Published online Sep 16, 2025. doi: 10.4253/wjge.v17.i9.110850
Revised: July 11, 2025
Accepted: August 19, 2025
Published online: September 16, 2025
Processing time: 87 Days and 15.5 Hours
Autoimmune gastritis (AIG) is recognized endoscopically by the presence of antrum-sparing corpus-dominant atrophy, known as reverse atrophy. However, a past Helicobacter pylori (H. pylori) infection can obscure this classic pattern. We present two cases of AIG with past H. pylori infection and highlight a novel endoscopic sign that may aid AIG recognition when typical features are absent.
One patient reported postprandial fullness, while the other was asymptomatic. Neither had a history of H. pylori eradication therapy. Both tested negative on a urea breath test and positive for anti-parietal cell antibodies. In both patients, endoscopy revealed mucosal atrophy involving both the corpus and antrum, which was counter to the characteristic reverse atrophy pattern typically seen in AIG. Beyond the atrophic border, we observed a distinct pattern of gyrus-like changes, manifesting as elevated mucosa between deep fissures. Histologically, targeted biopsies from these gyrus-like areas revealed parietal cell degeneration, lymphocytic infiltration, and hyperplasia of enterochromaffin-like cells, consistent with early histopathologic changes seen in AIG. These results supported diag
Gyrus-like changes may serve as a novel endoscopic clue of AIG with past H. pylori infection.
Core Tip: We present two patients diagnosed with autoimmune gastritis (AIG) after past Helicobacter pylori (H. pylori) infection. In both, the reverse atrophy characteristic of AIG was absent. We identified gyrus-like changes, characterized by mildly elevated mucosa between deep fissures, as a novel endoscopic feature. Histology confirmed the early pathology of AIG in these areas. This previously unrecognized endoscopic pattern offers a valuable clue of detecting AIG in patients with past H. pylori infection that may facilitate the early recognition and management of this condition.
- Citation: Tan CC, Shangguan XL, Lei XM, Deng FF, Wu YP, Zhang GM. Gyrus-like endoscopic changes in autoimmune gastritis with past Helicobacter pylori infection: Two case reports and review of literature. World J Gastrointest Endosc 2025; 17(9): 110850
- URL: https://www.wjgnet.com/1948-5190/full/v17/i9/110850.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i9.110850
Autoimmune gastritis (AIG) is characterized by oxyntic mucosal atrophy and the production of anti-parietal cell antibodies (PCA) and/or anti-intrinsic factor antibodies (IFA). This is due to a CD4+ T cell-mediated immune response targeting gastric parietal cells[1-4]. Epidemiological studies have shown AIG prevalences ranging from 0.3% to 2.7%, with variation across geographic regions[5]. While early research suggested a lower prevalence in Asian populations[6-8], recent data indicate this was an underestimation, likely due to the high prevalence of Helicobacter pylori (H. pylori) gastritis in these regions[9,10], where H. pylori infection disrupts the clinical manifestations of AIG, particularly its endoscopic features[11]. When both conditions coexist, antral atrophy caused by H. pylori often masks the reverse atrophy characteristic of AIG. In patients with resolved H. pylori infection (either through eradication or spontaneous clearance), persistent antral changes may still interfere with endoscopic evaluation. A large Japanese cohort study (n = 10822) reported significantly lower rates of endoscopically-detected AIG in post-eradication patients than in H. pylori-negative controls[12,13]. Therefore, the identification of additional endoscopic indicators beyond reverse atrophy is essential. We present two cases of AIG with past H. pylori infection and introduce gyrus-like changes as a novel endoscopic feature that may facilitate the recognition of AIG.
Case 1: A 40-year-old Chinese male underwent endoscopy due to complaints of postprandial fullness.
Case 2: A 34-year-old Chinese woman underwent endoscopy during a routine health screening.
Case 1: The patient developed postprandial fullness for two weeks prior to presentation, without obvious precipitating factors. Associated symptoms such as abdominal pain, nausea, vomiting, or belching were absent. A urea breath test (UBT) performed at a local hospital was negative for H. pylori, and no specific treatment was administered. He presented to our hospital for further evaluation via upper gastrointestinal endoscopy.
Case 2: The patient was asymptomatic and underwent endoscopy as part of a routine health evaluation. She reported no gastrointestinal symptoms at the time of examination.
Case 1: The patient was healthy, with no history of H. pylori eradication therapy or proton pump inhibitor use. He had no known history of autoimmune disease, gastrointestinal disorders, chronic illnesses, surgery, or trauma.
Case 2: A UBT performed a year before presentation was negative, and there was no history of H. pylori eradication therapy. The patient reported no prior use of acid-suppressive agents, including proton pump inhibitors or H2-receptor antagonists. She had no history of autoimmune disorders or gastrointestinal disease. No other significant medical, surgical, or medication history was noted.
Cases 1 and 2: There was no significant personal or family history.
Cases 1 and 2: Physical examination revealed no significant abnormalities.
Case 1: Serological testing demonstrated positive PCA with a titer of 210.90 U/mL, while IFA was negative. Complete blood count, serum pepsinogen I and II, iron, and vitamin B12 levels showed values within normal limits.
Case 2: Serological tests for antibodies revealed a positive PCA result and a negative IFA result. The patient’s complete blood count, serum pepsinogen, iron, and vitamin B12 levels were all within the normal range.
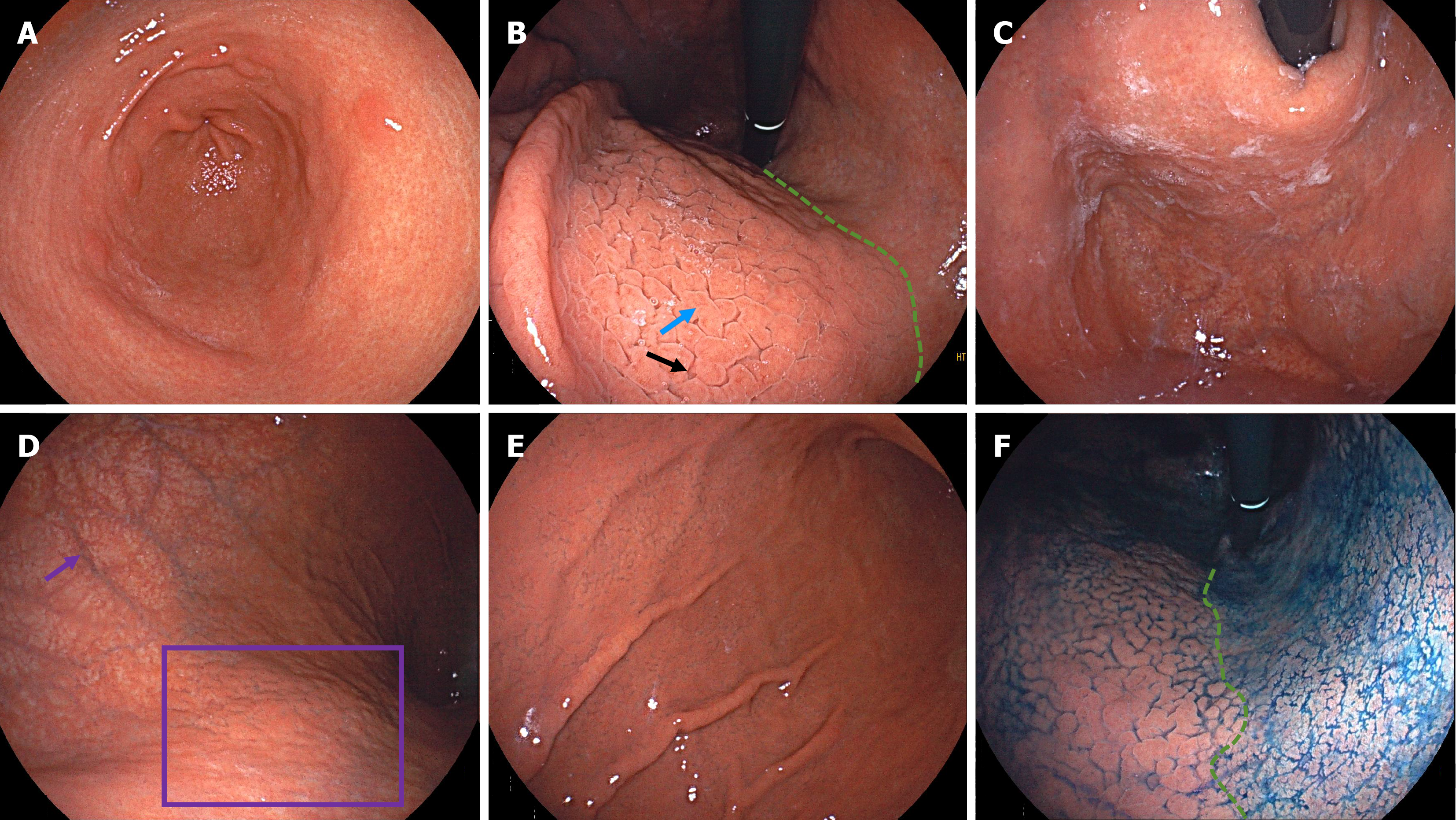
Case 1: Endoscopic examination demonstrated atrophy in the antrum, with xanthomas and circular wrinkle-like patterns (Figure 1A). The atrophy extended upward along the lesser curvature of the corpus, with a faintly visible atrophic border. The anterior wall of the corpus exhibited gyrus-like mucosal elevations and deep fissures (Figure 1B). In the fundus, there was white adherent mucus (Figure 1C). In the upper greater curvature of the corpus, we observed severe mucosal atrophy with visible submucosal vessels, and there was a grid pattern of scattered atrophy on the posterior wall of the corpus (Figure 1D). Extensive gyrus-like changes were apparent throughout the lower greater curvature of the corpus (Figure 1E). Indigo carmine staining clearly delineated the atrophic border along the lesser curvature of the corpus (Figure 1F).
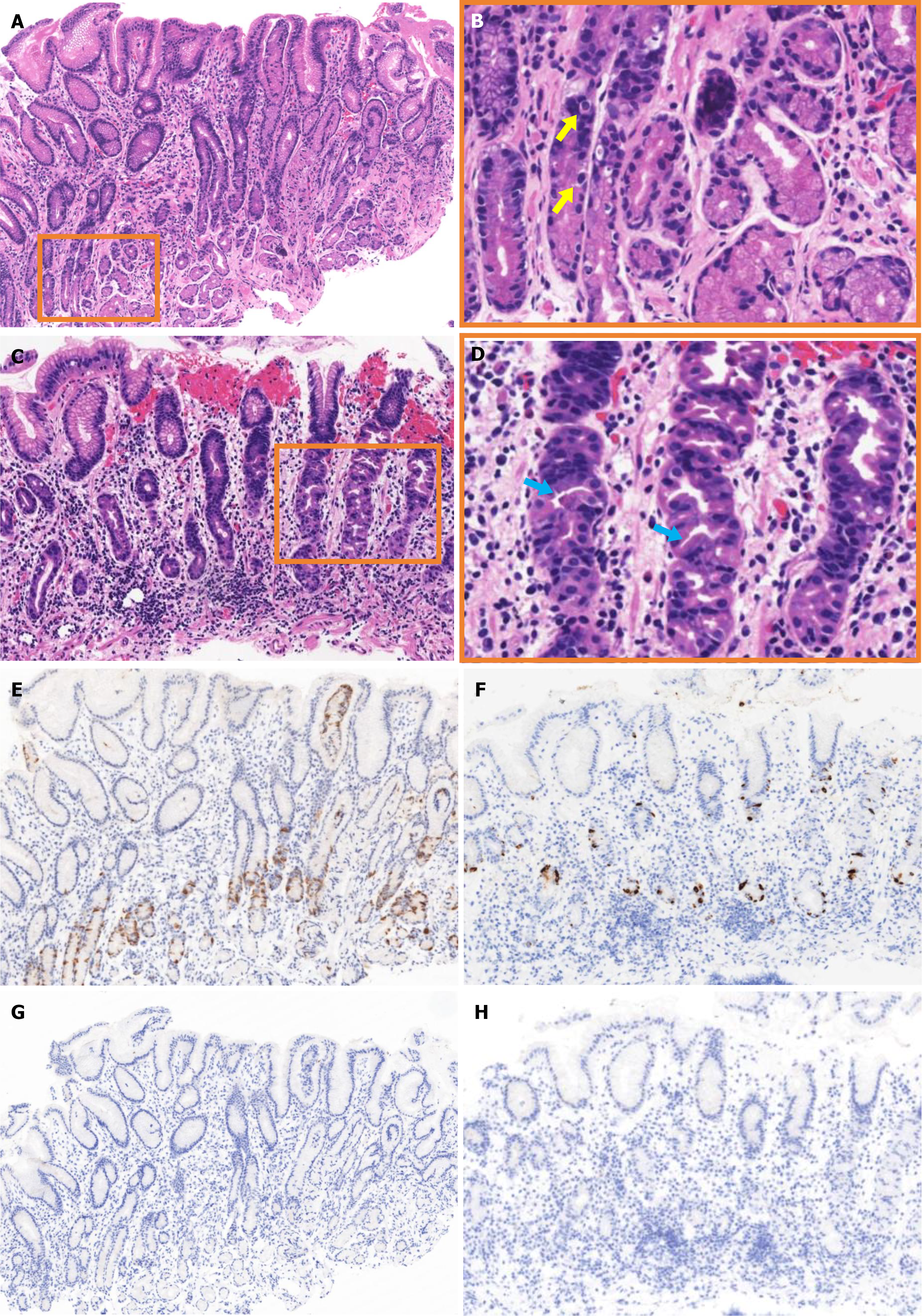
Histopathological examination of biopsy specimens from the antrum showed no significant inflammation or atrophy but evident G-cell hyperplasia (Figure 2A and B). Biopsy specimens from the corpus showed lymphocytic infiltration, with a decreased number of parietal cells and hyperplasia and protrusions into parietal cell lumen (Figure 2C and D). Gastrin staining revealed G-cell hyperplasia (Figure 2E). Chromogranin A staining showed enterochromaffin-like cells in both punctate and linear patterns (Figure 2F). H. pylori staining found no H. pylori in specimens from either the antrum (Figure 2G) or the corpus (Figure 2H).
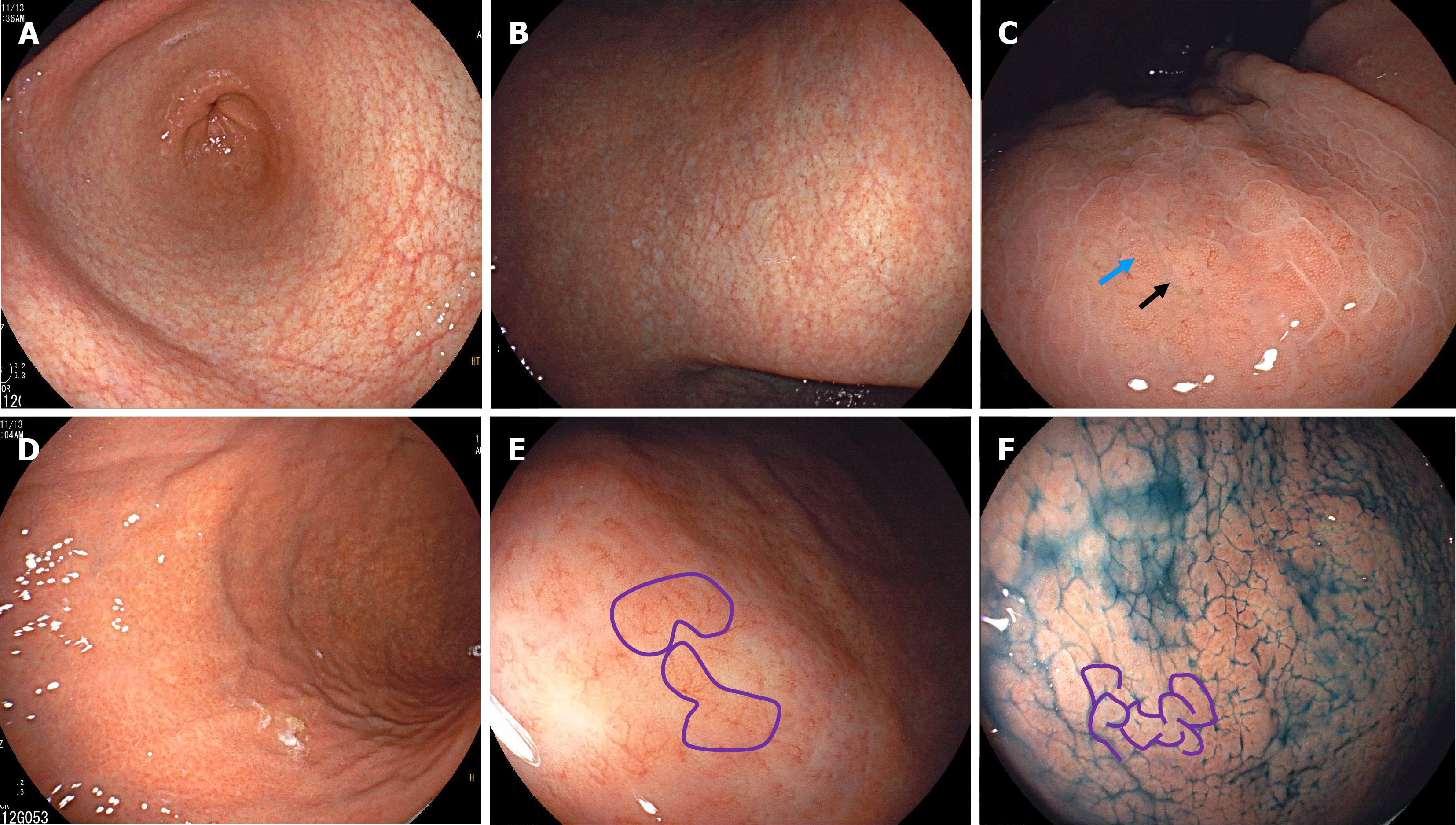
Case 2: Endoscopic examination showed atrophy in both the antrum and the lesser curvature of the corpus, with atrophic borders (Figure 3A and B). The anterior wall of the corpus displayed elevations and deep fissures presenting as gyrus-like changes (Figure 3C). With adequate air insufflation, the greater curvature of the corpus revealed an alternating pattern of distribution between remnant oxyntic mucosa and atrophic mucosa. The remnant oxyntic mucosa exhibited gyrus-like morphology (Figure 3D and E). Indigo carmine staining showed prominent gyrus-like changes in the greater curvature of the corpus (Figure 3F).
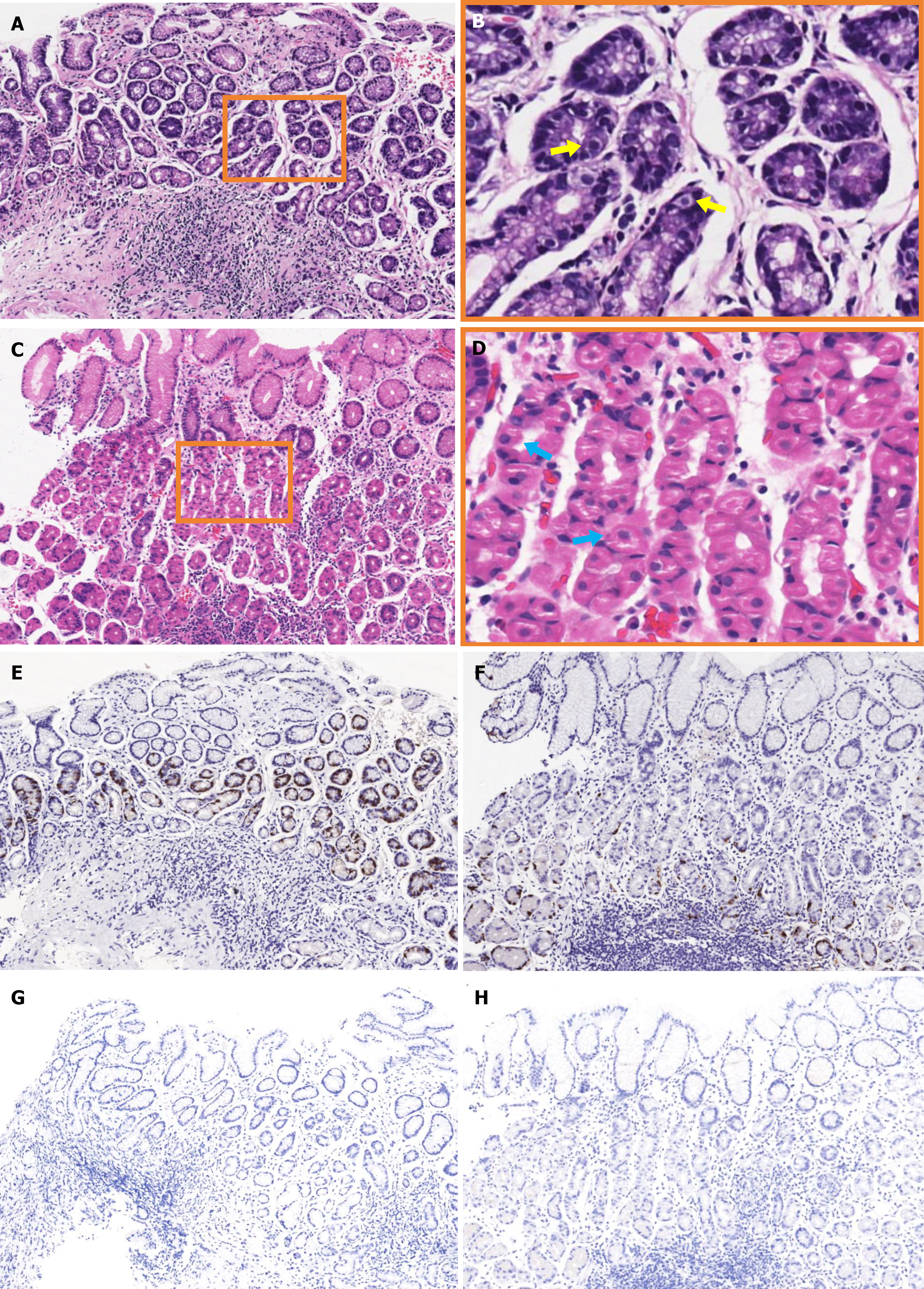
Histopathological examination of specimens from the antrum revealed no significant atrophy, but lymphoid follicles, distorted gastric pits, and hyperplastic G-cells (Figure 4A and B). Specimens from the corpus showed lymphocytic infiltration, with hyperplasia and protrusions into parietal cell lumen (Figure 4C and D). Gastrin staining revealed focal G-cell hyperplasia (Figure 4E), while chromogranin A staining showed linear hyperplasia of enterochromaffin-like cells (Figure 4F). H. pylori staining of antral (Figure 4G) and corporal (Figure 4H) specimens was negative.
Based on their endoscopic findings, histopathological evaluations, serological test results, and clinical histories, both patients were diagnosed with AIG with past H. pylori infection.
The patient was treated with compound digestive enzyme capsules for symptom relief.
No treatment was given.
At a 3-month follow-up, the patient’s symptoms had improved. He was advised to undergo endoscopic follow-up within 3 years.
Endoscopic follow-up was recommended within 3-5 years.
In 2023, Japanese scholars suggested that a diagnosis of AIG should require both positive PCA or IFA results and endoscopic or histological findings consistent with AIG[14]. The AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review (2021) suggests that the early pathological stage of AIG is strongly indicated by lymphocytic infiltration targeting parietal cells, along with a corpus-predominant pattern of inflammation and atrophy, with PCA and IFA serving as helpful adjunctive diagnostic markers[15]. While the Japanese criteria focus on changes in cellular morphology and cell counts, the AGA guidelines emphasize the corpus-predominant atrophy pattern in AIG. However, this pattern may be obscured in cases complicated by past H. pylori infection, particularly in East Asian populations where H. pylori prevalence is high. Given these differences, the Japanese criteria were considered more suitable for our cases. Both patients demonstrated the early pathological features of AIG, including lymphocytic infiltration, parietal cell degeneration, and linear hyperplasia of the enterochromaffin-like cells. Both were also positive for PCA. Given the absence of historic eradication therapy in either patient, we utilized the Japanese diagnostic criteria for previous H. pylori-infected atrophic gastritis[16]. Both cases met the required conditions: (1) No history of H. pylori eradication; (2) Endoscopic atrophy beyond Kimura-Takemoto classification C2; (3) Antral atrophy inconsistent with isolated AIG; and (4) Negative UBT results. Both cases met these criteria, supporting diagnoses of AIG with past H. pylori infection. To exclude other conditions, we performed relevant differential diagnoses. First, the long-term use of proton pump inhibitors can cause endoscopically observable fissure-like changes. However, neither patient had a history of proton pump inhibitor use, and the pathological findings supported an autoimmune-driven mechanism. Second, collagenous gastritis can present with diffusely distributed nodular changes, but its histological hallmark is the presence of subepithelial collagen bands, which were not observed in our patients. Hence, diagnoses of AIG with past H. pylori infection were made.
Identifying past H. pylori infection is essential to the recognition of this type of AIG. If past infection is overlooked, masking of the typical reverse atrophy of AIG by antral atrophy may prevent accurate diagnosis. To determine whether there has been a past H. pylori infection, a thorough medical history is crucial. This should include details of any eradication therapy, its timing, and the treatment outcomes. However, approximately 10% of adult H. pylori infections do not receive eradication therapy. This can be due to unintentional eradication without treatment, unreported successful eradication, or spontaneous bacterial clearance[16]. A comprehensive assessment for past H. pylori infection should combine patient history, endoscopic features, UBT results, and serological test results. Although neither patient in our study had a history of eradication therapy, their negative UBT results combined with the endoscopic findings from the antrum supported diagnoses of past H. pylori infections.
When reverse atrophy is absent, careful assessment of corporal mucosal atrophy becomes critical. Atrophy in the corpus may result from either past H. pylori infection or AIG, which can be difficult to differentiate. Typically, H. pylori-related atrophy progresses from the lesser curvature toward the fundus and greater curvature. It follows the Kimura-Takemoto classification system and presents with an atrophic border[17]. In contrast, AIG-induced atrophy generally lacks a distinct distribution pattern. This is due to the autoimmune system attacking the parietal cells widely distributed throughout the corpus. Consequently, mucosal atrophy beyond the atrophic border is more suggestive of AIG. In our study, we noted prominent deep fissures in the corporal mucosa outside the atrophic border. Miyamoto et al[18] have reported gastric mucosal fissures in patients with long-term proton pump inhibitor use, which they attribute to mild dilation of the oxyntic gland. These are different from the deep fissures observed in our cases, with the latter being more like the furrows formed by gastric mucosa atrophy. We posit that, as the atrophic mucosal regions expand, scattered atrophic areas gradually emerge.
Evaluation of non-atrophic mucosa is also essential to the identification of atypical AIG. In early AIG, endoscopic examination of the non-atrophic mucosa shows various characteristic changes, including swollen gastric pits, a mosaic pattern, pseudopolyp-like reddish nodules, and salmon-roe-like nodular lesions[19]. In advanced AIG, there are non-atrophic mucosa, known as remnant oxyntic mucosa, with less severe pathological changes than the adjacent atrophic areas[20]. Regenerative fundic glands resulting from past H. pylori infection can appear similar to remnant oxyntic mucosa, although their borders are often indistinct. A Japanese multicenter study identified five remnant oxyntic mucosa morphologies: Flat localized, pseudopolypoid, island, widespread, and granular[21]. However, these were proposed based on AIG with well-established atrophy, while descriptions of earlier AIG stages remain limited. In our cases, we observed only fissure-like atrophy beyond the atrophic border, with non-atrophic mucosa showing mild swelling.
Through the integration of endoscopic findings from both atrophic and non-atrophic mucosa, we have identified a novel morphological feature of AIG in the form of gyrus-like changes in the corporal mucosa. These may represent transitional morphology, indicating progression from early to more advanced stages of AIG. The pattern is characterized by mildly elevated mucosa between deep fissures that resemble the cerebral sulcus and the cerebral gyrus. The feature becomes particularly prominent with indigo carmine staining due to the dye’s tendency to accumulate in fissured areas. Some collecting venules remain visible on the mucosal surface within these gyrus-like regions, suggesting the preservation of the fundamental structure of the fundic glands. Histological examination confirmed early pathological changes of AIG in these regions, including lymphocytic infiltration and pseudohypertrophy of parietal cells. This supported the possibility that these regions were remnant oxyntic mucosa.
To date, no consensus has been reached on the role of H. pylori infection in the pathogenesis of AIG. Some studies suggest that H. pylori may trigger an autoimmune response through molecular mimicry or cross-reactivity[22,23]. The β-subunit of H. pylori is highly homologous with the H+/K+-ATPase in parietal cells so may induce CD4+ T cells to attack gastric self-antigens, contributing to the development of AIG[24]. A case report has described AIG onset following the spontaneous clearance of H. pylori infection, indicating the possible contribution of H. pylori to the initiation of autoimmune processes[25]. However, another study found no significant difference in the rate of PCA positivity between H. pylori-positive and H. pylori-negative individuals[26]. AIG is also frequently reported in H. pylori-negative children[27], indicating that H. pylori is not a prerequisite for AIG. The impact of H. pylori eradication therapy on the disease course of AIG is also controversial. One retrospective study reported clinical improvements in some patients with active pre-atrophic AIG after H. pylori eradication[28], while another noted accelerated AIG progression following eradication[29]. Animal studies suggest that H. pylori infection may promote a Th2 response and upregulate TGF-β expression, thereby suppressing Th1-driven autoimmunity and exerting a protective effect against AIG progression[30]. Thus, H. pylori may act as both a trigger and a modulator of immune responses at different stages of AIG.
Combined with the above, we propose the following mechanism behind the formation of the gyrus-like changes observed in our patients. When H. pylori infection involves the gastric corpus, AIG may be induced through antigenic cross-reactivity. At this stage, H. pylori-driven inflammation dominates, suppressing AIG-related autoimmunity and resulting in well-defined atrophic borders. After the spontaneous clearance of H. pylori, the AIG-related autoimmunity become predominant. Any focal epithelial injury previously induced by H. pylori may enhance antigen exposure, promoting localized immune attacks and resulting in earlier glandular destruction, which manifests as deep fissures. Meanwhile, other areas may begin to show early features of AIG, such as pseudohypertrophy of parietal cells and hyperplasia of enterochromaffin-like cells, with relative preservation of the glandular architecture. This then manifests as slightly elevated gyrus-like changes. Taken together, the gyrus-like changes may reflect a shift in the immunological focus from H. pylori-related inflammation to an autoimmune response that is influenced by factors such as H. pylori clearance, the degree of antigen exposure, and the disease stage.
The latest Japanese diagnostic criteria for AIG recommend obtaining biopsy specimens from the upper greater curvature of the corpus and the greater curvature of the pylorus[14]. We followed this guideline in both of our cases and obtained biopsies from the recommended sites. In case 1, we performed an additional biopsy targeting the gyrus-like elevations, which appeared distinct from the surrounding atrophic areas. This decision was based on the concern that, in patients with past H. pylori infection, sampling only from atrophic areas could obscure the true etiology, as these areas have likely been affected by both H. pylori and AIG. In contrast, the non-atrophic regions are less likely to have been affected by past H. pylori damage, so are more likely to reflect early, localized features of AIG. This targeted sampling of gyrus-like areas successfully revealed early histological evidence of AIG in our cases, supporting the clinical value of this approach. We believe that targeted biopsies from non-atrophic regions can offer greater diagnostic clarity in cases of AIG with past H. pylori infection. This may be a useful supplement to existing guidelines.
This study was a case report with a limited sample size, did not include comparisons with pure AIG cases, and had an insufficient follow-up period. Therefore, we can draw no definitive conclusions regarding the generalizability and specificity of the gyrus-like changes. Nevertheless, despite these limitations, we believe these changes to be a potential means of recognizing AIG when its typical presentation is masked due to past H. pylori infection. Further studies with larger cohorts that include pure AIG patients and longitudinal follow-up data are needed to validate the diagnostic significance and clinical utility of our findings.
Based on two cases of AIG with past H. pylori infection, this study proposes an endoscopic evaluation and biopsy strategy when the reverse atrophy is unreliable. After confirming past H. pylori infection status, careful assessment of the corporal mucosa is essential, especially in regions beyond the atrophic border. We introduce gyrus-like changes as a potential endoscopic feature of AIG with past H. pylori infection. These changes can be histologically confirmed through targeted biopsy analysis. The findings from these two cases offer preliminary insights that warrant validation by larger studies.
| 1. | Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Whittingham S, Mackay IR. Autoimmune gastritis: historical antecedents, outstanding discoveries, and unresolved problems. Int Rev Immunol. 2005;24:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Soykan İ, Er RE, Baykara Y, Kalkan C. Unraveling the Mysteries of Autoimmune Gastritis. Turk J Gastroenterol. 2024;36:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Coati I, Fassan M, Farinati F, Graham DY, Genta RM, Rugge M. Autoimmune gastritis: Pathologist's viewpoint. World J Gastroenterol. 2015;21:12179-12189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (2)] |
| 5. | Rustgi SD, Bijlani P, Shah SC. Autoimmune gastritis, with or without pernicious anemia: epidemiology, risk factors, and clinical management. Therap Adv Gastroenterol. 2021;14:17562848211038771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Notsu T, Adachi K, Mishiro T, Fujihara H, Toda T, Takaki S, Kinoshita Y. Prevalence of Autoimmune Gastritis in Individuals Undergoing Medical Checkups in Japan. Intern Med. 2019;58:1817-1823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Zhang H, Jin Z, Cui R, Ding S, Huang Y, Zhou L. Autoimmune metaplastic atrophic gastritis in chinese: a study of 320 patients at a large tertiary medical center. Scand J Gastroenterol. 2017;52:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Autoimmune gastritis. Nat Rev Dis Primers. 2020;6:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Castellana C, Eusebi LH, Dajti E, Iascone V, Vestito A, Fusaroli P, Fuccio L, D'Errico A, Zagari RM. Autoimmune Atrophic Gastritis: A Clinical Review. Cancers (Basel). 2024;16:1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Furuta T, Baba S, Yamade M, Uotani T, Kagami T, Suzuki T, Tani S, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. Aliment Pharmacol Ther. 2018;48:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 11. | Arai J, Niikura R, Hayakawa Y, Hirata Y, Ushiku T, Fujishiro M. Autoimmune gastritis may be less susceptible to cancer development than Helicobacter pylori-related gastritis based on histological analysis. Gut. 2024;73:1037-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Nishizawa T, Yoshida S, Watanabe H, Toyoshima A, Kataoka Y, Takahashi Y, Kanazawa T, Ebinuma H, Suzuki H, Koike K, Toyoshima O. Clue of Diagnosis for Autoimmune Gastritis. Digestion. 2021;102:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Bloomquist MS, Powell J 3rd, Masand RP, Dhall D, Karamchandani DM, Jain S. Lack of uniformity in reporting autoimmune gastritis among a diverse group of pathologists. Ann Diagn Pathol. 2022;56:151840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kamada T, Watanabe H, Furuta T, Terao S, Maruyama Y, Kawachi H, Kushima R, Chiba T, Haruma K. Diagnostic criteria and endoscopic and histological findings of autoimmune gastritis in Japan. J Gastroenterol. 2023;58:185-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 16. | Kishikawa H, Ojiro K, Nakamura K, Katayama T, Arahata K, Takarabe S, Miura S, Kanai T, Nishida J. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter. 2020;25:e12669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Quach DT, Hiyama T. Assessment of Endoscopic Gastric Atrophy according to the Kimura-Takemoto Classification and Its Potential Application in Daily Practice. Clin Endosc. 2019;52:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Miyamoto S, Kato M, Tsuda M, Matsuda K, Muranaka T, Abiko S, Ono M, Mizushima T, Omori S, Yamamoto K, Mabe K, Ono S, Kudo T, Shimizu Y, Sakamoto N. Gastric mucosal cracked and cobblestone-like changes resulting from proton pump inhibitor use. Dig Endosc. 2017;29:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Kishino M, Nonaka K. Endoscopic Features of Autoimmune Gastritis: Focus on Typical Images and Early Images. J Clin Med. 2022;11:3523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Maruyama Y, Yoshii S, Terai T. [Endoscopic diagnosis of autoimmune gastritis]. Nihon Shokakibyo Gakkai Zasshi. 2022;119:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Terao S, Suzuki S, Yaita H, Kurahara K, Shunto J, Furuta T, Maruyama Y, Ito M, Kamada T, Aoki R, Inoue K, Manabe N, Haruma K. Multicenter study of autoimmune gastritis in Japan: Clinical and endoscopic characteristics. Dig Endosc. 2020;32:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 22. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D'Elios MM, Del Prete G. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Toh BH, Chan J, Kyaw T, Alderuccio F. Cutting edge issues in autoimmune gastritis. Clin Rev Allergy Immunol. 2012;42:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | D'Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Ihara T, Ihara N, Kushima R. Autoimmune Gastritis with a Long-term Course of Type B Gastritis: A Report of Two Cases. Intern Med. 2023;62:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Erdoğan A, Yilmaz U. Is there a relationship between Helicobacter pylori and gastric autoimmunity? Turk J Gastroenterol. 2011;22:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Demir AM, Berberoğlu Ateş B, Hızal G, Yaman A, Tuna Kırsaçlıoğlu C, Oğuz AS, Karakuş E, Yaralı N, Özbek NY. Autoimmune atrophic gastritis: The role of Helicobacter pylori infection in children. Helicobacter. 2020;25:e12716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Müller H, Rappel S, Wündisch T, Bayerdörffer E, Stolte M. Healing of active, non-atrophic autoimmune gastritis by H. pylori eradication. Digestion. 2001;64:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Ihara T, Ihara N, Kushima R, Haruma K. Rapid Progression of Autoimmune Gastritis after Helicobacter pylori Eradication Therapy. Intern Med. 2023;62:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Ohana M, Okazaki K, Oshima C, Kawasaki K, Fukui T, Tamaki H, Matsuura M, Asada M, Nishi T, Uchida K, Uose S, Nakase H, Iwano M, Matsushima Y, Hiai H, Chiba T. Inhibitory effects of Helicobacter pylori infection on murine autoimmune gastritis. Gut. 2003;52:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/