Published online Feb 16, 2026. doi: 10.4253/wjge.v18.i2.113502
Revised: September 26, 2025
Accepted: December 5, 2025
Published online: February 16, 2026
Processing time: 161 Days and 15.6 Hours
Gastric endoscopic submucosal dissection (ESD) is a minimally invasive the
To analyze the learning curve of gastric submucosal endoscopic dissection in one of the largest Western series reported to date.
A retrospective study was conducted, including patients who underwent ESD for superficial gastric neoplasms between 2009 and 2025. Patients were divided into 4 consecutive chronological phases to assess temporal trends in outcomes: (1) Phase I: From case 1 to case 40; (2) Phase II: From case 41 to case 80; (3) Phase III: From case 81 to case 120; and (4) Phase IV: From case 121 to case 162. The following par
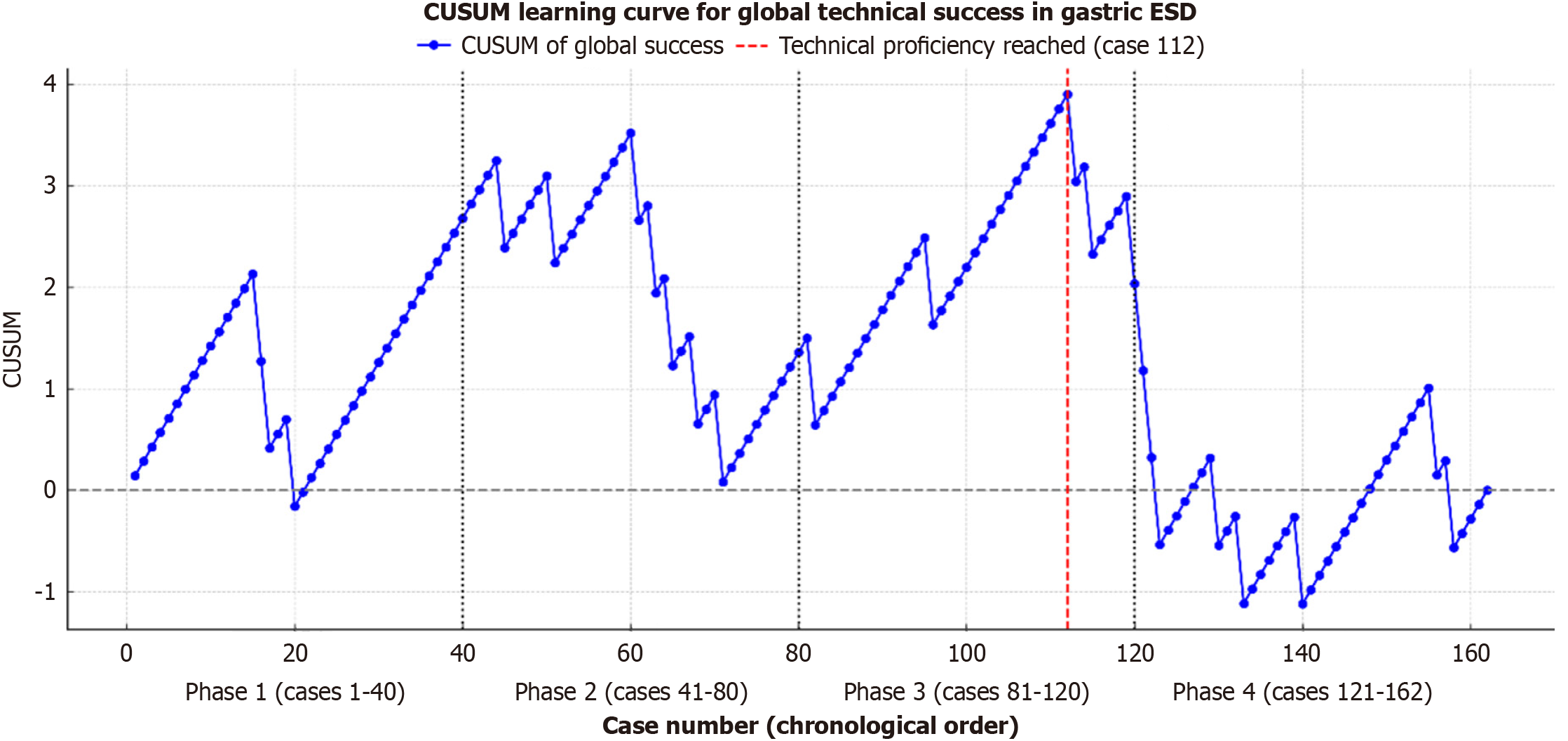
A total of 164 ESD procedures were performed. The duration of the first phase, second phase, third phase, and fourth phase was 77 months, 40 months, 37 months, and 50 months, respectively. In the first phase of the study (phase I) the mean tumor size, mean procedure time, en bloc resection rate, complete resection rate and curative resection rate were: 21.5 mm, 99.4 minutes, 97.5% (39/40), 92.5% (37/40) and 87.5% (35/40) respectively, while in the last phase of the study (phase IV) those features were: 25.6 mm, 107.7 minutes, 90.4% (38/42), 83.3% (35/42) and 78.5% (33/42) respectively. Likewise, the overall rate of adverse events in phase I was 0% and in phase IV was 7.1% (3/42) (P = 0.35) (mortality associated with the procedure: 0%). The Cumulative Sum Curve showed a turning point in the curve in case number 112.
Proficiency in gastric ESD takes more than one hundred procedures to be achieved in Western settings, with high standards of safety and efficacy, provided that rigorous and systematic training is combined with a planned pro
Core Tip: Currently, scientific evidence on the learning curve in gastric endoscopic submucosal dissection (ESD) is limited, and most of the available data comes from Asian countries. This lack of scientific evidence represents a significant barrier, especially given the progressive increase in the early diagnosis of gastric cancer in Western regions and the growing interest in effective and minimally invasive endoscopic therapeutic strategies. The learning curve in gastric ESD is feasible, safe, and effective in Western countries, provided that intensive training and adequate progressive case selection are in place. According to our case series, after 112 ESD procedures, consolidated technical mastery is achieved.
- Citation: Aliaga Ramos J, Nunes Arantes V. Mastering gastric endoscopic submucosal dissection: A learning curve analysis of over 100 consecutive cases performed by Western endoscopist. World J Gastrointest Endosc 2026; 18(2): 113502
- URL: https://www.wjgnet.com/1948-5190/full/v18/i2/113502.htm
- DOI: https://dx.doi.org/10.4253/wjge.v18.i2.113502
Gastric cancer is the fifth most common malignant neoplasm worldwide, with an estimated global prevalence of 5.7% of all diagnosed cancers, according to the most recent studies[1]. Its incidence is approximately 9.2 cases per 100000 inhabitants, and it is the third leading cause of cancer death globally, with a mortality rate ranging from 8% to 11% of all deaths from this disease[1]. Advances in endoscopic technology and the growing implementation of screening programs have facilitated the early diagnosis of gastric cancer, allowing for a more effective therapeutic approach using minimally invasive techniques. In this context, endoscopic submucosal dissection (ESD) has established itself as the first-line treatment for superficial gastric neoplasms. This technique has demonstrated efficacy and safety profiles comparable to those of gastrectomy in expert hands, with the additional advantage of preserving the affected organ, reducing post-surgical morbidity, and maintaining an optimal quality of life after the procedure. However, its correct performance involves a high level of technical complexity and requires a long and specialized learning curve[2-6].
Studies conducted in Asia have shown that technical skill in gastric ESD improves progressively with accumulated experience[6]. According to international consensus, mostly from Eastern countries, it is considered necessary to perform at least 30 procedures to achieve basic competence in ESD of the lower third of the stomach, evaluated in terms of en bloc resection, procedure time, and complication management. To achieve an advanced level of competence in the resection of more complex lesions located in the middle-upper third of the stomach, it is estimated that between 50 and 100 cases are required. Internationally, and especially in Eastern training programs, these ranges have been fundamental in structuring effective training programs, establishing objective criteria for technical competence, and reducing the gap in the adoption of this technique in regions where its implementation is still limited, such as in the West and Latin America[7-11].
In the West, one of the main limitations to the effective eradication of superficial gastric neoplasms through ESD is the scarcity of centers specializing in highly complex endoscopic procedures that offer structured and accessible training programs for endoscopists interested in acquiring experience in this advanced technique[12-16]. Currently, scientific evidence on the learning curve in gastric ESD is limited, and most of the available data comes from Asian countries, where the prevalence of early gastric cancer is higher and ESD is more widely used[17-21]. This lack of scientific evidence represents a significant barrier, especially given the progressive increase in the early diagnosis of gastric cancer in Western regions and the growing interest in effective and minimally invasive endoscopic therapeutic strategies. In this context, it is a priority to understand and adequately characterize the learning curve in gastric ESD in order to validate and standardize training programs adapted to local realities. Our study aims to analyze the learning curve in gastric ESD in one of the largest Western cohorts, through a detailed analysis divided into four consecutive chronological phases.
This is a retrospective study. Data were collected from a prospectively generated database of patients admitted in chronological order from 2009 to 2025 who underwent ESD for superficial gastric neoplasms performed by a single endoscopist (Arantes VN). The inclusion criteria were patients admitted for endoscopic resection for early gastric neoplasms. It is important to note that all participants enrolled in our study were thoroughly evaluated prior to ESD by an expert operator using high-definition white light endoscopy and image-enhanced endoscopy (digital chromoendoscopy and endoscopic magnification) to determine the degree of neoplastic invasion in deep layers. Only in doubtful cases were endoscopic ultrasonography and computed tomography used to optimize preoperative staging. Patients with advanced neoplasms that showed massive submucosal invasion or invasion into deeper layers during preoperative staging, as well as individuals with clinical conditions unacceptable for anesthesia or the endoscopic procedure, were excluded from the study.
Patients were divided into four chronological phases in order to obtain a similar number of participants in each group: The first period included patients from case 1 to case 40, the second period included patients from case 41 to case 80, the third period included patients from case 81 to case 120, and the fourth period included patients from case 121 to case 162. The following parameters were calculated for each study period: Age, gender, average procedure time, tumor size, en bloc resection rate, complete resection rate (R0), curative resection rate, adverse event rate, histopathological report of the resected specimen, and location of the lesion.
The rates of curative resection, en bloc resection, and complete resection with tumor-free margins were assessed based on the most recent Japanese clinical guidelines[2]. The parameters for defining curative treatment of gastric carcinoma were established as follows: (1) Endoscopic curability A: Predominantly differentiated type, tumors limited to the mucosa (pT1a), negative resection margins (complete R0 resection), absence of lympho-vascular invasion, absence of ulceration or ulcer scar, regardless of size. Long diameter ≤ 2 cm, predominantly undifferentiated type, pT1a, absence of ulceration or ulcer scar, negative resection margins, and absence of lympho-vascular invasion. Long diameter ≤ 3 cm, predominantly differentiated type, pT1a, finding of ulceration or ulcer scar, negative resection margins, and absence of lympho-vascular invasion; and (2) Endoscopic curability B: Resected en bloc, long diameter ≤ 3 cm, predominantly of the differentiated type, negative resection margins, absence of lympho-vascular invasion and tumors limited to the superficial submucosa [pT1b/SM1 (superficial submucosa)] < 500 μm from the muscularis mucosae. Histopathological assessment was per
All ESD procedures were performed under general anesthesia. The operator who conducted all gastric ESDs in our series received specialized training from experienced endoscopists at high-volume referral centers in Japan and has performed over 350 ESD procedures across various segments of the gastrointestinal tract to date. Following a thorough evaluation using high-definition white-light endoscopy and virtual chromoendoscopy, lesions eligible for ESD were classified according to the Paris classification system. All ESD procedures were carried out using standardized equipment, which included either a Flush Knife BT 2.0 or 2.5 (Fujifilm Co., Tokyo, Japan), connected to an electrosurgical unit (ERBE VIO 200S, 200D, or 300D, Tübingen, Germany), a 4-mm distal cap (Elastic Touch, Top Co., Tokyo, Japan) affixed to the en
All statistical analyses were performed using SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, United States) and Python version 3.11 for advanced graphical representation. The variables included in the analysis were compared between the four phases of the study (phase I: Case 1-40; phase II: Case 41-80; phase III: Case 81-120; phase IV: Case 121-162) to assess significant differences along the learning curve. For the comparison of the continuous variables among the four phases, a one-way analysis of variance (ANOVA) test was used due to the design with multiple in
In addition to the comparative analysis of the clinical and demographic variables between the phases of the study, statistical analyses were performed to evaluate the evolution of the operator’s technical performance during the learning curve in gastric ESD. To evaluate the operator’s global learning curve, a Cumulative Sum Curve (CUSUM) was con
The authors declare that this study entailed a retrospective evaluation of the learning curve in gastric ESD and was conducted in accordance with the Declaration of Helsinki. The analysis was confined to the statistical assessment across four defined chronological periods. The research was conducted at two endoscopic referral centers in Brazil, with prior informed consent obtained from all patients and approval from the Institutional Review Board granted on May 6, 2020. The study adhered strictly to established ethical standards for scientific research, including the protection of patient confidentiality.
During the aforementioned study period, 162 gastric ESDs in 160 patients were performed; 89 patients were women (55.6%) and 71 were men (44.3%). The duration of the first phase, second phase, third phase, and fourth phase was 77 months, 40 months, 37 months, and 50 months, respectively. In the first phase of the study (phase I), it took a longer period of time to achieve 40 cases, as compared to the other 3 periods. In phase I the mean tumor size, the mean pro
Likewise, the overall rate of adverse events in phase I was 0% and in phase IV was 7.1% (3/42) (P = 0.35). In phases II and III, there was a decrease in the overall rate of adverse events from 5% (2/40) to 2.5% (1/40), without showing statistically significant results. All adverse events were successfully managed endoscopically by thermal coagulation and clips closure (mortality associated with the procedure: 0%). Table 1 shows a detailed analysis of all these parameters in each of the study phases in sequential order. The CUSUM showed a turning point in case number 112 (maximum CUSUM peak: 3.90 and overall technical success rate: 85.8%). From this case onwards, there was a sustained decline in the curve, indicating the achievement of technical proficiency of gastric ESD in this series. Figure 1 shows a graphical representation of the CUSUM.
| Phase I (case 1-40) | Phase II (case 41-80) | Phase III (case 81-120) | Phase IV (case 121-162) (n = 42) | P value | |
| Age | 65.2 years (23-86) | 65.8 years (30-85) | 65.6 years (19-89) | 69.5 years (24-88) | 0.71 |
| Male/female | 18/22 | 16/24 | 23/16 | 14/27 | 0.14 |
| En bloc resection | 97.5 (39/40) | 95 (38/40) | 92.5 (37/40) | 90.4 (38/42) | 0.58 |
| Complete resection (R0) | 92.5 (37/40) | 87.5 (35/40) | 87.5 (35/40) | 83.3 (35/42) | 0.66 |
| Curative resection | 87.5 (35/40) | 80 (32/40) | 82.5 (33/40) | 78.5 (33/42) | 0.73 |
| Size | 21.5 mm (10-40) | 32.1 mm (15-60) | 26.2 mm (10-60) | 25.6 mm (10-80) | < 0.01 |
| Procedure time (minute) (mean ± SD) | 99.4 ± 40.9 | 105.8 ± 60.2 | 110 ± 52.4 | 107.7 ± 57.5 | 0.87 |
| Adverse events | |||||
| Overall adverse event rate | 0 | 5 (2/40) | 2.5 (1/40) | 7.1 (3/42) | 0.35 |
| Digestive bleeding | 0 | 5 (2/40) | 0 | 7.1 (3/42) | 0.14 |
| Perforation | 0 | 0 | 2.5 (1/40) | 0 | 0.38 |
| Histological type of lesions | |||||
| Benign neoplasms | 32.5 (13/40) | 20 (8/40) | 15 (6/40) | 7.1 (3/42) | 0.02 |
| Low grade dysplasia | 12.5 (5/40) | 10 (4/40) | 27.5 (11/40) | 26.1 (11/42) | 0.09 |
| High grade dysplasia | 17.5 (7/40) | 20 (8/40) | 30 (12/40) | 35.7 (15/42) | 0.19 |
| Adenocarcinoma | 37.5 (15/40) | 50 (20/40) | 27.5 (11/40) | 30.9 (13/42) | 0.16 |
| Intramucosal | 27.5 (11/40) | 40 (16/40) | 22.5 (9/40) | 23.8 (10/42) | 0.28 |
| SM1 invasion | 2.5 (1/40) | 0 | 2.5 (1/40) | 2.3 (1/42) | 0.80 |
| SM2 invasion | 7.5 (3/40) | 10 (4/40) | 2.5 (1/40) | 4.7 (2/42) | 0.53 |
| Undifferentiated type (signet ring) | 2.5 (1/40) | 0 | 2.5 (1/40) | 9.5 (4/42) | 0.11 |
| Location of the lesion | |||||
| Lower third of the stomach | 57.5 (23/40) | 72.5 (29/40) | 70 (28/40) | 66.6 (28/42) | 0.50 |
| Upper-middle third of the stomach | 42.5 (17/40) | 27.5 (11/40) | 30 (12/40) | 33.3 (14/42) | 0.50 |
Factors that increase the technical difficulty of gastric ESD include the location of the lesion and the histopathology of the resected lesion. It was observed that in phase I, lesions located in the distal and proximal thirds of the stomach were: 57.5% (23/40) and 42.5% (17/40), respectively, while in phase IV, lesions located in the distal and proximal thirds of the stomach were 66.6% (28/42) and 33.3% (14/42), respectively, showing no statistically significant differences. In phases II and III, there was an increase in the number of lesions located in the upper-middle third of the stomach and a decrease in the number of lesions located in the lower third of the stomach, both without showing statistically significant differences.
Regarding histopathological analysis of the resected specimen, the following distribution was observed in the first phase of the study: Benign neoplasms - 32.5% (13/40) and adenocarcinoma - 37.5% (15/40); intramucosal - 27.5% (11/40). With deep submucosal invasion (SM2) - 7.5% (3/40) and undifferentiated type (signet ring) - 2.5% (1/40). In the last phase of the study, the following distribution was observed: Benign neoplasms - 7.1% (3/42) and adenocarcinoma - 30.9% (13/42); intramucosal - 23.8% (10/42). With deep submucosal invasion (SM2) - 4.7% (2/42) and undifferentiated type (signet ring) - 9.5% (4/42). In phases II and III, there was a decrease in the number of benign neoplasms and an increase in the number of lesions with high- and low-grade dysplasia. Likewise, a decrease in the number of adenocarcinoma cases was observed, in this group of lesions also showing a reduction in the number of intramucosal adenocarcinomas and an increase in cases of adenocarcinomas with superficial and deep submucosal invasion. There was also an increase in cases of undifferentiated adenocarcinomas; however, none of these showed statistically significant results. It is important to highlight that a statistically significant difference in the number of benign neoplasms was observed between phases I and IV.
Systematic analysis of the learning curve in gastric ESD is essential to accurately define the point at which technical competence in this procedure is consolidated. Although the eradication of superficial gastric neoplasms using ESD is routinely performed in Asia, its implementation in Western countries has been slower due to the reduced incidence of early gastric cancer, the high technical complexity of the procedure, and the limited availability of structured endoscopic training programs. This study analyzed 162 consecutive cases of gastric ESD performed by a single Western operator, constituting one of the largest series reported in the West. The cases were grouped into four successive phases of 40 to 42 procedures each to evaluate the technical evolution and possible turning points associated with the progressive mastery of gastric ESD.
In terms of technical success, our results show consistently high rates of en bloc, complete, and curative resection from phase I to phase IV with no statistically significant differences, showing a slight downward trend in the later phases. This phenomenon could be explained by a progressive increase in the complexity of the selected cases, as evidenced by the increase in the average size of lesions in phase II (32.1 mm) compared to phase I (21.5 mm) (P < 0.01). This demonstrates that a structured learning curve can lead to highly effective implementation of gastric ESD in Western contexts. These findings are consistent with the proposal of the European Society of Gastrointestinal Endoscopy (ESGE), which em
Regarding the duration of the procedure, no significant decrease was observed between stages (P = 0.87), which can be explained by the progressive increase in the technical difficulty of the selected cases, as demonstrated by the increase in tumor size and the higher proportion of advanced neoplasms (high-grade dysplasia/adenocarcinoma) in later stages. This trend is consistent with the findings of Yano et al[20], who described that as endoscopists gain experience, they tend to take on more technically challenging cases, which may mask improvements in operational efficiency when procedure time is evaluated as the single indicator.
In analyzing the learning curve for gastric ESD, adverse events are a critical indicator for evaluating the safety of the procedure in the hands of endoscopists in training. In our cohort, the overall rate of adverse events associated with the procedure was low, with a predominant concentration in the initial phases (P = 0.35). This pattern is consistent with that described by Hong et al[21], who found that after the first 60 gastric ESD procedures, the frequency of adverse events decreased considerably, especially in the third and fourth phases of learning (P < 0.05). Our study reaffirms these results, highlighting the need for supervised training protocols and gradual evaluation to reduce clinical risks during the progressive acquisition of technical competence, and showing that gastric ESD can be safely implemented in Western centers.
Regarding the CUSUM curve analysis, our results identified a turning point in case number 112 (total = 162 cases), from which a sustained decrease in the technical failure rate was observed, defined as the inability to achieve complete resection, en bloc, and without associated complications. Yoshida et al[22] analyzed the learning curve in 334 gastric ESDs performed by seven endoscopists in training (42-50 consecutive cases per endoscopist in training), demonstrating through a CUSUM curve a higher level of technical competence after 30 cases. This reinforces the validity of our results and supports the use of this methodological approach to determine the moment of technical consolidation in gastric ESD, but in a Western context and with a greater number of consecutive cases per endoscopist in training.
During the longitudinal analysis of our case series, a progressive variation was observed in the histopathological profile of lesions treated by gastric ESD throughout the learning curve. In the initial phase, neoplasms with benign histology predominated (phase I: 32.5%; phase IV: 7.1%; P = 0.02), while in the final phases there was an increase in the proportion of lesions with histology showing greater oncological progression (low-grade dysplasia, high-grade dysplasia, intramucosal adenocarcinoma, adenocarcinoma with superficial/deep submucosal invasion, undifferentiated adenocarcinoma). This progressive pattern suggests a stepwise selection of histological complexity, in accordance with the pedagogical principle of therapeutic endoscopy, in which the safety of the operator in training is prioritized by initially addressing less aggressive lesions. The most representative studies do not systematically report an evolution of the histopathological profile throughout training, focusing mainly on technical aspects (efficacy-safety). Therefore, our findings would provide a novel perspective to the field of gastric ESD training, highlighting the importance of con
One of the most decisive factors in the technical evolution of gastric ESD is the anatomical topography of the lesion. Not all regions of the stomach present the same technical challenge or require the same degree of surgical skill, and this aspect is highly relevant in the context of the learning curve[23-26]. Recently, Aliaga Ramos et al[27] demonstrated that proximal gastric lesions require longer procedure times and have significantly lower rates of en bloc, complete, and curative resection compared to distal gastric lesions. The authors also observed that proximal gastric lesions showed a higher incidence of submucosal invasion, demonstrating that these lesions pose a technical-oncological challenge. These findings reinforce the notion that technical progression in gastric ESD should consider a strategic anatomical sequence, in which initial learning focuses on the management of more accessible distal lesions before advancing to more challenging regions such as the upper-middle third of the stomach. Recognizing this topographical variability not only allows for safe and efficient planning of endoscopist training but also helps to minimize the risk of adverse events and optimize on
The topographical distribution of gastric cancer shows significant geographical differences between the East and the West. In Western countries, a relative increase in neoplasms located in the proximal third of the stomach (cardia and gastroesophageal junction) has been observed in recent decades[28]. This pattern is mainly attributed to the increasing prevalence of risk factors characteristic of these populations, such as visceral obesity, gastroesophageal reflux disease, autoimmune gastritis (type A), and Epstein-Barr virus infection[28-30]. In addition, the greater efficacy in eradicating Helicobacter pylori in Western countries has contributed to the progressive decline in distal gastric cancers, modifying the topographic distribution toward a relative predominance of tumors located in the proximal segments of the stomach. In contrast, in Eastern countries, the trend continues to show a predominance of neoplasms located in the distal third of the stomach (antrum and body), where Helicobacter pylori infection and atrophic gastritis remain epidemiological determinants. These differences reflect the fact that geographical distribution has a decisive influence on the topography of gastric cancer and emphasize the need for prevention and early detection strategies tailored to each region[31,32]. Our casuistry shows that most cases of gastric cancer are located in the distal third of the stomach, confirming that this region continues to be the most affected in our population. However, recently, a considerable percentage of tumors has been progressively detected in the proximal third of the stomach, reflecting a growing trend toward the presentation of gastric neoplasms in this location in our community.
Our study has certain limitations that must be acknowledged when interpreting its results. First, our sample size could be considered small compared to similar Asian studies previously published; however, to our knowledge, our gastric ESD cohort is one of the largest in the West. Another limitation is that the analysis was based on the experience of a single operator with background training in Japan, which, while ensuring consistency in technique and clinical judgment, limits the generalizability of our results to endoscopy centers with different levels of training or endoscopic infrastructure. Finally, the retrospective design of the study imposes inherent restrictions on variable control and standardized follow-up; thus, future studies with a prospective design should be conducted to validate these results.
In conclusion, our results confirm that the learning curve in gastric ESD is feasible, safe, and effective in Western countries, provided that intensive training and adequate progressive case selection are in place. According to our case series, starting with case 112, consolidated technical mastery is achieved, with optimal therapeutic success rates and no clinical failures, even in complex lesions. This finding confirms that excellence in gastric ESD is an achievable goal outside Asia, provided that technical discipline, strategic anatomical progression, and a commitment to continuous improvement are integrated.
Endoscopy Unit, Alfa Institute of Gastroenterology; School of Medicine, Federal University of Minas Gerais; Hospital Mater Dei Contorno, Belo Horizonte, Minas Gerais, Brazil
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68588] [Article Influence: 13717.6] [Reference Citation Analysis (201)] |
| 2. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 3. | Aliaga Ramos J, Arantes V, Abdul Rani R, Yoshida N. Off-label use of 0.4 % sodium hyaluronate teardrops: a safe and effective solution for submucosal injection in gastric endoscopic submucosal dissection. Endosc Int Open. 2020;8:E1741-E1747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 479] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 5. | Al-Haddad MA, Elhanafi SE, Forbes N, Thosani NC, Draganov PV, Othman MO, Ceppa EP, Kaul V, Feely MM, Sahin I, Ruan Y, Sadeghirad B, Morgan RL, Buxbaum JL, Calderwood AH, Chalhoub JM, Coelho-Prabhu N, Desai M, Fujii-Lau LL, Kohli DR, Kwon RS, Machicado JD, Marya NB, Pawa S, Ruan W, Sheth SG, Storm AC, Thiruvengadam NR, Qumseya BJ; (ASGE Standards of Practice Committee Chair). American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: methodology and review of evidence. Gastrointest Endosc. 2023;98:285-305.e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin Endosc. 2020;53:142-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (3)] |
| 7. | Pimentel-Nunes P, Pioche M, Albéniz E, Berr F, Deprez P, Ebigbo A, Dewint P, Haji A, Panarese A, Weusten BLAM, Dekker E, East JE, Sanders DS, Johnson G, Arvanitakis M, Ponchon T, Dinis-Ribeiro M, Bisschops R. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2019;51:980-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2747] [Article Influence: 457.8] [Reference Citation Analysis (3)] |
| 9. | Kushima R. The updated WHO classification of digestive system tumours-gastric adenocarcinoma and dysplasia. Pathologe. 2022;43:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | McCarty TR, Aihara H. Current state of education and training for endoscopic submucosal dissection: Translating strategy and success to the USA. Dig Endosc. 2020;32:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Yamamoto Y, Fujisaki J, Ishiyama A, Hirasawa T, Igarashi M. Current status of training for endoscopic submucosal dissection for gastric epithelial neoplasm at Cancer Institute Hospital, Japanese Foundation for Cancer Research, a famous Japanese hospital. Dig Endosc. 2012;24 Suppl 1:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Simsek C, Aihara H. Training in Endoscopic Submucosal Dissection in the United States: The Current Paradigm. Gastrointest Endosc Clin N Am. 2023;33:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Ebigbo A, Messmann H. How can we make the learning curve of endoscopic submucosal dissection for (Western) endoscopists less steep? Endoscopy. 2016;48:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Ly EK, Nithyanand S, Modayil RJ, Khodorskiy DO, Neppala S, Bhumi S, DeMaria M, Widmer JL, Friedel DM, Grendell JH, Stavropoulos SN. Learning Curve for Endoscopic Submucosal Dissection With an Untutored, Prevalence-Based Approach in the United States. Clin Gastroenterol Hepatol. 2020;18:580-588.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter D. Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion. 2014;89:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Kim JH, Nam HS, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, Lee SH. Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc. 2017;31:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Higuchi K, Katagiri A, Nakatani S, Kikuchi K, Fujiwara T, Gocho T, Inoki K, Konda K, Yamamura F, Yoshida H. Risk Factors Indicating Difficulty During Gastric Endoscopic Submucosal Dissection for Inexperienced Endoscopists: A Retrospective Study. Cureus. 2022;14:e32713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Ribeiro-Mourão F, Veloso N, Dinis-Ribeiro M, Pimentel-Nunes P. Endoscopic Submucosal Dissection of Gastric Superficial Lesions: Predictors for Time of Procedure in a Portuguese Center. GE Port J Gastroenterol. 2015;22:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Yano T, Hasuike N, Ono H, Boku N, Ogawa G, Kadota T, Oda I, Doyama H, Hori S, Iishi H, Takahashi A, Takizawa K, Muto M. Factors associated with technical difficulty of endoscopic submucosal dissection for early gastric cancer that met the expanded indication criteria: post hoc analysis of a multi-institutional prospective confirmatory trial (JCOG0607). Gastric Cancer. 2020;23:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Hong KH, Shin SJ, Kim JH. Learning curve for endoscopic submucosal dissection of gastric neoplasms. Eur J Gastroenterol Hepatol. 2014;26:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Yoshida M, Kakushima N, Mori K, Igarashi K, Kawata N, Tanaka M, Takizawa K, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H, Ono H. Learning curve and clinical outcome of gastric endoscopic submucosal dissection performed by trainee operators. Surg Endosc. 2017;31:3614-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Yoon JY, Shim CN, Chung SH, Park W, Chung H, Lee H, Shin SK, Lee SK, Lee YC, Park JC. Impact of tumor location on clinical outcomes of gastric endoscopic submucosal dissection. World J Gastroenterol. 2014;20:8631-8637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kim SJ, Choi CW, Kang DH, Kim HW, Park SB, Nam HS, Ryu DG. Clinical outcomes of endoscopic submucosal dissection for lesions on the proximal location between remnant and entire stomach. Surg Endosc. 2020;34:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Kang DH, Choi CW, Kim HW, Park SB, Kim SJ, Nam HS, Ryu DG. Location characteristics of early gastric cancer treated with endoscopic submucosal dissection. Surg Endosc. 2017;31:4673-4679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Cho JH, Jin SY, Park S. Resection speed of endoscopic submucosal dissection according to the location of gastric neoplasia: a learning curve using cumulative sum analysis. Surg Endosc. 2023;37:2969-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Aliaga Ramos J, Arantes VN. Impact of gastric neoplasms location on clinical outcome of patients treated by endoscopic submucosal dissection. World J Gastrointest Endosc. 2025;17:107911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Inoue M. Epidemiology of Gastric Cancer-Changing Trends and Global Disparities. Cancers (Basel). 2024;16:2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 29. | Noh JH, Shin JY, Lee JH, Park YS, Lee IS, Kim GH, Na HK, Ahn JY, Jung KW, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. Clinical Significance of Epstein-Barr Virus and Helicobacter pylori Infection in Gastric Carcinoma. Gut Liver. 2023;17:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, Yamasaki T, Sakaida I. Clinical Importance of Epstein⁻Barr Virus-Associated Gastric Cancer. Cancers (Basel). 2018;10:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 484] [Reference Citation Analysis (1)] |
| 32. | Han Z, Liu J, Zhang W, Kong Q, Wan M, Lin M, Lin B, Ding Y, Duan M, Li Y, Zuo X, Li Y. Cardia and non-cardia gastric cancer risk associated with Helicobacter pylori in East Asia and the West: A systematic review, meta-analysis, and estimation of population attributable fraction. Helicobacter. 2023;28:e12950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/