Published online Feb 16, 2026. doi: 10.4253/wjge.v18.i2.114779
Revised: October 31, 2025
Accepted: December 10, 2025
Published online: February 16, 2026
Processing time: 128 Days and 22.4 Hours
Eosinophilic esophagitis (EoE) is a chronic, antigen-mediated inflammatory condition of the esophagus, characterized by signs of esophageal dysfunction and characteristic endoscopic and histological features. Currently, EoE has developed from a rare disease entity to a prevalent condition with a rising incidence and pre
Core Tip: Eosinophilic esophagitis is being increasingly diagnosed in children. However, clear guidelines to diagnose and manage these cases are limited. We presented a concise update of the guidelines to simplify management protocols, thus improving patient outcomes.
- Citation: Samanta A, Singh V, Chakraborty B, Ray G. Eosinophilic esophagitis in children: Clinical perspectives and evolving therapeutic strategies. World J Gastrointest Endosc 2026; 18(2): 114779
- URL: https://www.wjgnet.com/1948-5190/full/v18/i2/114779.htm
- DOI: https://dx.doi.org/10.4253/wjge.v18.i2.114779
Eosinophilic esophagitis (EoE) is a chronic, antigen-mediated inflammatory disease of the esophagus, characterized by eosinophil-predominant inflammation and symptoms of esophageal dysmotility. It was first described in 1978 but has been increasingly diagnosed over the past three decades[1]. EoE causes significant impairment of quality of life (QoL) and poses a substantial burden on healthcare resources. An increasing number of studies have highlighted mucosal inflammatory patterns, newer therapeutic targets, and the natural history[2-4]. Two Food and Drug Administration (FDA)-approved medications are available so far for EoE, necessitating further research. The advent of omics technologies, including genomics, transcriptomics, proteomics, etc, provides the latest insights into the genetic and immunological mechanisms. In this review, we discussed novel diagnostic and treatment strategies and future directions in monitoring EoE in children.
A literature search was performed in PubMed and Google Scholar for the period between January 1, 2003 and August 31, 2025. The objective criteria for inclusion were: (1) English language; (2) Peer reviewed; and (3) PubMed cited studies on children. Key concepts were EoE, therapy, diagnosis, epidemiology, randomized clinical trials, and reviews. Keywords associated with each concept were examined and combined.
EoE has changed from a rare disease to a common cause of esophageal dysmotility[3]. Following gastroesophageal reflux disease (GERD), it is the second most frequent cause of reflux in children. Furthermore, it now accounts for the majority of cases of impaction of food bolus in older children in the Western population[3,4]. A meta-analysis of population-based studies from North America, Europe, and Australia reported an overall incidence of 3.7/100000 per year [95% confidence interval (CI): 1.7-6.5), which was higher in adults (7/100000 per year; 95%CI: 1.0-18.3) than in children (5/100000 per year; 95%CI: 1.5-10.9)[4]. The prevalence also appeared to be higher in adults than in children (43.4, 95%CI: 22.5-71.2 vs 29.5, 95%CI: 17.5-44.7, respectively) and in the American population compared with the European population[4]. More re
Several recent studies from developing countries have reported an increase in the incidence and prevalence of EoE in children, similar to allergic diseases (atopic dermatitis, allergic rhinitis, etc)[6,7]. The latest study from India found a prevalence of 3.5% in children who underwent elective upper gastrointestinal endoscopy (UGIE)[7]. The European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 2024 guideline has highlighted that cli
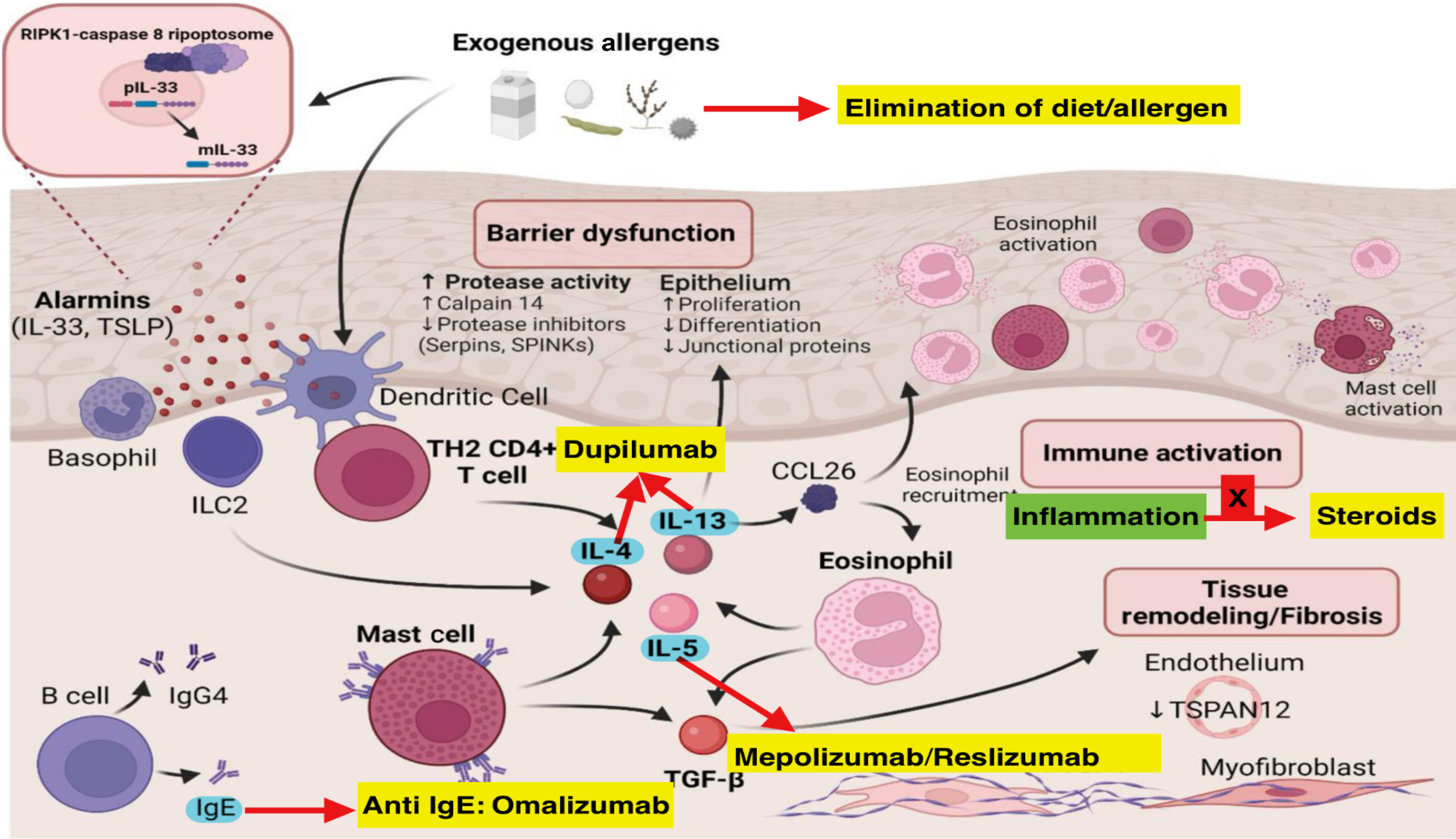
EoE is caused by a complex interplay between genetic predisposition, environmental triggers, and immune dysregulation[8,9]. Figure 1 depicts the interaction between genetic susceptibility, environmental stimulants, and immune dysregulation in the pathogenesis. Esophageal epithelial barrier dysfunction allows food allergens to induce thymic stromal lymphopoietin (TSLP)/interleukin (IL)-33, leading to stimulation of type 2 helper T (Th2) cells, natural killer cells, mast cells, and basophils[10,11]. These cells stimulate IL-4, which induces Th2 differentiation. IL-4 and IL-13 induce chemokine (C-C motif) ligand 26 (CCL26), which further stimulates eosinophils to secrete IL-5. IL-5, secreted by Th2 cells and mast cells, also stimulates eosinophils. Mast cells also induce transforming growth factor-beta 1, which stimulates eosinophils and fibroblasts responsible for fibrosis and tissue remodeling[11].
Recent studies have identified the role of additional cytokines in the pathogenesis of EoE[12-14]. Collison et al[12] in an interesting study found that tumor necrosis factor-related, apoptosis-inducing ligand controlled midline-1 and TSLP expression, inflammation, and fibrosis in experimental models. de Souza et al[13] in another study found macrophage migration inhibitory factor as an inducing agent for eosinophils in a murine model. Dutt et al[14] established that allergen-induced IL-18 promoted IL-5-and natural killer-T-dependent EoE pathology.
With fast advances in technologies, such as genomics, epigenetics, transcriptomics, etc, new insights into the genetic and immunologic mechanisms are being unfolded[11,15-18]. Six studies have identified genetic loci associated with EoE, proving the genetic causality[10,11,16-19]. A genome-wide association study by Rothenberg et al[20] involving 181 European children with EoE and 170 healthy controls found an important genetic association with chromosome 5q22 encoding TSLP.
Two meta-analyses and four genome-wide association studies identified 41 variant loci and 27 suggestive loci[20-25]. A whole exome sequencing study by Rochman et al[26] involving 33 unrelated patients with EoE identified 39 rare mu
Epigenetics is an emerging field in genetic research, and studies have identified changes in DNA methylation that have an effect on gene expression[27,28]. DNA methylation of 20 children with and without EoE identified the 25 methylated CpG loci as biomarkers to differentiate active disease from controls[29]. These loci helped in diagnostics but maintained distinct profiles from healthy controls. The findings of this study shed light on their potential as a useful diagnostic and monitoring tool.
Our understanding of EoE pathogenesis evolved when gene expression analysis of esophageal tissues noticed a unique transcriptome profile[30-32] that distinguishes active EoE from inactive EoE and healthy controls[33-36]. In-depth transcriptomic study have unveiled a unique gene expression profile in EoE, associated with a type 2 immunity response and the epithelial barrier. CCL26, the eosinophil chemoattractant, has been found to be the uniquely upregulated gene among the EoE transcriptome, establishing a clear association with eosinophil levels in esophageal biopsies[37].
The EoE transcriptome shows differential expression of various genes linked to mast cells (CPA3 and TPSAB1), emphasizing the significant role of mast cells in the EoE inflammatory response[30,38]. Genes such as filaggrin and involucrin, which are crucial for epithelial differentiation and barrier function, also exhibit unique expression patterns in EoE[37]. Interestingly, certain transcriptomic changes persist despite EoE remission, such as periostin (POSTN), which is involved in fibrosis and tissue remodeling[39,40]. Upregulation of POSTN in esophageal fibroblasts causes fibrosis[41]. The high levels of POSTN promote eosinophil adhesion, collagen production, and fibrosis, leading to tissue remodeling.
Higher levels of CCL26 and tryptase in pretreatment esophageal biopsies have been linked with better response to steroid therapy. On the contrary, upregulated IL-5 and IL-13 pathways promote profibrotic cascade and are linked with steroid resistance and more fibrostenosis.
Proteomic studies have helped in further understanding EoE pathogenesis, identifying potential biomarkers and therapeutic targets. The minichromosome maintenance complex, seen in proliferative epithelial cells, had higher expression in the esophagus of EoE[42]. Another proteomic research study by Kaymak et al[43] identified elevated levels of IL-20 subfamily cytokines in patients with active EoE. This study further suggested that these cytokines should be studied as novel therapeutic targets[43]. Another study by Hsieh et al[44] identified thrombospondin-1 as a pathogenic mediator of EoE fibrosis.
The clinical features of EoE vary depending on the age of the child[45-47]. Infants and young children usually have nonspecific symptoms such as vomiting, refusal to feed, and failure to thrive, making the diagnosis difficult. This is the reason a higher index of suspicion is required in such cases. Older children and adolescents can have dysphagia, chest pain, heartburn, regurgitation, and food bolus impaction as a more fibrostenotic disease phenotype is seen in them due to longer disease duration.
In a study of 43 children with EoE (86% male, mean age 8.4 years), Rodrigues et al[48] found that the most common symptoms were nausea, vomiting, and abdominal pain (100%) in children younger than 7 years while heartburn (52%) and food impaction (48%) were the most common symptoms in children older than 7 years. The prevalence of EoE among UGIE performed in children with food bolus impaction and/or dysphagia was high (63%-88%)[49-51]. Therefore, the index of suspicion should be high, and these patients should undergo UGIE and esophageal biopsy. When symptoms are refractory to medical treatments for GERD, EoE should be strongly considered.
Making a diagnosis of EoE is difficult because of variable clinical presentation and less awareness about endoscopic features and taking appropriate biopsies. Furthermore, esophageal eosinophilia can be found in several other diseases, leading to a diagnostic dilemmas[3,46].
The diagnostic criteria of EoE have undergone several changes in the past two decades with a better understanding of the disease pathophysiology and emerging evidence. In the 2014 guidelines a PPI trial was an essential criterion before making a diagnosis of EoE[52]. The 2018 consensus rewrote the diagnostic criteria and removed the PPI trial from it[2]. The latest internationally accepted diagnostic criteria is: The presence of ≥ 15 eosinophils/high power field (hpf) (× 400 magnification) in either half of the esophagus with the absence of eosinophils in the gastric and duodenal mucosa[2]. Following suit, the ESPGHAN 2024 guideline adopted the latest internationally accepted definition[3]. It is imperative to note that at least six biopsies from ≥ 2 esophageal levels, including proximal and distal as well as additional gastric and duodenal biopsies, should be taken for definitive diagnosis. The lack of gastric/duodenal eosinophilia is supportive of EoE; however, the presence of gastric/duodenal eosinophilia is not sufficient to rule out EoE when the clinical context fits. According to the latest guidelines, patients without clinical symptoms of esophageal dysfunction cannot be diagnosed as EoE even in the presence of esophageal eosinophilia[2].
EoE is an antigen-driven, eosinophil-predominant disorder with Th2 cytokine profile. The majority of patients with EoE (43%-70%)[3,7] may have a family history of allergic disease[53-57]. Peripheral eosinophilia could be seen in 8.6%-33.0% of patients with EoE[7,55,58].
Testing for allergic sensitization has been used in patients with EoE. Various methods are advocated: Skin prick test; atopy patch testing; or allergen-specific IgE levels. However, an EoE trigger has not been identified. Additionally, their positive predictive value and clinical correlation remain poor and uncertain as a non-IgE medicated inflammatory response seems to be the predominant pathway for EoE pathogenesis[52,59]. Hence, these tests are not currently recommended for the diagnosis of EoE[3,52]. Considering the limitations of applying allergy-based testing in the pe
Similarly, the EoE Histology Scoring System (EoEHSS), comprising of peak eosinophil count (PEC), basal zone hyperplasia, esophageal atresia (EA), eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis, has been newly developed and validated in the pediatric po
Both EREFS and EoEHSS correlate well with PEC and can be used for disease activity monitoring. Interobserver agreement for both is generally fair, but it varies by endoscopic/microscope features. Table 1 summarizes the differences between the 2014 and 2024 ESPGHAN guideline recommendations.
| 2024 ESPGHAN guidelines | 2014 ESPGHAN guidelines | |
| Trail of PPI as part of diagnostic criteria | PPIs no longer used as a diagnostic tool; rather as a treatment option | PPIs used to define EoE by documenting nonresponse to PPI |
| Eosinophilic involvement of other segments of the GI tract does not exclude the diagnosis of EoE | EoE may coexist with eosinophilic infiltration of other segments of the GI tract | Not addressed |
| Clinical symptom assessment tools (Pediatric EoE Symptom Score) | Advocated for assessing treatment response and monitoring | Not discussed |
| EREFS | EREFS score discussed; needs further evaluation | Subjective observations discussed |
| Biopsy protocol and reporting | Follow-up endoscopy with biopsies generally recommended 8-12 weeks after initiating or making a major change to therapy or symptom flare. Every 1-3 years in asymptomatic cases during maintenance | Not discussed in detail |
| Histology scores | Eosinophilic Esophagitis Histology Severity Score discussed; need for report format that is comparable between centers | Peak eosinophil cutoff/high power field discussed and subjective assessment of ancillary findings |
| Systemic steroids | A short course of systemic steroids may be used as treatment option for severe pediatric EoE associated strictures | Not specifically mentioned for use |
| Biologics | First biologic approved by Food and Drug Administration and for children > 1 years of age | No biologics advocated |
| Biomarkers and noninvasive techniques | Discussed in current guideline | Not discussed |
| Quality of life | Addressed in current guideline | Not discussed |
Children with successful repair of EA are known to carry higher risk of EoE[63-65]. Various reasons have been po
Both EoE and inflammatory bowel disease (IBD) share common immune dysfunction with abnormalities in helper T cells and epithelial barrier function[68]. Co-occurrence of these diseases has been reported[69,70]. Moore et al[69] reported that the prevalence of EoE among 4515 American children with IBD as approximately 1.5%. A study found EoE in 11/3090 (0.35%) Italian children with IBD[70]. More genetic studies should be performed.
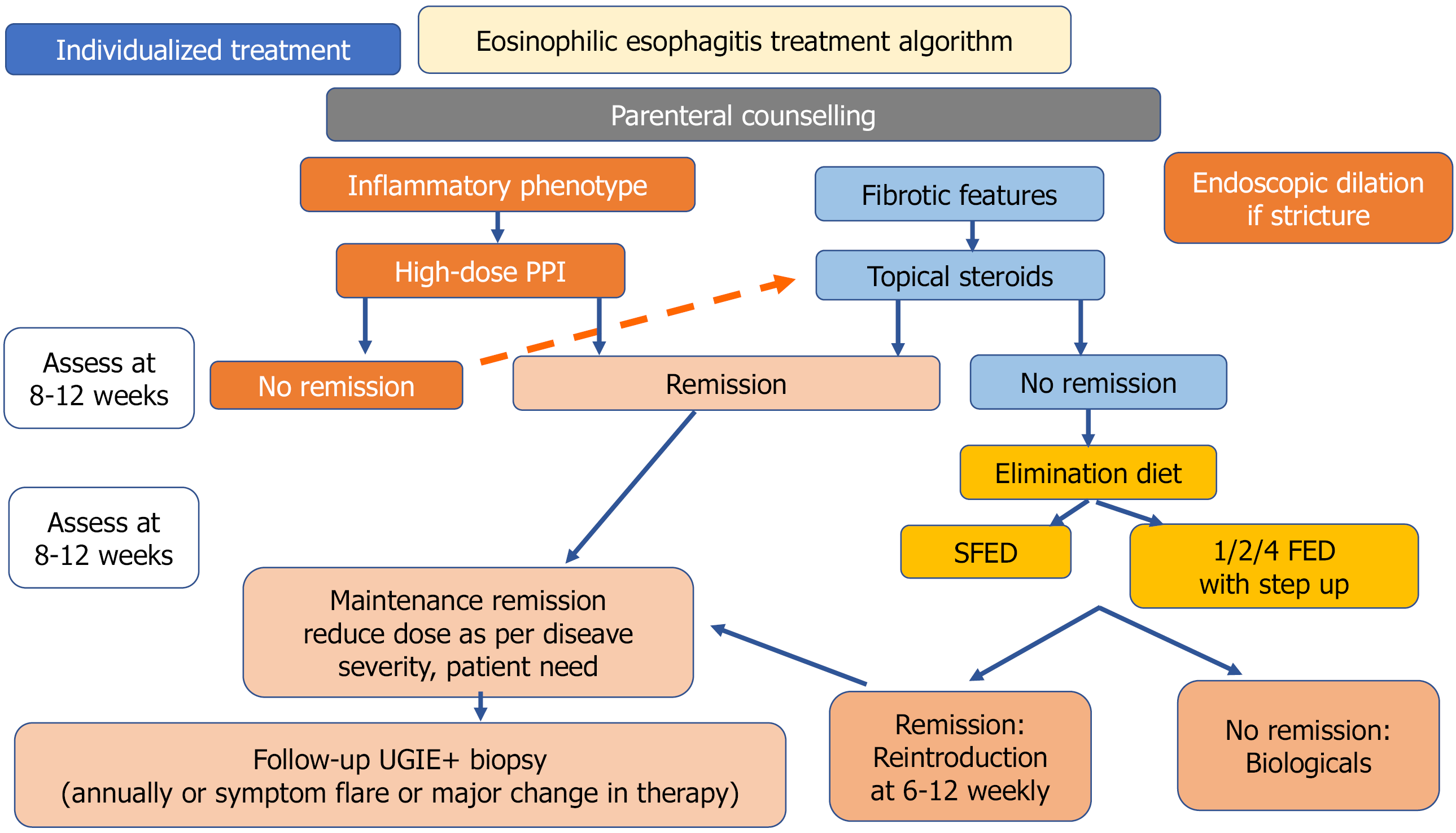
The main objectives of the management of EoE are the achievement of histological remission that may avoid long-term complications, improvement of clinical symptoms, and restoration of normal growth[3]. In the pediatric population the current EoE treatment strategies can be categorized into three ‘D’s: (1) Dietary therapy; (2) Drugs; and (3) Dilatation for strictures. There is a scarcity of randomized trials to define the best approach. Choosing the best treatment option among them depends on the disease endotype (presence of strictures, nutritional status) after discussion with the family with an emphasis on shared decision-making. Figure 2 describes a stepwise approach to deciding the initial treatment modality, monitoring, and long-term maintenance plan in patients with EoE.
Given the role of food allergens in triggering EoE, dietary elimination may provide symptom resolution, improvement in growth parameters, esophageal inflammation, and potentially esophageal subepithelial fibrosis[71]. Three different approaches for food avoidance are available: (1) An empirical elimination diet; (2) A testing-directed diet; and (3) An elemental diet. The role of a clinical nutritionist is of paramount importance in this regard.
The most commonly followed dietary approach is a six-food elimination diet (SFED) that accounts for six major food groups involved in EoE (milk, egg, soy, wheat, peanuts/tree nuts, and seafood). The efficacy of SFED ranges from 57%-81%[3,72]. However, adherence with such a restrictive dietary approach is difficult to follow, raising the question of a less-restrictive dietary protocol as the initial step. Studies have found that peanut/tree nut and seafood are rare triggers for EoE[73]. Subsequently, less restrictive dietary approaches with a four-food group elimination diet (4FED) and two-food elimination diet have been used with more favorable adherence and comparable efficacy[3,74]. A one-food elimination diet (1FED) (only milk) was tried successfully in children with a histological remission rate of 50%-65%[75,76]. More recently, Kliewer et al[77] performed a randomized controlled trial (RCT) among 63 children divided into 2 groups: (1) 1FED (n = 38); and (2) 4FED (n = 25). The histological remission rates (41% vs 44%; P = 1.00), histology scoring system (-0.25 vs -0.29; P = 0.77), endoscopic reference score (-1.10 vs -0.58; P = 0.47), QoL scores, and transcriptomic outcomes were comparable between the two groups. The authors concluded that 1FED is a reasonable first-choice the
Elimination diets suggest an overall success rate that varies between 45% and 90% depending on the different approaches. The SFED and 4FED achieve high remission rates but with high patient nonadherence because of low palatability. In addition, detrimental effects of nutritional deficiency, decreased QoL, psychological impact, and the risk of developing feeding disorders (e.g., anorexia and bulimia, especially in adolescents) needs to be considered. A detailed discussion with family members should be done to decide the initial therapeutic choices.
PPIs are now one of the biggest advances in the management of EoE[78]. Over the last few years, PPIs have evolved from being an agent for a PPI trial to the main factor of PPI-responsive esophageal eosinophilia and a definitive therapeutic option for EoE[79]. Downregulation of CCL26, IL-5, and IL-3 in biopsies was found for PPI, similar to patients receiving steroids[79]. PPIs restore the integrity of the leaky esophageal epithelium and help in reducing endoscopic features of circular rings[80]. Given the ease of administration and good safety profile, PPIs are the first-line therapy, especially among patients with milder symptoms and an inflammatory endotype. They are used at high dosages (e.g., 1-2 mg/kg, often twice daily) for their anti-inflammatory properties. The efficacy of PPIs to induce clinical and histopathological remission of EoE has been shown in multiple observational studies[80-82]. A systematic review of 33 studies found that double doses of PPI led to histopathological remission in nearly half the patients and symptomatic improvement in 60% of children at week 12, regardless of patient age, study design, or type of PPI evaluated[83]. According to the 2024 guidelines, PPIs are the first-line therapy on the same level as steroids and diet[3].
Owing to its high efficacy in patients with EoE, topical corticosteroids are often used as first-line treatment in more severe cases or in those with failed PPI therapy. The most common topical steroids are swallowed/inhaled fluticasone propionate 88-440 μg BID-QID (maximum 880-1760 μg). Various studies have shown the efficacy of topical steroids to range from 45%-82%[84-86]. Recently, oral viscous budesonide [dosage < 10 years of age: 0.5 mg BID (maximum 4 mg), > 10 years of age: 1 mg BID (maximum 4 mg)] has received FDA approval for use in patients with EoE with a histological remission rate of 54% at 6 weeks and 84% at 12 weeks[87]. In the latest ESPGHAN guidelines, the use of short-term systemic steroids (1-2 mg/kg/day; twice daily dosing) is advocated to reduce the sessions for dilation in moderate to severe strictures[3]. Topical steroids can cause local adverse events such as esophageal candidiasis while adrenal insufficiency could be a side effect in those receiving long-term systemic steroids.
Given the role of different immunological pathways in the pathogenesis of EoE, various biologics have been used in patients with EoE with mixed results. Anti-IgE agents (omalizumab), anti-IL-5 agents (mepolizumab), anti-IL-13 agents (QAX576), and anti-tumor necrosis factor-alpha agents (infliximab) have been used but with no encouraging results[88-91].
Dupilumab targets the IL-4 receptor alpha subunit, inhibiting both IL-4 and IL-13 signaling. It has shown promising results in children with refractory EoE[92,93]. Chehade et al[93] in a RCT in pediatric patients with EoE showed that dupilumab resulted in histologic remission (PEC < 15/hpf, primary end point) in a significantly higher percentage of children (aged 1-11 years) with EoE than placebo at week 16 (68% with higher-exposure vs 58% with lower-exposure subcutaneous dupilumab regimen vs 3% in placebo group placebo, P < 0.001). Minor adverse events such as headache, nausea, injection site reaction, urticaria, and upper respiratory tract infections were reported. On May 20, 2022, du
Pediatric natural history studies are scarce for EoE. However, long-term studies in adult patients clearly suggest that untreated active EoE can lead to fibrostenotic complications, needing more endoscopic interventions, and poor QoL[94]. Alexander et al[94] followed up 101 patients with EoE over a median follow-up duration of 17 months and found that the overall histological relapse rate was 44%. In addition, they found that the relapse rate among those patients on high-dose and low-dose PPI maintenance therapy was 33% and 35%, respectively, while the relapse rate for those not on any maintenance was 86%. The majority of the relapses were asymptomatic. Hence, both adult and pediatric guidelines recommend periodic monitoring and maintenance therapy[3,95]. The monitoring targets are histologic remission (PEC < 15 eos/hpf), EREFS improvement, symptom resolution, and improvement of growth parameters. No consensus has been reached about the ideal medication, dosage, and duration. Low-dose PPI, topical steroid, and dupilumab are certain drugs used for this purpose[3]. Montelukast is not effective as a maintenance therapy[3].
The ESPGHAN 2024 guideline has recommended UGIE and histopathology every 1-3 years in patients with stable clinical remission[3]. Endoscopy is also advocated in cases of symptomatic flare or major treatment changes. However, many experts advocate for a personalized monitoring schedule based on the individual disease endotype and transcriptomic profile.
Endoscopy and histopathology remain the gold standard monitoring tools[3]. For endoscopic evaluation EREFS, an accurate and validated tool, should be used and each third of the esophagus should be graded separately as per the EREFS[60,61]. In addition to monitoring the PEC/hpf, EoEHSS should be used for better identification of disease activity[62]. Additionally, it can also predict the therapeutic responsiveness of individual patients[96].
The Pediatric EoE Symptom Score has been endorsed for evaluating treatment response and disease monitoring[3,97]. However, there is a poor correlation between the symptoms score and histopathological findings. The reasons could be: (1) Post-inflammatory esophageal remodeling; (2) Esophageal hypersensitivity; and (3) Avoidant eating behavior. To identify the avoidant eating behavior, the IMPACT questionnaire is usually used. The IMPACT questionnaire includes: (1) Imbibe more water with food; (2) Modify meal (cut piece, pore); (3) Prolonged meal time; (4) Avoid hard-textured food; (5) Chew excessively; and (6) Turn away tablet/pills. These adaptive behaviors correlate well with active EoE and decreased esophageal distensibility. The Pediatric EoE Symptom Score correlates poorly with tissue activity, and endoscopy with histology remains the reference standard.
Multiple endoscopies during the food reintroduction put extra financial and psychological burdens on the patient. Hence, transnasal endoscopy is being increasingly used for performing biopsy during follow-up. Transnasal endoscopy can be done with a 2.8 mm or 4.0 mm diameter bronchoscope in awake conditions without anesthesia and as an outpatient procedure, thus reducing the cost. Studies on children with EoE have shown that the tissues obtained by this technique is comparable with those generated during standard endoscopy with good histological correlation, fewer adverse events, lesser cost (approximately half), and high patient satisfaction[98-101]. There are no absolute contraindications for transnasal endoscopy [relative contraindications. Patients with nasal trauma, prior nasal surgery, and recurrent nasal bleeding can safely undergo this procedure. It is safe in children as young as 4 years[3].
Minimally/noninvasive disease-monitoring methods and biomarkers (eosinophilic cationic protein; eosinophil-derived neurotoxin, eosinophil peroxidase; major basic protein, CCL26, POSTN, mast cell tryptase) are being investigated. Cytosponge is a sponge-containing capsule that collects esophageal tissue as it is pulled back after being swallowed. It can be an easy method to assess inflammation[3,102]. Another monitoring tool is the esophageal string test that monitors eosinophil-derived granule proteins from secretions for the esophagus that stick to the string as it is removed[103,104]. However, these studies are not RCTs, had a small sample size, and used outdated diagnostic criteria for EoE. Esophageal string test and cytosponge are promising but still investigational in children. Hence, these methods need further validation studies for definitive recommendations in routine clinical practice.
Numerous biomarkers such as eosinophil peroxidase, eosinophil cationic protein, IL-10, and anti-NC1A142 (collagen XVII) IgG4 are under investigation[3]. They remain investigational, and no definitive recommendation exists for their use in diagnosis or clinical monitoring. Table 2 summarizes all the potential biomarkers and the tissues where they are tested.
| Biomarker | Tissue specimen | Clinical utility | Evidence maturity (clinical use vs investigational) | ||
| Mucosa | Blood | Feces | |||
| Eosinophil cationic protein | Yes | Yes | Yes | Disease activity monitoring, predict endoscopy severity, predict treatment response | Investigational |
| Eosinophil-derived neurotoxin | Yes | Yes | Yes | Disease activity monitoring, predict treatment response | Investigational |
| Eosinophil peroxidase | Yes | Yes | No | Disease activity monitoring, predict treatment response | Investigational |
| Major basic protein | Yes | Yes | No | Disease activity monitoring | Investigational |
| Mast cell tryptase | Yes | No | No | Disease activity monitoring | Investigational |
| Chemokine (C-C motif) ligand 26 | Yes | Yes | No | Disease activity monitoring | Investigational |
| Periostin | Yes | No | No | Disease activity monitoring | Investigational |
| Microbiome | Yes | No | No | Disease activity monitoring | Investigational |
Functional imaging, such as endoluminal functional imaging system have been used as adjuncts in adult patients with EoE to assess the esophageal distensibility[105]. Mucosal biopsy gives an idea about the epithelial inflammation, but there is little information about submucosal fibrosis that is responsible for distensibility and stricture formation. The pediatric literature is limited in the form of case series[106]. Mucosal impedance is a promising, rapid, and less invasive method to monitor EoE activity[107]. However, these methods need larger studies with more data about its clinical utility.
In resource-limited settings, caregivers need to take into consideration the nutritional access, endoscopy capacity, family counselling, and shared decision making before deciding therapeutic choices and monitoring schedules.
The field of EoE is ever changing with new innovations. Table 3 summarizes all the practice changers that happened from 2023 to 2025. Newer medications (CRTH2 antagonist, lirentelimab) are being tested as a potential treatment for EoE. The KRYPTOS trial found that lirentelimab (siglec-8 blockers) leads to significant eosinophil reduction in esophageal biopsy[108]. The long-term complications can be responsible for poor QoL, necessitating it to make psychosocial support an integral part of patient care[3]. Also, transcriptome analysis has revolutionized the management of EoE, identifying characteristic molecular signatures. Challenges in integrating diverse omics data persist. Machine learning is emerging as a valuable tool. The integration of multi-omics data promises significant advancements in our understanding of EoE by identifying biomarkers for diagnosis, predicting therapeutic responses, and monitoring natural history. Finally, in the era of artificial intelligence, EoE provides a concrete pediatric use-case that could benefit from the artificial intelligence capabilities such as endoscopic image scoring, histology quantification, treatment response prediction, and the methodological and translational toolkit needed to operationalize the EoE recommendations.
| EoE: Practice changers in 2023-2025 |
| Removal of mandatory proton pump inhibitor trial |
| Routine use of endoscopic reference score/EoE histologic scoring system |
| Pragmatic 1-food elimination as a first step |
| Food and Drug Administration approval of dupilumab for refractory cases |
| Transnasal endoscopy for monitoring endoscopies during maintenance |
EoE is no longer a rare disease. It is riddled with considerable diagnostic and therapeutic challenges. Ongoing research has led to deeper understanding of the disease process and newer therapeutic targets. Dupilumab holds promise as a treatment modality. It is of the utmost importance to evaluate the least/noninvasive diagnostic tool for the assessment of disease activity and the optimal treatment regimen to develop the latest treatment options.
| 1. | Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 293] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154:319-332.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 609] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 3. | Amil-Dias J, Oliva S, Papadopoulou A, Thomson M, Gutiérrez-Junquera C, Kalach N, Orel R, Auth MK, Nijenhuis-Hendriks D, Strisciuglio C, Bauraind O, Chong S, Ortega GD, Férnandez SF, Furman M, Garcia-Puig R, Gottrand F, Homan M, Huysentruyt K, Kostovski A, Otte S, Rea F, Roma E, Romano C, Tzivinikos C, Urbonas V, Velde SV, Zangen T, Zevit N. Diagnosis and management of eosinophilic esophagitis in children: An update from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2024;79:394-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 4. | Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Navarro P, Arias Á, Arias-González L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 6. | Kinoshita Y, Ishimura N, Oshima N, Ishihara S. Systematic review: Eosinophilic esophagitis in Asian countries. World J Gastroenterol. 2015;21:8433-8440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Samanta A, Poddar U, Kumari N, Sen Sarma M, Srivastava A, Mishra P. Eosinophilic esophagitis in children: A cross-sectional study from a tertiary care center. JGH Open. 2024;8:e13024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Ryu S, Lee KH, Tizaoui K, Terrazzino S, Cargnin S, Effenberger M, Shin JI, Kronbichler A. Pathogenesis of Eosinophilic Esophagitis: A Comprehensive Review of the Genetic and Molecular Aspects. Int J Mol Sci. 2020;21:7253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Clayton F, Peterson K. Eosinophilic Esophagitis: Pathophysiology and Definition. Gastrointest Endosc Clin N Am. 2018;28:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Vinit C, Dieme A, Courbage S, Dehaine C, Dufeu CM, Jacquemot S, Lajus M, Montigny L, Payen E, Yang DD, Dupont C. Eosinophilic esophagitis: Pathophysiology, diagnosis, and management. Arch Pediatr. 2019;26:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Davis BP. Pathophysiology of Eosinophilic Esophagitis. Clin Rev Allergy Immunol. 2018;55:19-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Collison AM, Sokulsky LA, Sherrill JD, Nightingale S, Hatchwell L, Talley NJ, Walker MM, Rothenberg ME, Mattes J. TNF-related apoptosis-inducing ligand (TRAIL) regulates midline-1, thymic stromal lymphopoietin, inflammation, and remodeling in experimental eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | de Souza HS, Tortori CA, Lintomen L, Figueiredo RT, Bernardazzi C, Leng L, Bucala R, Madi K, Buongusto F, Elia CC, Castelo-Branco MT, Bozza MT. Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol. 2015;8:1154-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Dutt P, Shukla JS, Ventateshaiah SU, Mariswamy SJ, Mattner J, Shukla A, Mishra A. Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice. Immunol Cell Biol. 2015;93:849-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Matsuyama K, Yamada S, Sato H, Zhan J, Shoda T. Advances in omics data for eosinophilic esophagitis: moving towards multi-omics analyses. J Gastroenterol. 2024;59:963-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | Del Giacco L, Cattaneo C. Introduction to genomics. Methods Mol Biol. 2012;823:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Sherrill JD, Rothenberg ME. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J Allergy Clin Immunol. 2011;128:23-32; quiz 33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | O'Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, Rothenberg ME. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 382] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 19. | Kottyan LC, Parameswaran S, Weirauch MT, Rothenberg ME, Martin LJ. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, Gober L, Kim C, Glessner J, Frackelton E, Thomas K, Blanchard C, Liacouras C, Verma R, Aceves S, Collins MH, Brown-Whitehorn T, Putnam PE, Franciosi JP, Chiavacci RM, Grant SF, Abonia JP, Sleiman PM, Hakonarson H. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 21. | Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, Weirauch MT, Vaughn S, Lazaro S, Rupert AM, Kohram M, Stucke EM, Kemme KA, Magnusen A, He H, Dexheimer P, Chehade M, Wood RA, Pesek RD, Vickery BP, Fleischer DM, Lindbad R, Sampson HA, Mukkada VA, Putnam PE, Abonia JP, Martin LJ, Harley JB, Rothenberg ME. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46:895-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, Bredenoord AJ, Furuta GT, Spergel JM, Hakonarson H. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Kottyan LC, Maddox A, Braxton JR, Stucke EM, Mukkada V, Putnam PE, Abonia JP, Chehade M, Wood RA, Pesek RD, Vickery BP, Furuta GT, Dawson P, Sampson HA, Martin LJ, Kelly JA, Kimberly RP, Sivils K, Gaffney PM, Kaufman K, Harley JB, Rothenberg ME. Genetic variants at the 16p13 locus confer risk for eosinophilic esophagitis. Genes Immun. 2019;20:281-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Kottyan LC, Trimarchi MP, Lu X, Caldwell JM, Maddox A, Parameswaran S, Lape M, D'Mello RJ, Bonfield M, Ballaban A, Mukkada V, Putnam PE, Abonia P, Ben-Baruch Morgenstern N, Eapen AA, Wen T, Weirauch MT, Rothenberg ME. Replication and meta-analyses nominate numerous eosinophilic esophagitis risk genes. J Allergy Clin Immunol. 2021;147:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Chang X, March M, Mentch F, Nguyen K, Glessner J, Qu H, Liu Y, Furuta G, Aceves S, Gonsalves N, Nadeau K, Cianferoni A, Spergel J, Sleiman P, Hakonarson H. A genome-wide association meta-analysis identifies new eosinophilic esophagitis loci. J Allergy Clin Immunol. 2022;149:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Rochman M, Travers J, Miracle CE, Bedard MC, Wen T, Azouz NP, Caldwell JM, Kc K, Sherrill JD, Davis BP, Rymer JK, Kaufman KM, Aronow BJ, Rothenberg ME. Profound loss of esophageal tissue differentiation in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;140:738-749.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Salma M, Andrieu-Soler C, Deleuze V, Soler E. High-throughput methods for the analysis of transcription factors and chromatin modifications: Low input, single cell and spatial genomic technologies. Blood Cells Mol Dis. 2023;101:102745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3929] [Cited by in RCA: 4757] [Article Influence: 365.9] [Reference Citation Analysis (0)] |
| 29. | Strisciuglio C, Payne F, Nayak K, Andreozzi M, Vitale A, Miele E, Zilbauer M. Disease-associated DNA methylation signatures in esophageal biopsies of children diagnosed with Eosinophilic Esophagitis. Clin Epigenetics. 2021;13:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 31. | Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, Buckmeier BK, Jameson SC, Greenberg A, Kaul A, Franciosi JP, Kushner JP, Martin LJ, Putnam PE, Abonia JP, Wells SI, Rothenberg ME. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033-4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Caldwell JM, Blanchard C, Collins MH, Putnam PE, Kaul A, Aceves SS, Bouska CA, Rothenberg ME. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol. 2010;125:879-888.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Sherrill JD, Kiran KC, Blanchard C, Stucke EM, Kemme KA, Collins MH, Abonia JP, Putnam PE, Mukkada VA, Kaul A, Kocoshis SA, Kushner JP, Plassard AJ, Karns RA, Dexheimer PJ, Aronow BJ, Rothenberg ME. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes Immun. 2014;15:361-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Shoda T, Morita H, Nomura I, Ishimura N, Ishihara S, Matsuda A, Matsumoto K, Kinoshita Y. Comparison of gene expression profiles in eosinophilic esophagitis (EoE) between Japan and Western countries. Allergol Int. 2015;64:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Menard-Katcher C, Liu C, Galbraith MD, Benson T, Burger C, Dobias D, Larsen L, O'Brien C, Spencer LA, Furuta GT, Masterson JC. Fibrostenotic eosinophilic esophagitis phenotype is defined by a proliferative gene signature. Allergy. 2023;78:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 375] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 37. | Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 682] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 38. | Kliewer KL, Murray-Petzold C, Collins MH, Abonia JP, Bolton SM, DiTommaso LA, Martin LJ, Zhang X, Mukkada VA, Putnam PE, Kellner ES, Devonshire AL, Schwartz JT, Kunnathur VA, Rosenberg CE, Lyles JL, Shoda T, Klion AD, Rothenberg ME. Benralizumab for eosinophilic gastritis: a single-site, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:803-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 39. | Ruffner MA, Shoda T, Lal M, Mrozek Z, Muir AB, Spergel JM, Dellon ES, Rothenberg ME. Persistent esophageal changes after histologic remission in eosinophilic esophagitis. J Allergy Clin Immunol. 2024;153:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Muir AB, Ackerman SJ, Pan Z, Benitez A, Burger C, Spergel JM, Furuta GT, Rothman J, Wilkins BJ, Arnold MA, Dolinsky L, Grozdanovic M, Menard-Katcher C. Esophageal remodeling in eosinophilic esophagitis: Relationships to luminal captured biomarkers of inflammation and periostin. J Allergy Clin Immunol. 2022;150:649-656.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Collins MH, Alexander ES, Martin LJ, Grotjan TM, Mukkada VA, Sheil A, Abonia JP, Putnam PE, Rothenberg ME. Acquired Esophageal Strictures in Children: Morphometric and Immunohistochemical Analyses. Pediatr Dev Pathol. 2022;25:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Rochman M, Rochman Y, Caldwell JM, Mack LE, Besse JA, Manes NP, Yoon SH, Shoda T, Nita-Lazar A, Rothenberg ME. The minichromosome maintenance complex drives esophageal basal zone hyperplasia. JCI Insight. 2023;8:e172143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 43. | Kaymak T, Kaya B, Wuggenig P, Nuciforo S, Göldi A; Swiss EoE Cohort Study Group (SEECS), Oswald F, Roux J, Noti M, Melhem H, Hruz P, Niess JH. IL-20 subfamily cytokines impair the oesophageal epithelial barrier by diminishing filaggrin in eosinophilic oesophagitis. Gut. 2023;72:821-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Hsieh LY, Chiang AWT, Duong LD, Kuo CC, Dong SX, Dohil R, Kurten R, Lewis NE, Aceves SS. A unique esophageal extracellular matrix proteome alters normal fibroblast function in severe eosinophilic esophagitis. J Allergy Clin Immunol. 2021;148:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Roh JH, Ryoo E, Tchah H. Clinical Manifestations of Eosinophilic Esophagitis in Children and Adolescents: A Single-Center, Matched Case-Control Study. Pediatr Gastroenterol Hepatol Nutr. 2020;23:319-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | De Matteis A, Pagliaro G, Corleto VD, Pacchiarotti C, Di Giulio E, Villa MP, Parisi P, Vassallo F, Ziparo C, Di Nardo G. Eosinophilic Esophagitis in Children: Clinical Findings and Diagnostic Approach. Curr Pediatr Rev. 2020;16:206-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Rodrigues M, D'Amico MF, Patiño FR, Barbieri D, Damião AO, Sipahi AM. Clinical manifestations, treatment, and outcomes of children and adolescents with eosinophilic esophagitis. J Pediatr (Rio J). 2013;89:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Hurtado CW, Furuta GT, Kramer RE. Etiology of esophageal food impactions in children. J Pediatr Gastroenterol Nutr. 2011;52:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Ettyreddy AR, Sink JR, Georg MW, Kitsko DJ, Simons JP. Association between Eosinophilic Esophagitis and Esophageal Food Impaction in the Pediatric Population. Otolaryngol Head Neck Surg. 2018;159:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Cheung KM, Oliver MR, Cameron DJ, Catto-Smith AG, Chow CW. Esophageal eosinophilia in children with dysphagia. J Pediatr Gastroenterol Nutr. 2003;37:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, Murch SH, Chong S, Gottrand F, Husby S, Lionetti P, Mearin ML, Ruemmele FM, Schäppi MG, Staiano A, Wilschanski M, Vandenplas Y; ESPGHAN Eosinophilic Esophagitis Working Group and the Gastroenterology Committee. Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr. 2014;58:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 53. | Franciosi JP, Liacouras CA. Eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:19-27, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Tan LN, Srivastava S, Teh M, Quak SH, Aw MM. Eosinophilic oesophagitis in children: an uncommon occurrence in a predominantly Chinese population in Singapore. Singapore Med J. 2017;58:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Saeed A, Assiri AM, Al Asmi M, Ullah A. Trend, clinical presentations and diagnosis of eosinophilic esophagitis in Saudi children. Saudi Med J. 2018;39:668-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Momen T, Saneian H, Amini N. Demographic, Clinical, and Allergic Characteristics of Children with Eosinophilic Esophagitis in Isfahan, Iran. Iran J Allergy Asthma Immunol. 2018;17:533-539. [PubMed] |
| 57. | Assiri AM, Saeed A. Incidence and diagnostic features of eosinophilic esophagitis in a group of children with dysphagia and gastroesophageal reflux disease. Saudi Med J. 2014;35:292-297. [PubMed] |
| 58. | Hasosah MY, Sukkar GA, Alsahafi AF, Thabit AO, Fakeeh ME, Al-Zahrani DM, Satti MB. Eosinophilic esophagitis in Saudi children: symptoms, histology and endoscopy results. Saudi J Gastroenterol. 2011;17:119-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Carr S, Chan ES, Watson W. Correction to: Eosinophilic esophagitis. Allergy Asthma Clin Immunol. 2019;15:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Arnim UV, Biedermann L, Aceves SS, Bonis PA, Collins MH, Dellon ES, Furuta GT, Gonsalves N, Gupta S, Hirano I, Lucendo AJ, Miehlke S, Oliva S, Schlag C, Schoepfer A, Straumann A, Vieth M, Bredenoord AJ; EUREOS and TIGERs. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023;21:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 61. | Wechsler JB, Bolton SM, Amsden K, Wershil BK, Hirano I, Kagalwalla AF. Eosinophilic Esophagitis Reference Score Accurately Identifies Disease Activity and Treatment Effects in Children. Clin Gastroenterol Hepatol. 2018;16:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 62. | Jevtić J, Ristić N, Pavlović V, Svorcan J, Milovanovich I, Radusinović M, Popovac N, Simić L, Ćirović A, Đuknić M, Životić M, Poljašević N, Obradović D, Filipović J, Janković R. The Usefulness of the Eosinophilic Esophagitis Histology Scoring System in Predicting Response to Proton Pump Inhibitor Monotherapy in Children with Eosinophilic Esophagitis. Diagnostics (Basel). 2023;13:3445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Dhaliwal J, Tobias V, Sugo E, Varjavandi V, Lemberg D, Day A, Bohane T, Ledder O, Jiwane A, Adams S, Henry G, Dilley A, Shi E, Krishnan U. Eosinophilic esophagitis in children with esophageal atresia. Dis Esophagus. 2014;27:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Gorter RR, Heij HA, van der Voorn JP, Kneepkens CM. Eosinophilic esophagitis after esophageal atresia: is there an association? Case presentation and literature review. J Pediatr Surg. 2012;47:e9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Yamada Y, Nishi A, Kato M, Toki F, Yamamoto H, Suzuki N, Hirato J, Hayashi Y. Esophagitis with eosinophil infiltration associated with congenital esophageal atresia and stenosis. Int Arch Allergy Immunol. 2013;161 Suppl 2:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Yasuda JL, Clark SJ, Staffa SJ, Blansky B, Ngo PD, Hamilton TE, Smithers CJ, Jennings R, Manfredi MA. Esophagitis in Pediatric Esophageal Atresia: Acid May Not Always Be the Issue. J Pediatr Gastroenterol Nutr. 2019;69:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Pagliara C, Zambaiti E, Antoniello LM, Gamba P. Eosinophilic Esophagitis in Esophageal Atresia: Is It Really a New Disease? Children (Basel). 2022;9:1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Eid R, Mounzer C, Mendoza M, Middleton J, Barnes B, Mcgowan E. Increased Prevalence of Eosinophilic Esophagitis (EoE) in Children with Inflammatory Bowel Disease (IBD). J Allergy Clin Immunol. 2021;147:AB91. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 69. | Moore H, Wechsler J, Frost C, Whiteside E, Baldassano R, Markowitz J, Muir AB. Comorbid Diagnosis of Eosinophilic Esophagitis and Inflammatory Bowel Disease in the Pediatric Population. J Pediatr Gastroenterol Nutr. 2021;72:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Correction to: Occurrence and Clinical Impact of Eosinophilic Esophagitis in a Large Cohort of Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2023;29:183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1517] [Article Influence: 101.1] [Reference Citation Analysis (1)] |
| 72. | Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, Rothenberg ME. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 73. | Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, Davis CM, Manuel-Rubio M, Marcus S, Shaykin R, Sulkowski M, Johnson K, Ross JN, Riffle ME, Groetch M, Melin-Aldana H, Schady D, Palac H, Kim KA, Wershil BK, Collins MH, Chehade M. Efficacy of a 4-Food Elimination Diet for Children With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15:1698-1707.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 74. | Molina-Infante J, Lucendo AJ. Dietary therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Wechsler JB, Schwartz S, Arva NC, Kim KA, Chen L, Makhija M, Amsden K, Keeley K, Mohammed S, Dellon ES, Kagalwalla AF. A Single-Food Milk Elimination Diet Is Effective for Treatment of Eosinophilic Esophagitis in Children. Clin Gastroenterol Hepatol. 2022;20:1748-1756.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 76. | Grasso J, Radler DR, Zelig R. Single-food elimination of cow's milk as a treatment for eosinophilic esophagitis in children aged 2-18 years: A review of the literature. Nutr Clin Pract. 2024;39:824-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 77. | Kliewer KL, Abonia JP, Aceves SS, Atkins D, Bonis PA, Capocelli KE, Chehade M, Collins MH, Dellon ES, Fei L, Furuta GT, Gupta SK, Kagalwalla A, Leung J, Mir S, Mukkada VA, Pesek R, Rosenberg C, Shoda T, Spergel JM, Sun Q, Wechsler JB, Yang GY, Rothenberg ME. One-food versus 4-food elimination diet for pediatric eosinophilic esophagitis: A multisite randomized trial. J Allergy Clin Immunol. 2025;155:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 78. | Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 769] [Article Influence: 85.4] [Reference Citation Analysis (1)] |
| 79. | Molina-Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodríguez G, Mateos-Rodríguez JM, Dueñas-Sadornil C, Perez-Gallardo B, Ferrando-Lamana L, Fernandez-Gonzalez N, Bañares R, Zamorano J. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther. 2014;40:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 80. | Navarro P, Laserna-Mendieta EJ, Guagnozzi D, Casabona S, Perelló A, Savarino E, de la Riva S, Olalla JM, Ghisa M, Serrano-Moya N, Alcolea-Valero C, Ortega-Rabbione G, Majano P, Santander C, Arias Á, Lucendo AJ; EUREOS EoE CONNECT research group. Proton pump inhibitor therapy reverses endoscopic features of fibrosis in eosinophilic esophagitis. Dig Liver Dis. 2021;53:1479-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, Rayo A, Echeverría L, Quevedo S, Bracamonte T, Román E. High Prevalence of Response to Proton-pump Inhibitor Treatment in Children With Esophageal Eosinophilia. J Pediatr Gastroenterol Nutr. 2016;62:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, Rayo A, Echeverría L, Borrell B, Román E. Long-term Treatment With Proton Pump Inhibitors Is Effective in Children With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2018;67:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 83. | Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14:13-22.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 84. | Munoz-Osores E, Maldonado-Campos I, Olivares-Labbe MT, Villarroel L, Gana JC. Corticosteroids for Eosinophilic Esophagitis in Children: A Meta-analysis. Pediatrics. 2020;146:e20200874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 86. | Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271-9; quiz 2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 87. | Miehlke S, Lucendo AJ, Straumann A, Jan Bredenoord A, Attwood S. Orodispersible budesonide tablets for the treatment of eosinophilic esophagitis: a review of the latest evidence. Therap Adv Gastroenterol. 2020;13:1756284820927282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, Taber T, Kaushal S, Limgala R, Brown M, Gupta R, Balba N, Goker-Alpan O, Khojah A, Alpan O. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10:e0113483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 89. | Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, Dohil R, Broide DH, Aceves SS. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 90. | Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, Nadeau K, Kaiser S, Peters T, Perez A, Jones I, Arm JP, Strieter RM, Sabo R, Gunawardena KA. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 91. | Lam AY, Ma C, Lee JK, Bredenoord AJ. Eosinophilic esophagitis: New molecules, better life? Curr Opin Pharmacol. 2022;63:102183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Syverson EP, Rubinstein E. Real World Experience With Dupilumab in Eosinophilic Esophagitis in Children and Young Adults at a Tertiary Care Pediatric Medical Center. JPGN Rep. 2022;3:e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Chehade M, Dellon ES, Spergel JM, Collins MH, Rothenberg ME, Pesek RD, Hirano I, Liu R, Laws E, Mortensen E, Martincova R, Shabbir A, McCann E, Kamal MA, Kosloski MP, Hamilton JD, Samuely C, Lim WK, Wipperman MF, Farrell A, Patel N, Yancopoulos GD, Glotfelty L, Maloney J. Dupilumab for Eosinophilic Esophagitis in Patients 1 to 11 Years of Age. N Engl J Med. 2024;390:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 94. | Alexander R, Kassmeyer B, Lennon R, Alexander J, Snyder D, Ravi K. EoE Recurrence on PPI Maintenance Therapy: You Do Not Know if You Do Not Look! Dig Dis Sci. 2024;69:4048-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Dellon ES, Muir AB, Katzka DA, Shah SC, Sauer BG, Aceves SS, Furuta GT, Gonsalves N, Hirano I. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025;120:31-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 96. | Lin B, Rabinowitz S, Haseeb MA, Gupta R. Usefulness of the Eosinophilic Esophagitis Histologic Scoring System in Distinguishing Active Eosinophilic Esophagitis From Remission and Gastroesophageal Reflux Disease. Gastroenterology Res. 2021;14:220-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, Varni JW, Grotjan JT, Eby M, He H, Marsolo K, Putnam PE, Garza JM, Kaul A, Wen T, Rothenberg ME. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol. 2015;135:1519-28.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 98. | Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, Fleischer DM, Kramer RE, Deterding RR, Capocelli KE, Prager JD. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc. 2016;83:299-306.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Shaul E, Kennedy KV, Spergel ZC, Daneshdoost S, Mahon M, Thanawala S, Spergel JM, Wilkins B, Ryan MJ, Muir AB. Endoscopic and histologic utility of transnasal endoscopy in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2024;78:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Dollin YT, Mark JA, Andrews R, Pan Z, Ort C, Kramer RE, Nguyen N. Adverse events are lower in unsedated transnasal esophagoscopy versus sedated esophagogastroduodenoscopy. J Pediatr Gastroenterol Nutr. 2025;81:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, Prager JD, Leinwand K, Kobak GE, Kramer RE, Menard-Katcher C, Furuta GT, Atkins D, Fleischer D, Greenhawt M, Friedlander JA. Transnasal Endoscopy in Unsedated Children With Eosinophilic Esophagitis Using Virtual Reality Video Goggles. Clin Gastroenterol Hepatol. 2019;17:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 102. | Katzka DA, Smyrk TC, Alexander JA, Geno DM, Beitia RA, Chang AO, Shaheen NJ, Fitzgerald RC, Dellon ES. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am J Gastroenterol. 2017;112:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, Masterson JC, Ochkur S, Protheroe C, Moore W, Pan Z, Amsden K, Robinson Z, Capocelli K, Mukkada V, Atkins D, Fleischer D, Hosford L, Kwatia MA, Schroeder S, Kelly C, Lovell M, Melin-Aldana H, Ackerman SJ. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 104. | Ackerman SJ, Kagalwalla AF, Hirano I, Gonsalves N, Katcher PM, Gupta S, Wechsler JB, Grozdanovic M, Pan Z, Masterson JC, Du J, Fantus RJ, Alumkal P, Lee JJ, Ochkur S, Ahmed F, Capocelli K, Melin-Aldana H, Biette K, Dubner A, Amsden K, Keeley K, Sulkowski M, Zalewski A, Atkins D, Furuta GT. One-Hour Esophageal String Test: A Nonendoscopic Minimally Invasive Test That Accurately Detects Disease Activity in Eosinophilic Esophagitis. Am J Gastroenterol. 2019;114:1614-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 105. | Carlson DA, Hirano I, Zalewski A, Gonsalves N, Lin Z, Pandolfino JE. Improvement in Esophageal Distensibility in Response to Medical and Diet Therapy in Eosinophilic Esophagitis. Clin Transl Gastroenterol. 2017;8:e119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Almazan E, Liang TZ, Hohl B, Hoskins BJ, Birkness-Gartman JE, Ng K. EndoFLIP distensibility index correlates with histologic findings in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2025;80:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 107. | Lowry MA, Vaezi MF, Correa H, Higginbotham T, Slaughter JC, Acra S. Mucosal Impedance Measurements Differentiate Pediatric Patients With Active Versus Inactive Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2018;67:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Dellon E, Chehade M, Genta RM, Leiman DA, Peterson KA, Spergel J, Wechsler J, Bortey E, Chang AT, Hirano I. S446 Results from KRYPTOS, a Phase 2/3 Study of Lirentelimab (AK002) in Adults and Adolescents With EoE. Am J Gastroenterol. 2022;117:e316-e317. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/