Published online Sep 16, 2012. doi: 10.4253/wjge.v4.i9.421
Revised: August 1, 2012
Accepted: September 12, 2012
Published online: September 16, 2012
AIM: To investigate whether flexible spectral color enhancement (FICE) improves diagnostic yields of capsule endoscopy (CE) for obscure gastro-intestinal bleeding (OGIB).
METHODS: The study subjects consisted of 81 patients. Using FICE, there were three different sets with different wavelengths. Using randomly selected sets of FICE, images of CE were evaluated again by two individuals who were not shown the conventional CE reports and findings. The difference between FICE and conventional imaging was examined.
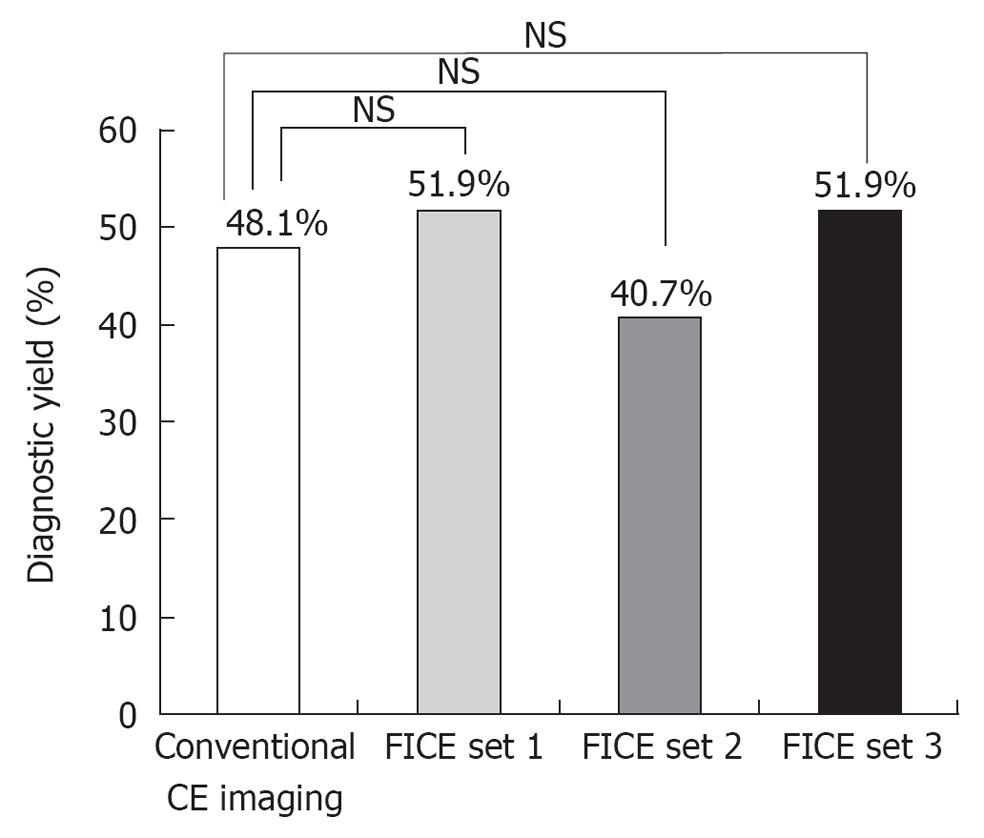
RESULTS: The overall diagnostic yields in FICE sets 1, 2, 3 and conventional imaging (48.1%) were 51.9%, 40.7%, 51.9% and 48.1%, respectively, which showed no statistical difference compared to conventional imaging. The total numbers of detected lesions per examination in FICE imaging and conventional imaging were 2.5 ± 2.1 and 1.8 ± 1.7, respectively, which showed a significant difference (P = 0.01).
CONCLUSION: The diagnostic yield for OGIB is not improved by FICE. However, FICE can detect significantly more small bowel lesions compared to conventional imaging.
- Citation: Matsumura T, Arai M, Sato T, Nakagawa T, Maruoka D, Tsuboi M, Hata S, Arai E, Katsuno T, Imazeki F, Yokosuka O. Efficacy of computed image modification of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc 2012; 4(9): 421-428
- URL: https://www.wjgnet.com/1948-5190/full/v4/i9/421.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i9.421
Obscure gastrointestinal bleeding (OGIB) is defined as recurrent or persistent bleeding with negative esophagogastroduodenoscopy (EGD), ileocolonoscopy and small bowel radiography[1]. Capsule endoscopy (CE) is the investigation of choice in OGIB, with a high diagnostic yield compared to other modalities. In a previous study, the diagnostic yield of CE in OGIB was reported to range from 30% to 80%[2-9], a higher value than that obtained by push enteroscopy[10,11], small bowel radiography[12] and computed tomography (CT)[13]. Based on these findings, CE has been recognized as the examination of choice for patients with OGIB after negative EGD and colonoscopy. However, there are some cases in which the bleeding lesion cannot be determined.
Computed virtual chromoendoscopy, both flexible spectral color enhancement (FICE) and narrow-band imaging (NBI), recently was introduced into gastrointestinal endoscopy, with the expectation that it would replace dye staining for heightening contrast and highlighting lesions. Over the past years, a multitude of reports have shown that modified imaging with FICE and NBI at high-resolution endoscopy improves detection of lesions in the upper gastrointestinal tract and enhances differentiation between neoplastic and non-neoplastic tissue[14-18]. Very recently, FICE software was implemented within the workstation of a video capsule system. However, it has rarely been investigated whether the FICE system can improve the diagnostic yields of CE for OGIB.
The aim of this study was to assess whether FICE can improve diagnostic yields better than conventional CE imaging in the examination of OGIB.
The study subjects consisted of 81 patients who underwent CE in Chiba University Hospital (Japan) for OGIB between September 2008 and June 2010. These patients had recently undergone at least two endoscopic examinations on the upper gastrointestinal tract and at least one colonoscopy, which showed negative findings. This study was reviewed and approved by the institutional review board of Chiba University School of Medicine. Informed consent was obtained from all patients.
CE was performed with Pillcam SB or Pillcam SB2 (Given Imaging, Yoqneam, Israel). CE studies were performed according to our unit’s protocol, which includes an overnight fast and bowel preparation (magnesium citrate 34 g). During a period from May 2010 until June 2010, patients were also administered 1 L of polyethylene glycol-electrolyte. A prokinetic agent (metoclopramide 10 mg) was added for patients with longstanding diabetes mellitus or a known slow transit was added.
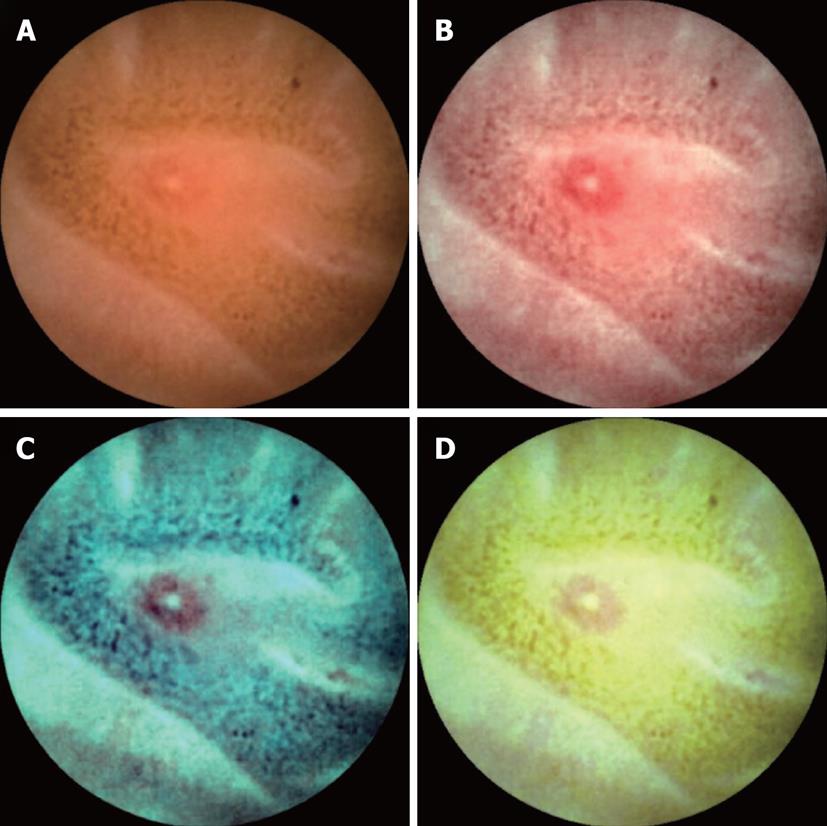
FICE is a spectral estimation technology based on arithmetical processing of ordinary images. Application of FICE for CE does not require any re-engineering of the capsule device, only integration of FICE software in the computer workstation. The wavelength spectrum used for creation of optical images is influenced by several factors: the spectrum of the light source, the optical device and the spectral sensitivity of the sensing element. However, these factors differ between flexible endoscopy and CE; therefore different FICE estimation algorithms with different estimation coefficients are required to optimize imaging. The spectral specifications (wavelengths) of the FICE settings that are useful in CE are as follows: Set 1: red 595 nm, green 540 nm, blue 535 nm; Set 2: red 420 nm, green 520 nm, blue 530 nm; and Set 3: red 595 nm, green 570 nm, blue 415 nm (Figure 1). With integration of the FICE digital processing system into the RAPID 6.0 workstation (Given Imaging), it enables a switchover between a conventional imaging and a FICE imaging immediately by a simple push of a button at the workstation. The three different settings make it possible to select the most suitable wavelengths required for evaluation of the capsule video.
The results of CE were evaluated separately by two endoscopists with experience of CE with and without the FICE system. If discrepancies occurred, the findings were reviewed simultaneously by both examiners and a consensus was reached. Two endoscopists were not shown the conventional CE reports and findings. Each set of FICE images was used randomly, on condition that the positive studies of conventional imaging were assigned equally. The differences between FICE imaging and conventional imaging of CE were examined to assess the clinical utility of FICE for diagnosing the cause of OGIB.
The outcome of CE was determined according to the definition reported by Macdonald et al[19] with a slight modification. CE findings were classified according to standard practice as highly relevant lesions (P2) or less-relevant lesions (P1, P0). An abnormal finding was classified as a P2 lesion when it was considered to be the cause of or an explanation for OGIB, such as angiodysplasia, Dieulafoy’s lesion, varix, arteriovenous malformation, tumor, polyp, ulceration, multiple (> 3) erosion, diverticulum or the presence of blood and/or blood clots in the lumen of the small bowel. When a definite abnormality was identified but was not thought to be the cause of or explanation for blood loss, it was assigned a P1 status. Minor mucosal changes or abnormalities that were not diagnostic were also categorized as P1 lesions and non-specific mucosal changes including isolated red spots, mucosal breaks and visible submucosal veins were regarded as of P1 status. A finding that definitely explained clinical symptoms was regarded as a “positive finding”. In this study, examinations that demonstrated one or more P2 lesions were recorded as positive findings, whereas those with only P1 lesions or no abnormality (P0) were negative. Diagnostic yield was established and the difference between FICE imaging and conventional imaging of CE were examined. Additionally, total numbers of detected lesions per CE examination were counted. If more than ten lesions were detected per examination, it was regarded as ten.
The baseline data are presented as mean ± SD. The differences in the values of clinical parameters between the three sets were analyzed by the χ2 test. The difference of the diagnostic yields was analyzed by the McNemar test. The difference of the numbers of lesions was analyzed by the Wilcoxon test. All analyses were performed with the statistical program SPSS 16.0 (SPSS Inc., Chicago, IL, United States); a P value of less than 0.05 was considered statistically significant.
The clinical characteristics of patients with OGIB are shown in Table 1. The patient group comprised 44 males and 37 females with ages ranging from 17 to 89 years (mean ± SD; 63.5 years ± 16.5 years). There was no statistical difference among each set of FICE imaging in the clinical characteristics.
| Conventional CE imaging (n = 81) | FICE set 1 (n = 27) | FICE set 2 (n = 27) | FICE set 3 (n = 27) | |
| Sex (male/female) | 44/37 | 14/13 | 12/15 | 11/18 |
| Age (yr, mean ± SD) | 63.5 ± 16.5 | 67.4 ± 13.1 | 60.3 ± 20.6 | 62.9 ± 14.7 |
| Type of OGIB | ||||
| Overt OGIB | 57 | 18 | 18 | 21 |
| Occult OGIB | 24 | 9 | 9 | 6 |
| Medication used | ||||
| Anti-coagulant | 13 | 6 | 3 | 4 |
| Anti-platelet drugs | 21 | 9 | 4 | 8 |
| NSAIDs (excluding low-dose aspirin ) | 14 | 3 | 4 | 7 |
| Comorbidity | ||||
| Liver cirrhosis | 5 | 1 | 2 | 2 |
| Chronic renal failure | 4 | 1 | 2 | 1 |
| Heart disease | 7 | 2 | 2 | 3 |
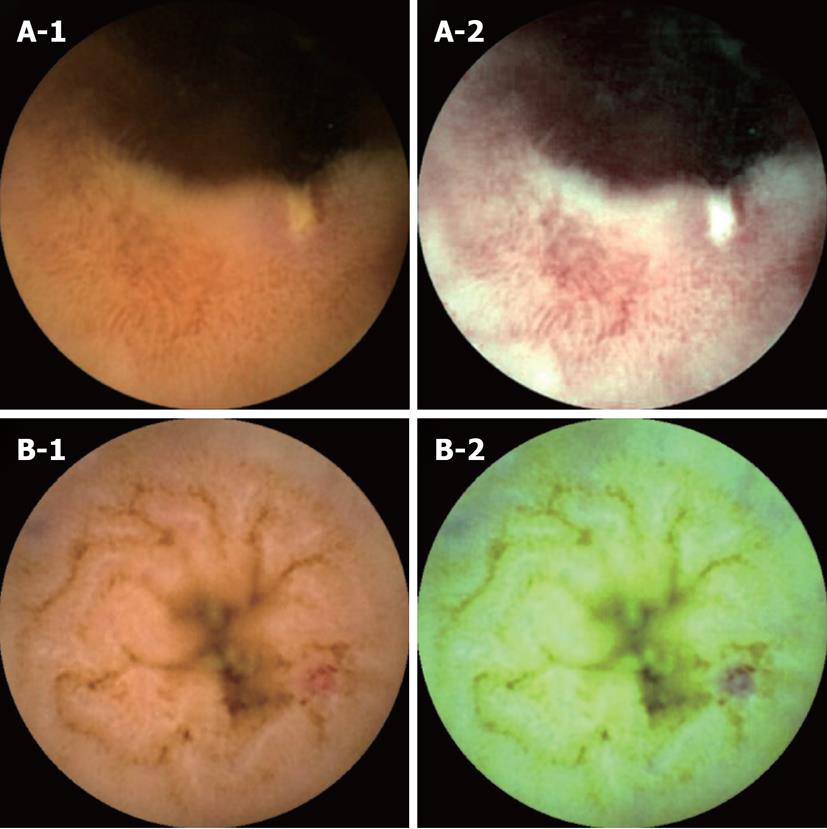
Positives of FICE imaging and the negatives of conventional CE imaging: There were two cases that FICE detected small bowel lesions that were missed with conventional CE imaging (Figure 2). In the first case (Figure 2A-1 and A-2), an ulcer was missed with conventional CE imaging and only detected with FICE imaging (set 1). With FICE imaging, it became easier to observe tissue characterization on surface parts compared with the conventional CE image. In the second case (Figure 2B-1 and B-2), an angioectasia was missed with conventional CE imaging and only detected with FICE imaging (set 3). With FICE imaging, angioectasia was clearly visualized when compared with conventional CE imaging.
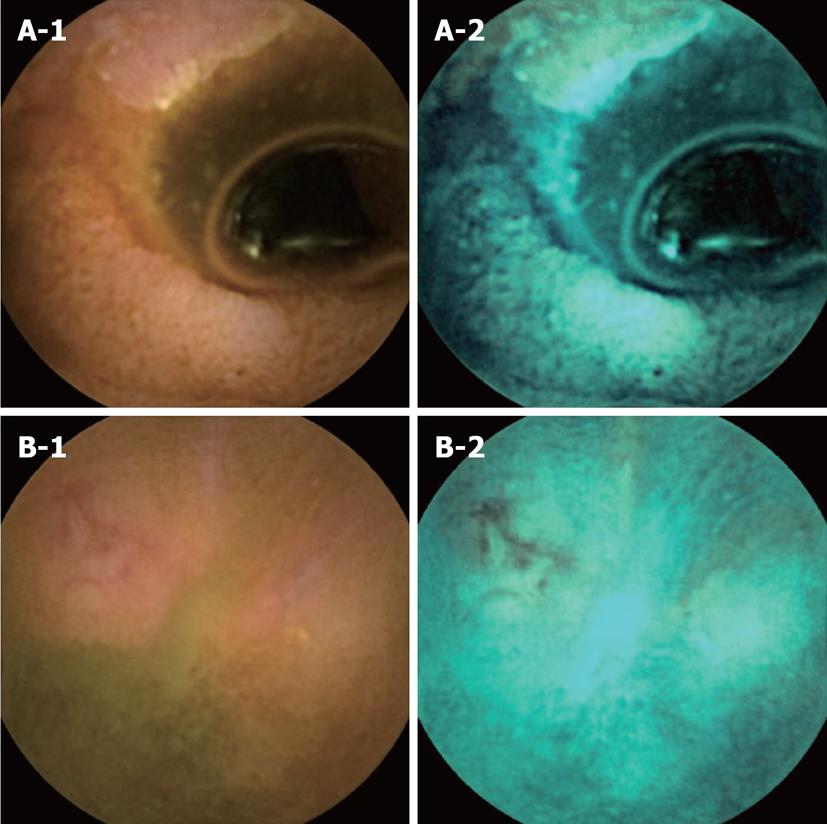
Negatives of FICE imaging and the positives of conventional CE imaging: There were two cases that FICE could not detect small bowel lesions that were detected with conventional CE imaging (Figure 3). In the first case (Figure 3A-1 and A-2), an annular ulcer was missed with FICE imaging (set 2) and only detected with conventional CE imaging. In the second case (Figure 3B-1 and B-2), an ulcer was missed with FICE imaging (set 2) and only detected with conventional CE imaging.
The total numbers of detected lesions per CE examination in FICE imaging and the conventional CE imaging were 2.5 ± 2.1 and 1.8 ± 1.7, respectively, which showed a significant difference (Wilcoxon test, P = 0.01, Table 2 ). Ulceration and erosion were defined as mucosal lesions. Angiodysplasia, Dieulafoy’s lesion, varix and arteriovenous malformation were defined as vascular lesions. Total numbers of detected lesions according to the type of lesions are shown in Table 2. The number of mucosal lesions differed significantly when comparing FICE imaging to conventional CE imaging (Wilcoxon test, P = 0.03). The total numbers of detected lesions according to the each set of FICE imaging are shown in Table 3. FICE sets 1 and 2 detected a lot more lesions but it was not a statistical difference (P = 0.068 and 0.069, respectively). FICE set 3 did not detect more compared to the conventional CE imaging (P = 0.35). The total numbers of mucosal lesions per CE examination according to the each set of FICE imaging are shown in Table 4. FICE set 1 detected more lesions but it was not a statistical difference (P = 0.08). The total numbers of vascular lesions per CE examination according to the each set of FICE imaging are shown in Table 5. There were no significant differences between each set using the FICE system.
When studying small bowel tumors, we detected tumors in seven patients. In four of these patients, a double balloon enteroscopy was performed after CE. The final diagnoses were ileal adenocarcinoma, ileal inflammatory polyp, malignant lymphoma and follicular lymphoma in one, respectively. Another three patients did not undergo additional examination because it had been thought that the polyp was not a cause of bleeding or any malignant tumors. In these seven patients, all of the same tumors were detected by both conventional CE imaging and the FICE imaging. There were no significant differences between each set using the FICE system.
The overall diagnostic yields of CE in FICE sets 1, 2, 3 and the conventional CE imaging were 51.9%, 40.7%, 51.9% and 48.1%, respectively (Figure 4), which showed no statistical difference compared to conventional imaging (P = 0.5, 0.23 and 0.5, respectively, McNemar test). There were no significant differences between each set using the FICE system in terms of diagnostic yields (χ2 test).
CE is used widely for detecting the cause of OGIB. However, there are some cases without an obvious bleeding lesion. In previous studies, some factors have been reported to be associated with diagnostic yield of CE in patients with OGIB; for example, the type of bleeding[2,4,10,11], the timing of performing CE[20-23], patient age[21], an obvious decrease in hemoglobin (Hb) value[24], the use of low-dose aspirin[9] and having another potential source of bleeding[25]. In CE, there are many reports about the diagnostic yield of OGIB. However, it is uncertain how much of a substantial miss rate exists and whether computed virtual chromoendoscopy can improve the diagnostic yield for OGIB. Recently, FICE software was implemented within the workstation of a video capsule system and there are some reports about the clinical usefulness of FICE in CE[26-28]. Imagawa et al[27] reported that FICE improves the visibility of angioectasia, erosion/ulceration and tumors in the small intestine and improves detectability of small bowel lesions[28]. In contrast, Gupta et al[29] reported that FICE was not better than conventional CE imaging for diagnosing and characterizing significant lesions on CE for OGIB. It was the first report of the diagnostic yields in CE for OGIB. However, they used only FICE set 1. In this study, we used FICE set 1, 2 and 3 and investigated the clinical utility of FICE, by dividing them by lesion type. In this study, the FICE system detected significantly more small bowel lesions when compared to the conventional CE imaging like the previous reports. However, diagnostic yields for OGIB were not improved by a computed virtual chromoendoscopy with the FICE system. It showed similar findings to the report from Gupta et al[29]. That is, even if the FICE system detected many lesions in the small bowel, it was uncertain whether these lesions coincided with the cause of OGIB. Therefore, the improvement of detectability did not always improve diagnostic yields. In addition, because two expert endoscopists with high ability in detection evaluated CE in this study, the FICE system might not be able to increase the diagnostic yields more. We need further analysis to clarify whether FICE can reduce the missing microlesions and improve diagnostic yield in beginners. In addition, this FICE technique may be possible to make the interpretation time short. Now in FICE, there were three different FICE sets in the Rapid 6.0 workstation and three different settings that make it possible to select the most suitable wavelengths required for evaluation of the capsule video. However, there are many situations of the capsule video per patient. For example, these situations include when there is or is not internal bleeding in the small bowel, food debris and bile acid. Therefore, it is troublesome to change settings to match these situations. So we investigated which set of wavelengths in FICE was the most suitable for determining the cause of OGIB. In this study, FICE sets 1 and 2 detected more lesions compared to the conventional CE imaging. However, there were no significant differences in terms of diagnostic yield. There were two cases that FICE set 2 could not detect ulcers which were detected with conventional CE imaging. This data might be caused by bad preparation and strong halation of the lesion from using the FICE system. However, it is uncertain whether these results could be avoided if we selected the most suitable wavelengths required for evaluation of the capsule video in these situations.
In conclusion, in CE, diagnostic yields for OGIB are not improved by computed virtual chromoendoscopy with the FICE system compared to conventional CE imaging. However, the FICE system can detect significantly more small bowel lesions when compared to conventional CE imaging.
Computed virtual chromoendoscopy with the flexible spectral color enhancement (FICE) system has been reported to improve visualization of the lesions in the gastrointestinal tract. However, it has rarely been investigated whether the FICE system can improve the diagnostic yields of capsule endoscopy (CE) for obscure gastro-intestinal bleeding (OGIB).
A recent study demonstrated that the FICE system improved visibility of small bowel lesions compared to conventional CE imaging. Thus, the authors thought that it was important to validate that FICE practically improves the diagnostic yield of small bowel lesions.
Diagnostic yields for OGIB are not improved by the FICE system in comparison with the conventional CE imaging in this study. However, the FICE system can detect significantly more small bowel lesions when compared to conventional CE imaging.
By understanding that FICE can detect significantly more small bowel lesions, this technique may make the interpretation time short and reduce the missing microlesions.
OGIB is defined as recurrent or persistent digestive bleeding of unknown origin that persists or recurs after a negative endoscopy work-up. CE is a non-invasive method allowing a complete investigation of the small bowel. FICE is a new chromoendoscopic tool that has been designed for enhancing visibility of lesions compared to conventional imaging.
This paper shows an influence of computed image modification for the patients with obscure GI bleeding in CE. The data are well analyzed and it could be informative and contribute to readers in the field of diagnostic endoscopy.
| 1. | Zuckerman GR, Prakash C, Askin MP, Lewis BS. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201-221. [PubMed] |
| 2. | Scapa E, Jacob H, Lewkowicz S, Migdal M, Gat D, Gluckhovski A, Gutmann N, Fireman Z. Initial experience of wireless-capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol. 2002;97:2776-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc. 2002;56:349-353. [PubMed] |
| 4. | Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 606] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Rastogi A, Schoen RE, Slivka A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest Endosc. 2004;60:959-964. [PubMed] |
| 7. | Viazis N, Papaxoinis K, Theodoropoulos I, Sgouros S, Vlachogiannakos J, Pipis P, Markoglou C, Avgerinos A. Impact of capsule endoscopy in obscure small-bowel bleeding: defining strict diagnostic criteria for a favorable outcome. Gastrointest Endosc. 2005;62:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Möller K, Jakobs R, Reitzig P. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005;61:826-832. [PubMed] |
| 9. | Matsumura T, Arai M, Sazuka S, Saito M, Takahashi Y, Maruoka D, Suzuki T, Nakagawa T, Sato T, Katsuno T. Negative capsule endoscopy for obscure gastrointestinal bleeding is closely associated with the use of low-dose aspirin. Scand J Gastroenterol. 2011;46:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 410] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 519] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Voderholzer WA, Ortner M, Rogalla P, Beinhölzl J, Lochs H. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Endoscopy. 2003;35:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | East JE, Tan EK, Bergman JJ, Saunders BP, Tekkis PP. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther. 2008;28:854-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kwon R, Mamula P, Rodriguez B, Shah RJ. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett's esophagus. Endoscopy. 2007;39:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Inoue M, Miyake Y, Odaka T, Sato T, Watanabe Y, Sakama A, Zenbutsu S, Yokosuka O. Objective evaluation of visibility in virtual chromoendoscopy for esophageal squamous carcinoma using a color difference formula. J Biomed Opt. 2010;15:056019. [PubMed] |
| 19. | Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Bresci G, Parisi G, Bertoni M, Tumino E, Capria A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: usefulness of early use. J Gastroenterol. 2005;40:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. [PubMed] |
| 23. | Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Estévez E, González-Conde B, Vázquez-Iglesias JL, de Los Angeles Vázquez-Millán M, Pértega S, Alonso PA, Clofent J, Santos E, Ulla JL, Sánchez E. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006;18:881-888. [PubMed] |
| 25. | Redondo-Cerezo E, Pérez-Vigara G, Pérez-Sola A, Gómez-Ruiz CJ, Chicano MV, Sánchez-Manjavacas N, Morillas J, Pérez-García JI, García-Cano J. Diagnostic yield and impact of capsule endoscopy on management of patients with gastrointestinal bleeding of obscure origin. Dig Dis Sci. 2007;52:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Pohl J, Aschmoneit I, Schuhmann S, Ell C. Computed image modification for enhancement of small-bowel surface structures at video capsule endoscopy. Endoscopy. 2010;42:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved visibility of lesions of the small intestine via capsule endoscopy with computed virtual chromoendoscopy. Gastrointest Endosc. 2011;73:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 28. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol. 2011;46:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 29. | Gupta T, Ibrahim M, Deviere J, Van Gossum A. Evaluation of Fujinon intelligent chromo endoscopy-assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol. 2011;17:4590-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
Peer reviewer: Naoki Muguruma, MD, PhD, Department of Gastroenterology and Oncology, The University of Tokushima Graduate School, 3-18-15, Kuramoto-cho, Tokushima 770-8503, Japan
S- Editor Song XX L- Editor Roemmele A E- Editor Zheng XM