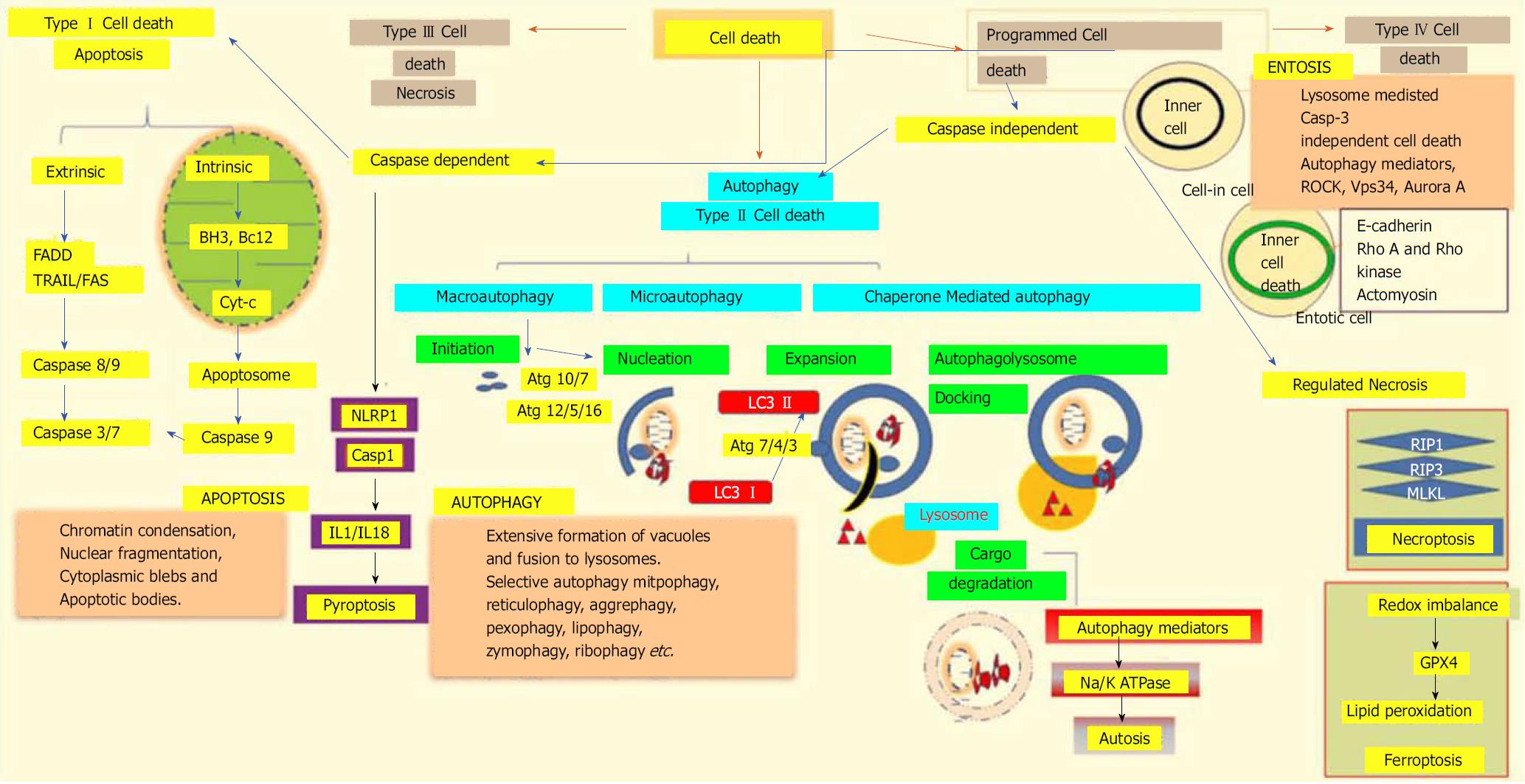
Figure 3 Different modalities of cell death.
Unpredictable perturbations in the extracellular or intracellular microenvironments of a cell can activate several signal transduction cascades that ultimately lead to various forms of cell death. Type I cell death apoptosis: the extrinsic pathway of apoptosis is mediated by Fas-associated death domain protein (FADD). Caspase 8, in turn, triggers caspase 3 and 7, which then activates caspase 9. Both intrinsic and mitochondrial pathways of apoptosis are mediated through the inhibition of anti-apoptotic Bcl2, which in turn activates Bax/Bak and induces release of cytochrome c from the mitochondria. The activation of caspase 9 in the apoptosome induces apoptotic cell death. Type II cell death autophagy: autophagy is an active lysosomal degradative flux, which can be divided into three distinct types; macroautophagy, microautophagy and chaperone-mediated autophagy. Macroautophagy involves four different steps: initiation, autophagosome nucleation, phagosome expansion and completion, and autolysosome docking. The tightly-regulated autophagy machinery is mediated through several autophagy-related (Atg) molecules. Regulated necrosis/necroptosis: regulated necrosis is mediated through the interaction of receptor interacting protein 1 (RIP1) with RIP3 upon caspase 8 inhibition. RIP3 and mixed-lineage kinase domain-like (MLKL) are phosphorylated and assembled into complex IIb, which is then translocated to the plasma membrane to mediate membrane permeabilization. Ferroptosis: this regulated form of cell death is driven by the loss of glutathione peroxidase 4 (GPX4) activity, a lipid repair enzyme, followed by the accumulation of lipid hydroperoxides. Autosis: a plasma membrane Na+/K+-dependent autophagy form of cell death. Entosis: internalized cells undergo entotic cell death through the formation of entotic vacuoles, which is mediated by autophagy proteins like Vps34, etc. Pyroptosis: a caspase-dependent cell death mechanism that is an intermediary variation of apoptosis and necrosis. Caspase 1 is activated by the NLRP3 inflammasome, which activates the inflammatory cytokines interleukin 1β and interleukin 18, which in turn mediate the lytic cycle.
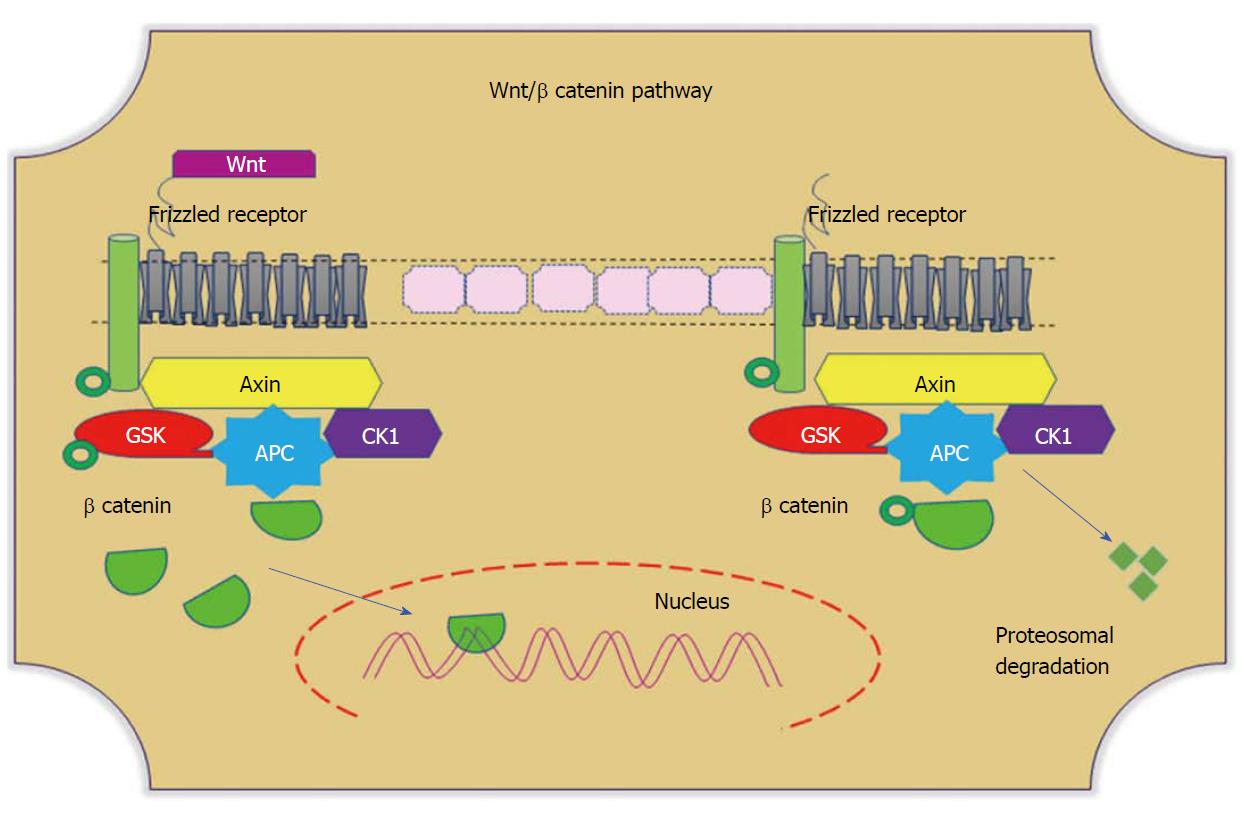
Figure 4 Wnt/β-catenin pathways.
In the absence of Wnt, cytoplasmic β-catenin forms a complex with Axin (yellow), APC (blue), GSK3 (red), and CK1 (purple). Phosphorylated β-catenin undergoes ubiquitin-mediated proteosomal degradation. In the presence of Wnt, Wnt binds to the frizzled receptor, which in turn recruits the Axin complex. This disrupts Axin-mediated proteosomal degradation of β-catenin. Cytoplasmic β-catenin then travels to the nucleus and functions as a co-activator with TCF to activate Wnt-regulated gene expression. GSK: Glycogen synthase kinase; APC: Adenomatous polyposis coli; CK1: Casein kinase 1.
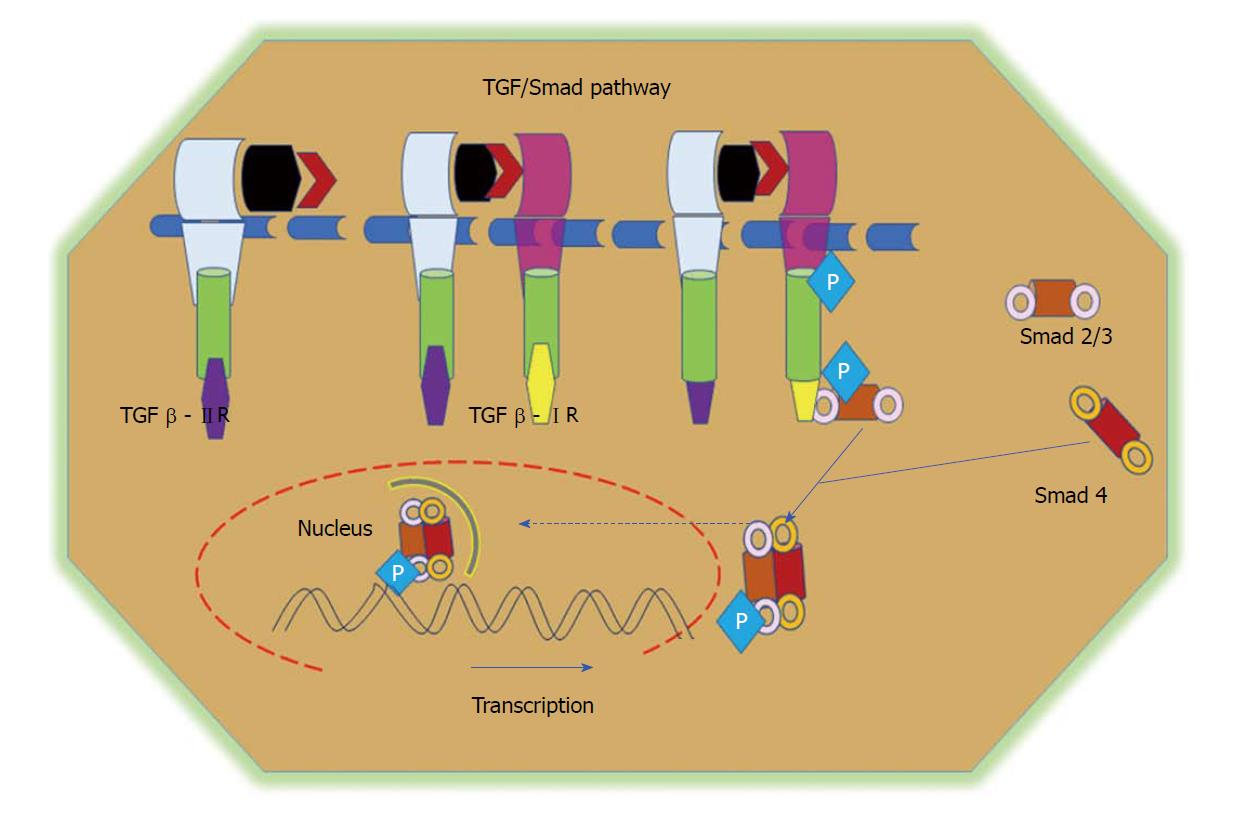
Figure 5 TGF/Smad pathway.
TGFβ signalling is initiated via the binding of the TGFβ1 ligand to receptor II, which promotes dimerization between receptor II and receptor I and the subsequent transphosphorylation of TGFβRI. Activated TGFβRI therein phosphorylates and activates receptor-regulated Smads (Smad2 and Smad3). Phosphorylated Smads, along with co-Smads, form a trimeric complex and translocate to the nucleus to induce the transcription of target genes and promote cell growth and survival. TGF: Transforming growth factor.
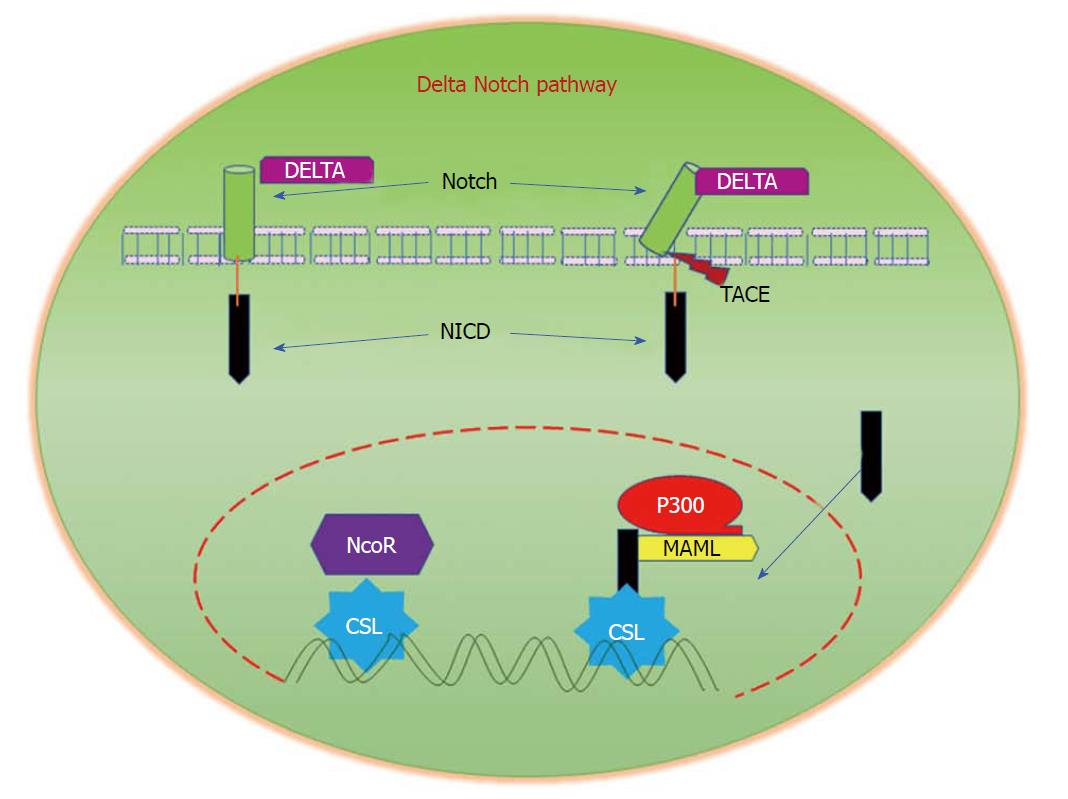
Figure 6 Delta/Notch Signalling.
Notch signalling is initiated by the binding of Notch on one cell to the transmembrane ligands Delta or Jagged on a neighbouring cell. This binding interaction promotes cleavage of the Notch receptor and releases the Notch intracellular domain (NICD), which travels to the nucleus and controls the transcription of Notch responsive genes. In the nucleus, NICD binds to the transcriptional repressor CBF1, which recruits Mastermind-like (MAML) and other co-activators to initiate the transcription of downstream Notch-regulated genes.
- Citation: Pandurangan AK, Divya T, Kumar K, Dineshbabu V, Velavan B, Sudhandiran G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J Gastrointest Oncol 2018; 10(9): 244-259
- URL: https://www.wjgnet.com/1948-5204/full/v10/i9/244.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i9.244