Published online Nov 16, 2017. doi: 10.4253/wjge.v9.i11.558
Peer-review started: June 13, 2017
First decision: July 10, 2017
Revised: July 11, 2017
Accepted: July 21, 2017
Article in press: July 24, 2017
Published online: November 16, 2017
Processing time: 158 Days and 12.6 Hours
Colonoscopy is a crucial diagnostic instrument for colorectal cancer screening and an adequate bowel preparation is definitely decisive for the success of the procedure. Especially in elderly patients, bowel cleansing is considered a big issue, because it is often poorly tolerated for many reasons (like inability to swallow large volume of liquids or unlikable taste); this can cause a suboptimal preparation that may lead to miss a neoplastic lesion. There is relatively little data about how to improve preparation tolerability. The purpose of our pilot study was to analyze the effect of prucalopride (Resolor®), a highly selective serotonin 5HT4 receptor agonist used for chronic constipation for its ability to stimulate gastrointestinal peristalsis, undertaken the day before colonoscopy, followed by half volume of polyethylene glycol solution. We found that this can be a good and safe method to achieve an adequate and better-tolerated colon cleansing.
Core tip: Efficacy of bowel cleansing is of crucial importance in screening colonoscopies for the prevention and early detection of colorectal cancer. Many categories of patients however cannot tolerate the large volume of liquids that make up standard bowel cleansing regimens. Aim of our pilot study was to test the efficacy of prucalopride, a highly selective 5HT4 receptor agonist that increases bowel movements, in improving bowel cleansing and reducing the necessary volume of liquids.
- Citation: Corleto VD, Antonelli G, Coluccio C, D’Alba L, di Giulio E. Efficacy of Prucalopride in bowel cleansing before colonoscopy: Results of a pilot study. World J Gastrointest Endosc 2017; 9(11): 558-560
- URL: https://www.wjgnet.com/1948-5190/full/v9/i11/558.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i11.558
Adequacy of preparation is one of the most important factors[1] in screening and early detection of colorectal cancer (CRC), which still has a high incidence and mortality. Poor colon cleansing however still affects as many as 20% of colonoscopies, increasing burden for patients and total costs of colon cancer screening programs[1,2]. Patient tolerability is strongly affected by the chosen preparation and manner in which it is administered. Many factors have been identified to influence bowel preparation such as unappealing taste of the solution or inability to swallow large volumes of liquids. There have been many efforts to improve bowel cleansing like smaller volume solutions, tablets consumed with water and split-dose regimens[3,4].
Prucalopride, a highly selective serotonin 5HT4 receptor agonist used for treatment of chronic constipation, stimulates gastrointestinal peristalsis and colon movements[5,6]. It is a generally well tolerated drug, contraindicated only in patients on dialysis or with bowel perforation or obstruction. The most common side effects are fatigue, appetite loss, diarrhea and headache at first dose administration[5,6]. In the present pilot study we tested the hypothesis that a previous dose of Prucalopride followed by a low volume of polyethylene glycol (PEG) solution, might achieve a satisfactory colon cleansing.
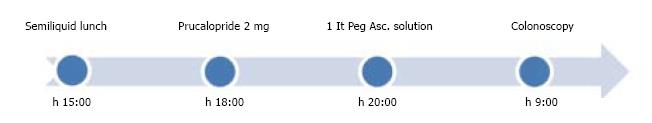
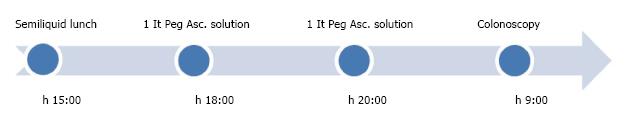
A total of 30 consecutive patients, 16F, 14M, mean age 55 years (48-62) (complete characteristics available in Table 1), all with regular bowel movements, after written informed consent, agreed to use the following preparation schedule: on the day before the examination, 3 h after a semi-liquid midday meal, 2 mg of Prucalopride, and later in the evening, 1 L of PEG-Asc. solution followed by the assumption of water or other clear liquids (> 1 L) (Figure 1). A control group of 30 patients with comparable characteristics followed the standard 2 L PEG-ASC. Preparation schedule (Figure 2). All patients underwent colonoscopy either for CRC screening or for periodical survey. All had four days of low fiber diet and all examinations were performed the following morning. The colonoscopies were performed by senior endoscopists who were unaware of the preparation schedule at the time of the examination. Twelve out of 14 patients of the Prucalopride group, who were undergoing colonoscopy for follow up of previous examinations, declared that the new preparation schedule was more acceptable compared to the standard one. Specifically, none of them reported nausea and/or retching during assumption of the PEG-ASC.
| Standard preparation (2 L PEG-ASC) (n = 30) | Prucalopride + 1 L PEG-ASC (n = 30) | |
| Age median (range) | 53 (46-67) | 55 (48-64) |
| Sex | 14 (47) | 14 (47) |
| BMI median (range) | 26.7 (18.4-32.8) | 25.4 (17.3-31) |
| Boston scale ≥ 7 | 26 (87) | 25 (83) |
| Boston scale ≤ 6 | 4 (13) | 5 (17) |
| Exam indication | ||
| Screening | 18 (60) | 16 (53) |
| Follow up | 12 (40) | 14 (47) |
| Adenoma detection rate (%) | 32 | 29 |
| Time to preparation (h) median (range) | 11.30 (10.45-12.30) | 11.45 (10.30-12.45) |
| Colonoscopy insertion time (min), median (range) | 8.2 (3.3-36) | 7.6 (3.1-47) |
Colonoscopy was completed (caecal intubation) in all patients studied. Insertion time is reported in Table 1. The colon cleansing was rated good/optimal (Boston scale 7-9) in 26/30 (87%) and in 25/30 (83%) in study and controls group respectively. The adenoma detection rate (ADR) among the two groups was comparable (Table 1). Among patients receiving Prucalopride, two patients had mild headache in the following three hours after its administration. Among patients receiving standard dose, two patients reported nausea and one patient reported mild abdominal pain during assumption.
Our pilot study shows that previous Prucalopride administration followed by half dose PEG solution produced comparable colon cleansing quality than regular standard dose. Faster intestinal transit with intestinal residuals removal stimulated by previous Prucalopride administration might explain why a reduced volume of preparation solution could achieve a satisfactory bowel cleansing. These results must be further investigated by a wider, prospective, randomized control trial that can confirm these preliminary findings and facilitate colon cleansing for those patients that are unable to drink large volumes of liquid.
| 1. | Prakash SR, Verma S, McGowan J, Smith BE, Shroff A, Gibson GH, Cheng M, Lowe Ii D, Gopal K, Mohanty SR. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700. [PubMed] |
| 2. | Hagège H, Laugier R, Nahon S, Coulom P, Isnard-Bagnis C, Albert-Marty A. Real-life conditions of use of sodium phosphate tablets for colon cleansing before colonoscopy. Endosc Int Open. 2015;3:E346-E353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015;149:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 4. | Rex DK. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol. 2014;12:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Bassotti G, Gambaccini D, Bellini M. Prucalopride succinate for the treatment of constipation: an update. Expert Rev Gastroenterol Hepatol. 2016;10:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Quigley EM, Neshatian L. Advancing treatment options for chronic idiopathic constipation. Expert Opin Pharmacother. 2016;17:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cavalcoli F, Chiba H, Marzano C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ