Published online Sep 16, 2012. doi: 10.4253/wjge.v4.i9.414
Revised: June 4, 2012
Accepted: September 12, 2012
Published online: September 16, 2012
AIM: To evaluate the diagnostic accuracy of confocal laser endomicroscopy (CLE) for the detection of dysplasia in long-standing ulcerative colitis (UC).
METHODS: We prospectively performed a surveillance colonoscopy in 51 patients affected by long-standing UC. Also, in the presence of macroscopic areas with suspected dysplasia, both targeted contrasted indigo carmine endoscopic assessment and probe-based CLE were performed. Colic mucosal biopsies and histology, utilised as the gold standard, were assessed randomly and on visible lesions, in accordance with current guidelines.
RESULTS: Fourteen of the 51 patients (27%) showed macroscopic mucosal alterations with the suspected presence of dysplasia, needing chromoendoscopic and CLE evaluation. In 5 macroscopically suspected cases, the presence of dysplasia was confirmed by histology (3 flat dysplasia; 2 DALMs). No dysplasia/cancer was found on any of the outstanding random biopsies. The diagnostic accuracy of CLE for the detection of dysplasia compared to standard histology was sensitivity 100%, specificity 90%, positive predictive value 83% and negative predictive value 100%.
CONCLUSION: CLE is an accurate tool for the detection of dysplasia in long-standing UC and shows optimal values of sensitivity and negative predictivity. The scheduled combined application of chromoendoscopy and CLE could maximize the endoscopic diagnostic accuracy for diagnosis of dysplasia in UC patients, thus limiting the need for biopsies.
- Citation: Rispo A, Castiglione F, Staibano S, Esposito D, Maione F, Siano M, Salvatori F, Masone S, Persico M, De Palma GD. Diagnostic accuracy of confocal laser endomicroscopy in diagnosing dysplasia in patients affected by long-standing ulcerative colitis. World J Gastrointest Endosc 2012; 4(9): 414-420
- URL: https://www.wjgnet.com/1948-5190/full/v4/i9/414.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i9.414
The risk of colorectal cancer (CRC) is increased in patients affected by inflammatory bowel disease (IBD), even if the exact magnitude of the risk is difficult to quantify because of several biases and methodological shortcomings[1,2]. According to the well-known meta-analysis by Eaden et al[3], the overall prevalence of CRC in patients with ulcerative colitis (UC) is approximately 3.7% and 5.4% for those with pancolitis, with a pooled estimate of cumulative CRC incidence of 2% at 10 years, 8% at 20 years, and 18% after 30 years of disease[3,4].
On the basis of these assumptions, patients affected by long-standing UC (and colic Crohn’s disease) are candidates for surveillance colonoscopy/histology and this issue has been clearly underlined in a variety of clinical and endoscopic practice guidelines[5-7].
In particular, UC patients should undergo a screening colonoscopy 8 years after the onset of symptoms and every other year after that (every 5 years for distal colitis)[6]. At present, the main goal of the surveillance program remains the recognition of flat dysplasia, usually detected microscopically in random biopsies from unremarkable mucosa. It has been calculated that when random biopsies are performed, 33 biopsy specimens are needed in order to exclude dysplasia with a sensitivity of 90%[8,9]. However, this diagnostic approach is considered time-consuming and of doubtful utility in terms of cost-efficacy[10].
More recently, chromoendoscopy has successfully been applied in this diagnostic field. Indeed, since the first study by Kiesslich et al[11], many reports have shown a clear diagnostic gain using the dye spray enhancement with indigo carmine (and methylene blue), with a 3 to 5-fold increased probability of detection of dysplasia in this context[11-14]. In accordance with these results, the European Crohn’s and Colitis Organisation (ECCO) guidelines define chromoendoscopy as an alternative procedure to random biopsies for appropriately trained endoscopists due to its superiority in the detection rate of neoplastic lesions[6].
In the last 5 years, confocal laser endomicroscopy (CLE) has been widely used for the diagnosis of superficial and early colorectal neoplasia, in view of its high agreement with the histopathology[15-17]. In addition, a small number of studies have recently suggested a possible role for this procedure in the diagnostic work-up of IBD[18,19]. In particular, a pilot study by Kiesslich et al[20] has shown the excellent diagnostic accuracy of CLE (sensitivity 95%; specificity 98%) in detecting dysplasia/neoplasia in UC patients.
On the basis of these considerations, we aimed to evaluate the diagnostic accuracy of CLE for the diagnosis of dysplasia in a group of patients affected by long-standing UC.
From March 2009 to March 2011, we prospectively performed a surveillance colonoscopy in patients affected by long-standing UC who were consecutively examined at our IBD Unit. The indication for the endoscopic examination was based on the ECCO guidelines[6]. Also, in the presence of macroscopic areas suspected for dysplasia (on flat mucosa or mass), both targeted contrasted indigo carmine endoscopic assessment and probe-based CLE (pCLE) were performed. Colic mucosal biopsies and histology, utilised as the gold standard, were carried out randomly, as well as on visible lesions.
Consecutive patients with clinically inactive, longstanding UC (minimum duration 8 years) were recruited from the outpatient clinic of our IBD Unit. Potential participants were identified using the previously reported inclusion and exclusion criteria[20]. In addition, the presence of diffuse pseudo-polyps was added as an exclusion criterion.
The potential participants were thus identified, their primary care physicians were invited to participate in the study and informed consent was obtained from all participants. The study was approved by the local ethical committee (prot.653/08).
CLE was performed using the Cellvizio® Endomicroscopy System (Mauna Kea Technologies, Paris, France) using a Coloflex UHD-type probe (1 μm lateral resolution; 12 frames/s).
This system uses a 2.5mm catheter probe (Coloflex UHD-type probe) that is inserted through the endoscope working channel to obtain dynamic imaging of the mucosa. This probe has a field of view of 240 μm × 200 μm, with a lateral resolution of 1 μm. pCLE imaging data were collected at a scan rate of 12 frames/s with a scanning field of 30 000 pixels. Single video frames were reconstructed into a single larger static image (4 mm × 2 mm) by a special computer software (‘‘mosaicing’’ Mauna Kea Technologies). Five-ten millilitres of 10% sodium fluorescein were injected intravenously as a contrasting agent before CLE image acquisition.
An experienced endoscopist, who had performed over 100 CLE procedures before index patient recruitment, performed all examinations. Twenty-four hours prior to the procedure, participating patients underwent colon preparation with 4 L of hypertonic polyethylene glycol solution. Conscious sedation with midazolam (5-10 mg iv) was administered at the patient’s request. Lesions and suspected areas were identified using white-light endoscopy (colonscope Olympus CF-Q145I), followed by targeted chromoendoscopic enhancement with indigo carmine 0.1% according to the SURFACE guidelines[21]. The mucosal areas with the suspected presence of dysplasia were studied by CLE. After localization of each lesion/area, a 10-20 mg intravenous bolus of Buscopan (hyoscine-N-butyl-bromide) was administered in order to limit peristaltic artifacts; 10 mL of 10% sodium fluorescein were also administered for CLE image acquisition. CLE image acquisition was performed by placing the tip of the probe in direct contact with the target tissue site using an endoscopic cap to stabilize the mucosa. CLE images of each observed lesion were stored digitally in specific folders in a database. CLE images were defined diagnostic for neoplastic tissue in presence of “dark” cells, with mucin depletion and goblet cell/crypt density attenuation, with irregular architectural pattern and epithelial thickness, villiform structures and “dark” epithelial border[15].
Endoscopically resected lesions and/or target biopsy specimens were evaluated by an experienced pathologist (SS) in a blinded fashion and graded in accordance with the Vienna modified classification of gastrointestinal epithelial neoplasia[22].
The diagnostic accuracy of CLE for the prediction of dysplasia when compared to standard histology was assessed by using Stats Direct statistical software.
By the end of the study, 55 patients affected by longstanding UC had been enrolled. Four patients were excluded from the investigation due to the presence of diffuse pseudo-polyposis and therefore the final analysis comprised of 51 patients (Table 1).
| Variable | UC |
| Number | 51 |
| Age (yr) | 52 (24-66) |
| Gender (M/F) | 28/23 |
| Extension (E1-E2-E3) | 0-23-28 |
| Length of disease (yr) | 18 (10-29) |
| Primary sclerosing cholangitis | 1 |
| Drugs | |
| 5-ASA | 37 |
| Immunosuppressors | 9 |
| Anti-TNF | 2 |
Fourteen of the 51 patients (27%) showed macroscopic mucosal alterations with the suspected presence of dysplasia, requiring targeted chromoendoscopic and CLE evaluation.
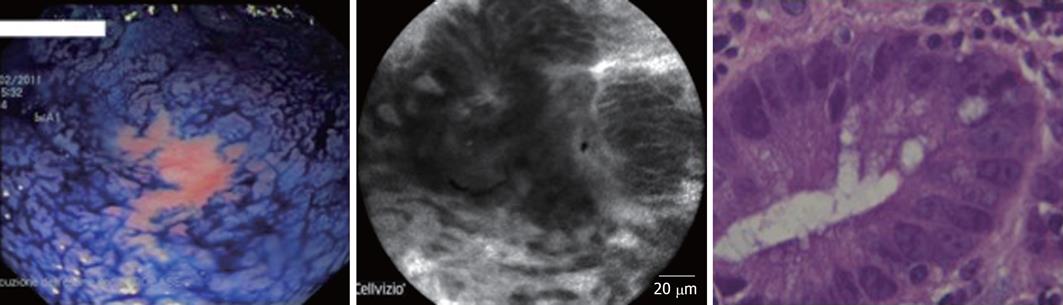
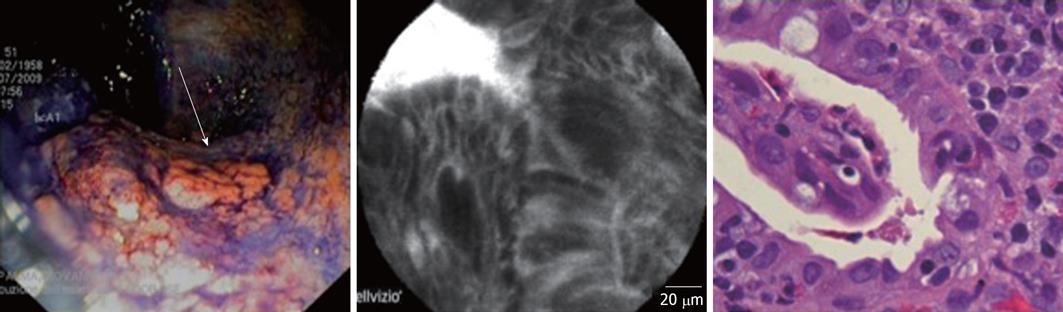
In 5 of these 14 macroscopically suspected cases (35%), the presence of dysplasia was confirmed by both histology and CLE examination. In 3 cases, the diagnosis of flat dysplasia (1 low-grade; 2 high-grade) was made (Figure 1), while the other 2 cases were diagnosed as dysplasia associated with lesion/mass (DALM) (Figure 2). All the cases of dysplasia were confirmed at subsequent surgery.
All five cases with dysplasia had an UC lasting at least 10 years. All patients were in maintenance treatment with 5-ASA derivates. Four of these 5 patients had a history of steroid-dependency. In the dysplastic group, 3 UC patients were affected by an extensive colitis, while the remaining two subjects suffered from a distal colitis. The two DALMs were both located at the sigmoid colon, while the other 3 cases of dysplasia were sited at the ascending colon, transverse colon and sigmoid colon, respectively.
When compared with standard histology, the targeted chromoendoscopy/CLE combination enabled the detection of dysplasia in 5 patients (true positive: 5; false positive: 1) and the ruling out of neoplastic complications in 9 subjects (true negative: 9; false negative: 0).
Finally, the diagnostic accuracy of CLE for the detection of dysplasia compared to standard histology was sensitivity 100%, specificity 90%, positive predictive value 83% and negative predictive value 100% (Table 2).
| % | 95% CI | |
| Sensitivity | 100 | 70-100 |
| Specificity | 90 | 65-98 |
| Positive predictive value | 83 | 43-96 |
| Negative predictive value | 100 | 70-100 |
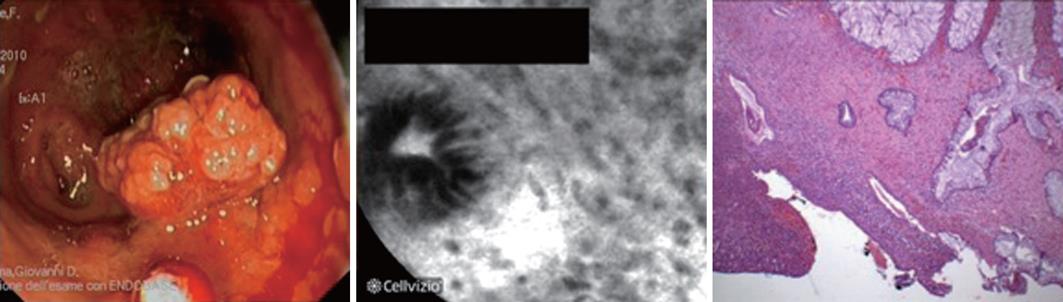
In three cases, we detected the presence of an isolated and irregular polypoid lesion which we then proceeded to resect (performing multiple biopsies around the base of the polyp). In one of these three cases, the diagnosis of DALM was made, while the other two cases were diagnosed as inflammatory pseudo-polyps. In all these circumstances, the CLE evaluation correctly predicted the histological outcome that followed (Figures 2 and 3).
With regards to the routine histological sampling, no dysplasia/cancer was found on any of the outstanding random biopsies.
Patients affected by long-standing UC have high relative risk of CRC and are candidates for endoscopic surveillance[1-4]. In the present study, the combined use of chromoendoscopy and pCLE led to the diagnosis of dysplasia in 5 of 51 patients affected by UC (3 cases of flat dysplasia, 2 of DALMs). The high percentage of dysplasia/cancer (9%) in our population is probably related to the presence of important risk factors for neoplastic complication (all cases of dysplasia in pancolitis; mean UC duration: 18 years; 1 case in PSC; UC patients afferent to a third-level IBD Unit). At present, the main aim of this surveillance scheme is the early diagnosis of dysplasia, which in many cases represents the indication for proctocolectomy[6]. However, the endoscopic and histological surveillance of UC patients is difficult, time-consuming and is considered of doubtful clinical significance[23] due to its inadequate profile of cost-efficacy. Hence, there is a need for more accurate and practical approaches.
One of the most important diagnostic goals in the management of patients with UC, especially of those who present risk factors for cancer development, should be the “real-time” endoscopic identification and diagnosis of dysplasia/neoplasia, as this would reduce the number of unnecessary biopsies with their associated time and costs. In view of this, recent studies on the use of dye spray chromoendoscopy have underlined the efficacy of this procedure in diagnosing dysplasia in UC patients, with a 4-5 fold diagnostic gain when compared with the standard procedure[24]. Furthermore, Kiesslich et al[20] have shown for the first time that the diagnosis of dysplasia/neoplasia in UC could be maximized by using both chromoendoscopy and CLE, with high values of diagnostic accuracy (sensitivity 94%, specificity 98%). This result has been recently confirmed, although with less remarkable diagnostic values, by van den Broek et al[25], who reported a diagnostic accuracy of 81% when comparing CLE with narrow-band imaging (NBI) plus high-definition endoscopy (HDE) (diagnostic accuracy 92%).
Our study mainly focused on the combined application of chromoendoscopy and CLE, confirming the high diagnostic potential of these procedures (sensitivity and negative predictive values of 100%). The striking diagnostic performance of pCLE in our hands compared to that observed in other studies is probably related to the experience of our first operator. Indeed, as shown in previous papers, the operator’s endoscopic expertise and learning curve represent the crucial issues and main limitation for the routine application of this endoscopic technique[15-17]. However, a recent report has highlighted that the ability to accurately interpret CLE images for predicting neoplastic lesions can be learned rapidly by a range of GI specialists[26]; similarly, the ability to acquire high-quality CLE images can also be learned quickly[26].
Some studies have investigated the utility of using NBI in endoscopic follow-up of UC. The majority of these reports have shown conflicting outcomes, most likely as the result of the confounding effect of baseline inflammation[27]. On the basis of this evidence, we decided to exclude NBI evaluation from our protocol.
The introduction of magnified HDE, and therefore the possibility to accurately analyze the “pit-pattern” of the colic glands, has significantly improved the diagnostic and prognostic accuracy of endoscopy in the study of sporadic polyps and colic neoplasms. However, data about the use of this method in the context of IBD are still lacking and it is not clear whether the pit-pattern evaluation will prove to be of the same significance in the presence of diffuse mucosal inflammation. In this field of research, a recent study has highlighted a possible role for this procedure, showing that HDE was highly accurate in the diagnosis of dysplasia in cases of UC (sensitivity 100%, specificity 82%)[25]. However, we decided not to routinely perform the high definition endoscopic examination (with magnification) in our patients in order to avoid introducing further diagnostic variables and therefore to simplify, as far as possible, the data on the combined use of CLE and chromoendoscopy. Nevertheless, one of the most significant aims of future studies should be the evaluation of the diagnostic efficacy of CLE in comparison with HDE with magnification, with a view to accurately define the value of new endoscopic technologies in this field of research.
Our results show that chromoscopy-guided pCLE is a procedure that could enable a rapid diagnosis of dysplasia in patients with long-standing UC, combining the advantages of both the above mentioned techniques. In our hands, CLE showed sensitivity and negative predictive values of 100%, with high specificity (90%). In particular, the combined use of the two procedures led to the diagnosis of dysplasia in 5 of 51 patients affected by UC (3 cases of flat dysplasia, 2 of DALMs), all confirmed by histology and subsequent surgery (proctocolectomy). In the future, the remarkable negative predictive value of this technique might enable us to avoid performing unnecessary biopsies and endoscopic resections in cases of CLE-negative suspected lesions/areas. We found 1 false positive case of dysplasia in the presence of high background inflammation; this issue should always be considered when performing CLE evaluation. In addition, this diagnostic approach proved effective in predicting histology after endoscopic resection of polypoid lesions. In all three cases of polypectomy, the pCLE evaluation (of the polyp and the surrounding mucosa) clearly predicted the diagnosis (1 case of DALM, 2 of inflammatory pseudo-polyps; Figures 2 and 3). In view of this result, chromoendoscopy/CLE evaluation could probably be used to better differentiate “adenoma-like mass” (ALM) from DALM lesions, confirming our previously reported experience in in vivo characterization of DALM in UC[28].
Our study presents some limitations. Firstly, the number of patients with the final diagnosis of dysplasia is quite small. However, this is a “real life” study and reflects the number of UC patients with dysplasia well that a third-level IBD Unit can diagnose during a 2 years period; so this shows the usefulness of such a procedure, even in every day clinical practice. According to the small number of patients with dysplasia, the 95% confidence intervals of the sensitivity, specificity and predictive values of CLE are likely wide. Furthermore, our study was mainly aimed at defining the diagnostic accuracy of using chromoendoscopy/CLE in the context of UC and did not focus on issues of feasibility; hence, several technical variables which have already been investigated in depth by other authors (e.g., time of endoscopic/chromoendoscopic procedure; total time of CLE imaging required to produce a video; proportion of total imaging time in which crypts/vessels were visible on the CLE images; and CLE video quality) were not fully recorded. However, about these concerns, our results would be not significantly different from those previously reported by other groups with well-known expertise[20,26]. Another critical issue in the present study is the small number of cases of patients with “low-grade dysplasia” in our UC population. Undoubtedly, this is an important issue if we aim to establish useful criteria for the endoscopic/histological surveillance of these patients. In effect, in the presence of this type of dysplastic lesion, the overall diagnostic accuracy of CLE could be less remarkable, even if in the Kiesslich’s experience this type of dysplastic lesions did not influence the diagnostic outcome of CLE[20,29]. However, starting from these considerations, our future aim will be a multicenter study able to significantly increase the number of this kind of lesions.
In conclusion, in view of its remarkable values of sensitivity and negative predictivity, confocal fluorescence microscopy could prove an accurate tool for the detection of dysplasia in cases of long-standing UC. The scheduled combined use of chromoendoscopy and CLE could maximize the endoscopic diagnostic accuracy for the diagnosis of dysplasia in UC patients. Further studies examining a wider population are needed to confirm our suggestion.
Patients affected by long-standing ulcerative colitis (UC) need a surveillance colonoscopy in view of the increased risk of colon cancer. Previous studies on confocal laser endomicroscopy (CLE) have shown that detection of dysplasia is significantly increased in sporadic colon cancer with good agreement with standard histology. However, data about the use of CLE in detecting dysplasia in UC are still scarce.
This study provides further results in favor of the use of high-tech endoscopy in detecting dysplasia in patients affected by UC.
The results of this study show that chromoscopy-guided probe-based confocal laser endomicroscopy (pCLE) is a procedure that could enable a rapid diagnosis of dysplasia in patients with long-standing UC. In our hands, CLE showed sensitivity and negative predictive values of 100%, with high specificity (90%). In the future, the remarkable negative predictive value of this technique might enable us to avoid performing unnecessary biopsies and endoscopic resections in cases of CLE-negative suspected lesions/areas.
Chromoscopy-guided pCLE can be utilized as an accurate tool for defining the presence of dysplasia in patients affected by UC.
Chromoscopy-guided pCLE refers to CLE performed at the level of suspected areas after targeted application of colorant (indigo carmine) during the endoscopic procedure.
This study evaluated the diagnostic accuracy of CLE for the detection of dysplasia in long-standing UC. The paper is of interest to readers of the journal and the comments are satisfactorily.
| 1. | Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Intestinal and extra-intestinal cancer in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther. 2004;19:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2130] [Article Influence: 85.2] [Reference Citation Analysis (2)] |
| 4. | Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1063] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 6. | Biancone L, Michetti P, Travis S, Escher JC, Moser G, Forbes A, Hoffmann JC, Dignass A, Gionchetti P, Jantschek G. European evidence-based Consensus on the management of ulcerative colitis: Special situations. J Crohns Colitis. 2008;2:63-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-74, 774.e1-4; quiz e12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 345] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 8. | Broström O, Löfberg R, Ost A, Reichard H. Cancer surveillance of patients with longstanding ulcerative colitis: a clinical, endoscopical, and histological study. Gut. 1986;27:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 10. | Harewood GC. Economic comparison of current endoscopic practices: Barrett's surveillance vs. ulcerative colitis surveillance vs. biopsy for sprue vs. biopsy for microscopic colitis. Dig Dis Sci. 2004;49:1808-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 559] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 12. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 353] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Obrador A, Ginard D, Barranco L. Review article: colorectal cancer surveillance in ulcerative colitis - what should we be doing. Aliment Pharmacol Ther. 2006;24 Suppl 3:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hurlstone DP, Sanders DS, McAlindon ME, Thomson M, Cross SS. High-magnification chromoscopic colonoscopy in ulcerative colitis: a valid tool for in vivo optical biopsy and assessment of disease extent. Endoscopy. 2006;38:1213-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | De Palma GD, Staibano S, Siciliano S, Persico M, Masone S, Maione F, Siano M, Mascolo M, Esposito D, Salvatori F. In vivo characterisation of superficial colorectal neoplastic lesions with high-resolution probe-based confocal laser endomicroscopy in combination with video-mosaicing: a feasibility study to enhance routine endoscopy. Dig Liver Dis. 2010;42:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | De Palma GD. Confocal laser endomicroscopy in the "in vivo" histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770-5775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Buchner AM, Shahid MW, Heckman MG, Krishna M, Ghabril M, Hasan M, Crook JE, Gomez V, Raimondo M, Woodward T. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Liu JJ, Wong K, Thiesen AL, Mah SJ, Dieleman LA, Claggett B, Saltzman JR, Fedorak RN. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Liu JJ, Madsen KL, Boulanger P, Dieleman LA, Meddings J, Fedorak RN. Mind the gaps: confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J Clin Gastroenterol. 2011;45:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 20. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 21. | Rutter M, Bernstein C, Matsumoto T, Kiesslich R, Neurath M. Endoscopic appearance of dysplasia in ulcerative colitis and the role of staining. Endoscopy. 2004;36:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1587] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 23. | Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;CD000279. [PubMed] |
| 24. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | van den Broek FJ, van Es JA, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Fockens P, Dekker E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011;43:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Buchner AM, Gomez V, Heckman MG, Shahid MW, Achem S, Gill KR, Jamil LH, Kahaleh M, Lo SK, Picco M. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc. 2011;73:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | De Palma GD, Staibano S, Siciliano S, Maione F, Siano M, Esposito D, Persico G. In-vivo characterization of DALM in ulcerative colitis with high-resolution probe-based confocal laser endomicroscopy. World J Gastroenterol. 2011;17:677-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 29. | Watanabe T. Efficacy of probe-based confocal laser endomicroscopy for surveillance in ulcerative colitis endomicroscopy for ulcerative colitis surveillance. Endoscopy. 2011;43:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Peer reviewers: Tony Chiew Keong Tham, MD, Consultant Gastroenterologist, Ulster Hospital, Dundonald, Belfast BT16 1RH, Northern Ireland, United Kingdom; Takayuki Yamamoto, MD, PhD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8, Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Song XX L- Editor Roemmele A E- Editor Zheng XM