©The Author(s) 2018.
World J Gastrointest Oncol. Jan 15, 2018; 10(1): 15-22
Published online Jan 15, 2018. doi: 10.4251/wjgo.v10.i1.15
Published online Jan 15, 2018. doi: 10.4251/wjgo.v10.i1.15
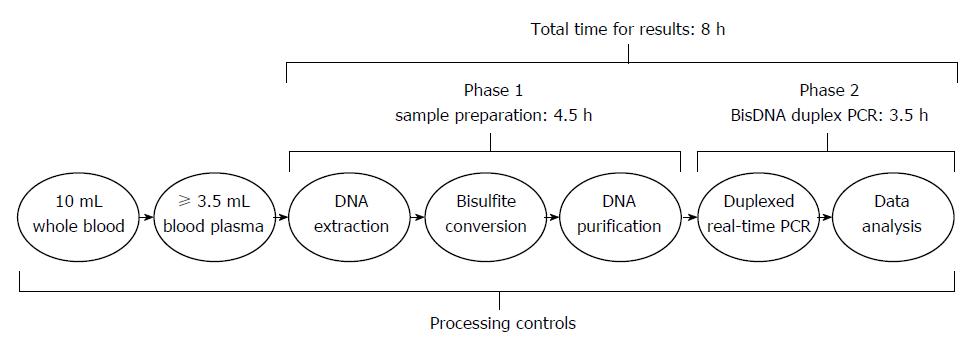
Figure 1 The outline of the Epi proColon work flow.
The test consists of the Epi proColon Plasma Quick kit, PCR kit, and Control kit. The total assay time is approximately 8 h. For the Plasma Quick kit, 3.5 mL of plasma was mixed with an equal volume of lysis buffer; after incubating for 10 min, magnetic beads and absolute ethanol were added. After 45 min, impurities were removed from the magnetic beads by centrifugation; the purified DNA was then released from the beads in the elution buffer and treated at 80 °C with a solution of ammonium bisulfite for deamination of cytosine[34]. After a series of washing steps, the converted DNA (bisulfite-modified DNA, bisDNA) was captured by magnetic beads. The bisDNA was assayed with the PCR kit on a Duplexed Real-Time PCR device. Finally, methylated SEPT9 and PCR results were recorded by the instrument software. In the whole working flow, the processing controls were included to monitor the execution of the procedure and ensure the validity of the test result and model[34].
- Citation: Wang Y, Chen PM, Liu RB. Advance in plasma SEPT9 gene methylation assay for colorectal cancer early detection. World J Gastrointest Oncol 2018; 10(1): 15-22
- URL: https://www.wjgnet.com/1948-5204/full/v10/i1/15.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i1.15