Published online Sep 16, 2025. doi: 10.4253/wjge.v17.i9.110424
Revised: July 2, 2025
Accepted: August 5, 2025
Published online: September 16, 2025
Processing time: 97 Days and 6.9 Hours
Pancreatic fluid leakage is a rare complication of pancreatic cancer and often requires drainage when conservative therapy fails. Endoscopic, percutaneous, and surgical drainage are options. Minimally invasive endoscopic procedures are generally considered the first-line treatment, with either a transpapillary approach or an endoscopic ultrasound-guided transmural approach selected depending on the case. Various dilators are used to dilate tracts to the leakage site. However, reports of dilation through a rigid trans-tumoral tract using a drill dilator remain extremely rare.
A 74-year-old woman with pancreatic body and tail cancer developed fever and left-sided chest pain after multiple courses of chemotherapy. Computed tomo
Even in rigid trans-tumoral tracts, the use of a drill dilator can facilitate successful tract expansion, enabling effective drainage.
Core Tip: We encountered a case of pancreatic fluid leakage with reactive left pleural effusion associated with pancreatic cancer. The guidewire traversed a relatively long distance through the tumor to reach the leakage site. Expanding this rigid tract was challenging with conventional dilators but was successfully achieved using a drill dilator. Placement of a drainage tube led to the resolution of both the pancreatic fluid leakage and reactive pleural effusion. When conventional or balloon dilators fail to expand the stenotic tract, a drill dilator may facilitate successful passage through the stricture.
- Citation: Makazu M, Koizumi K, Kubota J, Kimura K, Masuda S. Transpapillary drainage of pancreatic fluid leakage via a rigid trans-tumoral tract using a drill dilator: A case report. World J Gastrointest Endosc 2025; 17(9): 110424
- URL: https://www.wjgnet.com/1948-5190/full/v17/i9/110424.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i9.110424
Pancreatic fluid leakage (PFL) is a pathological condition in which pancreatic fluid extravasates outside the pancreatic duct due to rupture of the pancreatic duct or pancreatic cysts, owing to various factors. Common causes include surgical procedures[1], pancreatitis[2], and trauma[3], although pancreatic cancer can also be a causative factor[4].
When PFL does not improve with conservative treatments, such as bowel rest and pharmacotherapy, pancreatic fluid drainage becomes necessary. The available drainage methods include endoscopic, percutaneous, and surgical approaches. Endoscopic drainage methods involve transpapillary approaches via endoscopic retrograde cholangiopancreatography (ERCP) and transmural drainage using endoscopic ultrasonography (EUS).
Herein, we describe a case of PFL with a reactive left pleural effusion that developed during chemotherapy for pancreatic cancer. The PFL and pleural effusion improved with transpapillary pancreatic duct drainage via a rigid trans-tumoral tract. Although the tract could not be dilated with conventional dilators, successful dilation was achieved using a drill dilator.
A 74-year-old woman presented to the emergency department with fever and left-sided chest pain.
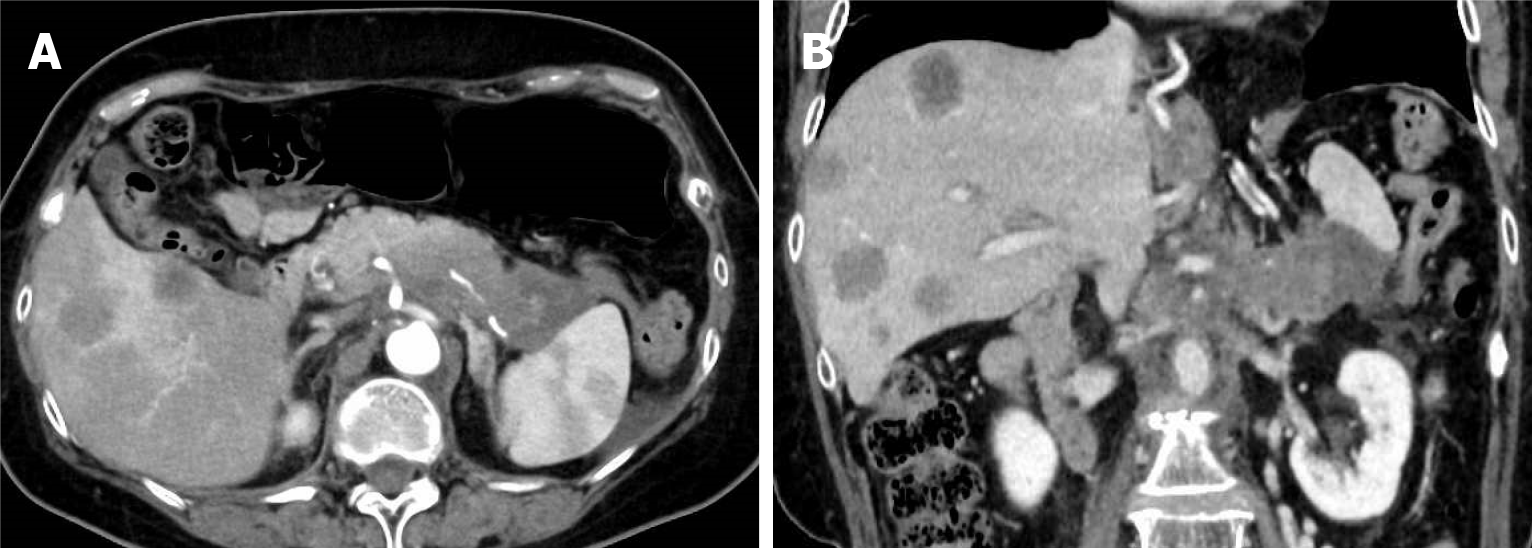
The patient had first presented to another hospital seven months prior with abdominal distension and epigastric pain. Abdominal non-contrast computed tomography (CT) had revealed suspected pancreatic body and tail cancer with multiple liver metastases, and she was referred to our hospital for further treatment. Dynamic contrast-enhanced CT demonstrated an irregularly shaped 7.5-cm tumor extending from the pancreatic body to the tail, along with multiple hepatic masses (Figure 1).
EUS was performed; however, due to a severe hiatal hernia, the stomach was almost entirely located within the thoracic cavity, making transgastric puncture of the pancreatic mass infeasible. Instead, EUS-guided fine-needle aspiration of the hepatic lesion was performed via the duodenum, and the pathological findings confirmed adenocarcinoma.
The patient was diagnosed with pancreatic cancer. Due to the presence of liver metastases, the disease was classified as stage IV according to the 8th edition of the TNM classification of malignant tumours of the Union for International Cancer Control classification[5]. Chemotherapy with gemcitabine and nab-paclitaxel was initiated. After two cycles, the patient developed bilateral lower extremity edema, which impaired her ability to walk. Consequently, chemotherapy was switched to nanoliposomal irinotecan (80 mg/m²) with fluorouracil and folinic acid every 2 weeks. After four courses, CT showed a reduction in the primary pancreatic lesion and liver metastases. However, after an additional four courses, CT revealed mild enlargement of the primary lesion, along with fluid accumulation along the pancreatic tail and spleen, which was partially encapsulated. A small amount of left pleural effusion was also observed.
Twelve days after the CT scan, the patient presented to the emergency department with fever and left-sided chest pain.
Her medical history included hypertension and an appendectomy.
The patient denied any family history of malignant tumors.
Body temperature, 37.7 °C; blood pressure, 156/100 mmHg; heart rate, 80 beats/min; respiratory rate, 20 breaths/min. Physical examination revealed moderate tenderness in the left upper quadrant without rebound tenderness.
On the day of admission, the white blood cell count was 5.4 × 109/L, which was within the normal range. The C-reactive protein level was elevated at 55.97 mg/L (reference range: 0-1.4 mg/L). The serum amylase level was also elevated at 103 U/L (reference range: 17-50). Pleural fluid analysis revealed a mildly elevated amylase level (241 U/L).
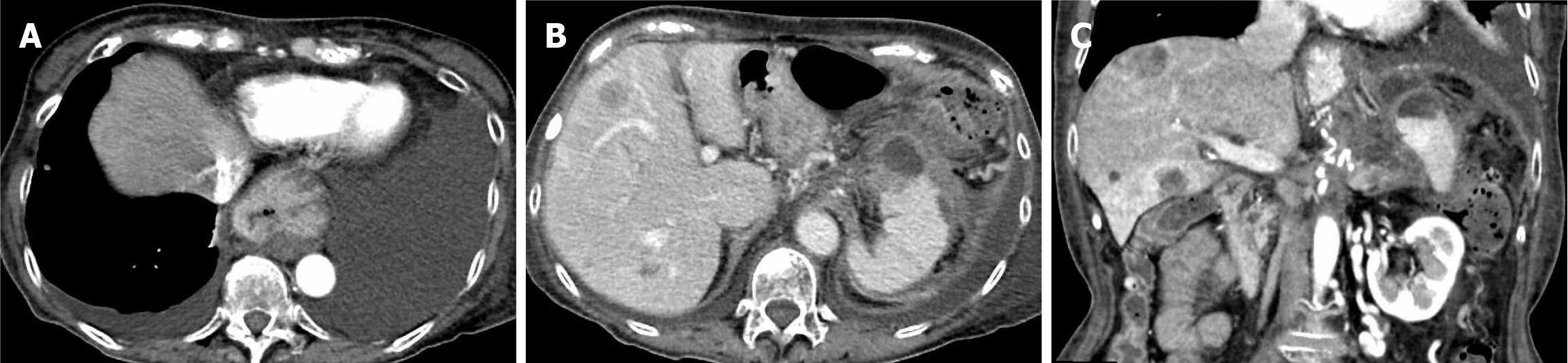
Dynamic pancreatic CT showed worsening fluid accumulation along the pancreatic tail and spleen (Figure 2) and an increase in the left pleural effusion.
The patient was diagnosed with PFL associated with pancreatic cancer and admitted. Given that the pleural effusion was on the same side as the pancreatic leakage and the amylase levels were only mildly elevated, she was ultimately diagnosed with reactive pleural effusion due to inflammatory changes rather than direct pancreatic fistulization.
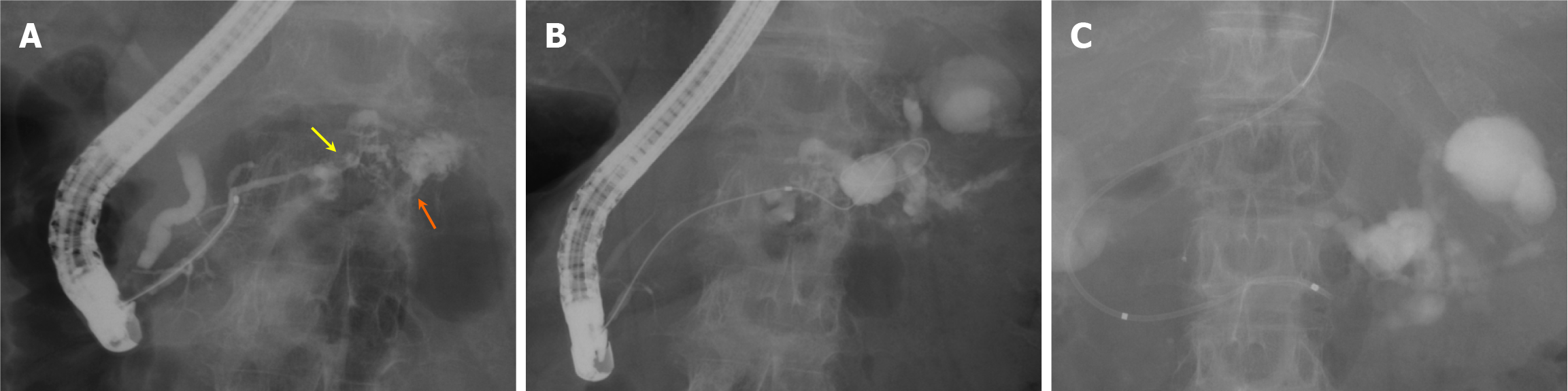
We decided to attempt minimally invasive endoscopic drainage for the pancreatic fluid leakage. However, because the leakage site was small and considered difficult to puncture, and due to the presence of a severe hiatal hernia, endoscopic ultrasound-guided transmural drainage was deemed challenging. Therefore, we selected ERCP as the drainage approach. On the second day of admission, the first ERCP was performed (Figure 3) using a TJF-Q290V (Olympus Optical Co., Ltd., Tokyo, Japan) and an ERCP catheter (MTW Co., Düsseldorf, Germany). ERCP revealed irregular stenosis of the main pancreatic duct in the pancreatic body. Distal to the stenosis, the main ductal structure was nearly obliterated by the tumor, and the contrast medium leaked into the area of PFL through several fine, disrupted ductal structures (Figure 3A). Guidewire insertion into the leakage site was challenging, even with multiple guidewires; eventually, the guidewire was successfully advanced through an extremely fine tract, which was not the main contrast-filled route (Figure 3B). Attempts to dilate this trans-tumoral passage using bougie and balloon dilators and an ultrathin catheter were unsuccessful.
At this stage, the use of a drill dilator was considered. However, whether the accessed site represented the original or newly created leakage site was unclear. A 5-French (Fr) endoscopic nasopancreatic drainage (ENPD) tube (Hanaco Medical Co., Saitama, Japan) was placed in the main pancreatic duct, proximal to the stenosis (Figure 3C).
A follow-up non-contrast CT scan confirmed contrast medium accumulation only at the preexisting leakage site, with no new extravasation.
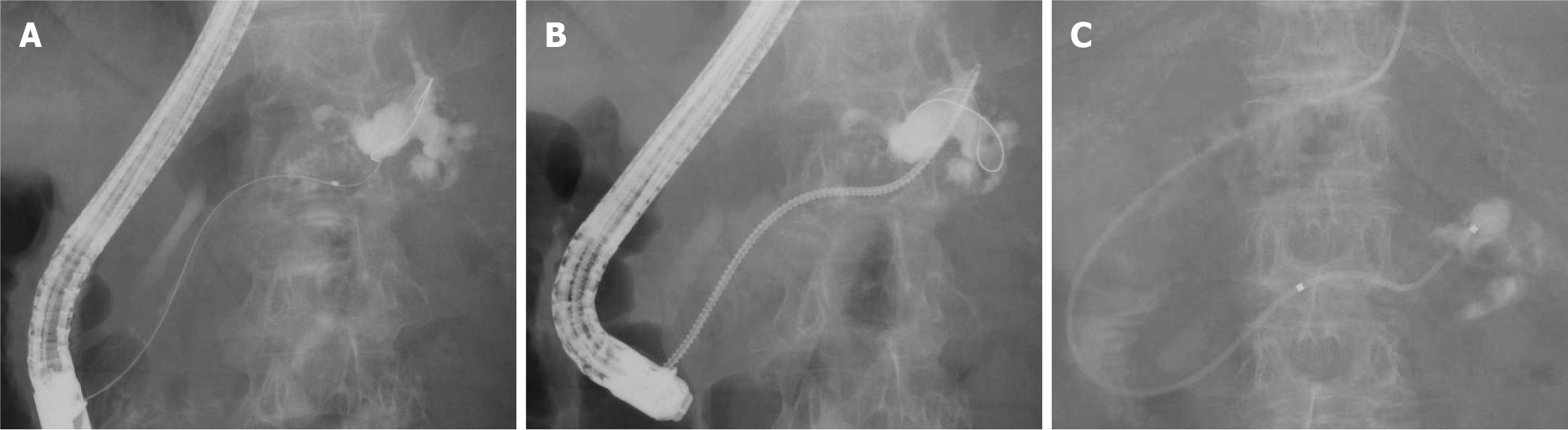
On the third day of admission, the patient underwent thoracentesis, and 700 mL of clear yellow pleural fluid was drained. On the fourth day, a second ERCP was performed (Figure 4). A guidewire was successfully advanced through the fine tract, which was presumed to be trans-tumoral, and reached the leakage site (Figure 4A). The tract was successfully dilated using a drill dilator (Tornus ES for a 0.025-inch guidewire; Asahi Intecc, Aichi, Japan) (Figure 4B). A 5-Fr ENPD tube was placed at the leakage site (Figure 4C). Oral intake was resumed on the following day, and the ENPD drained approximately 60-90 mL per day of the pancreatic fluid. Laboratory findings showed gradual improvement in the inflammatory markers.
On the ninth day of admission (5 days after the second ENPD placement), a follow-up plain CT scan showed a reduction in the PFL. The left pleural effusion persisted but did not increase.
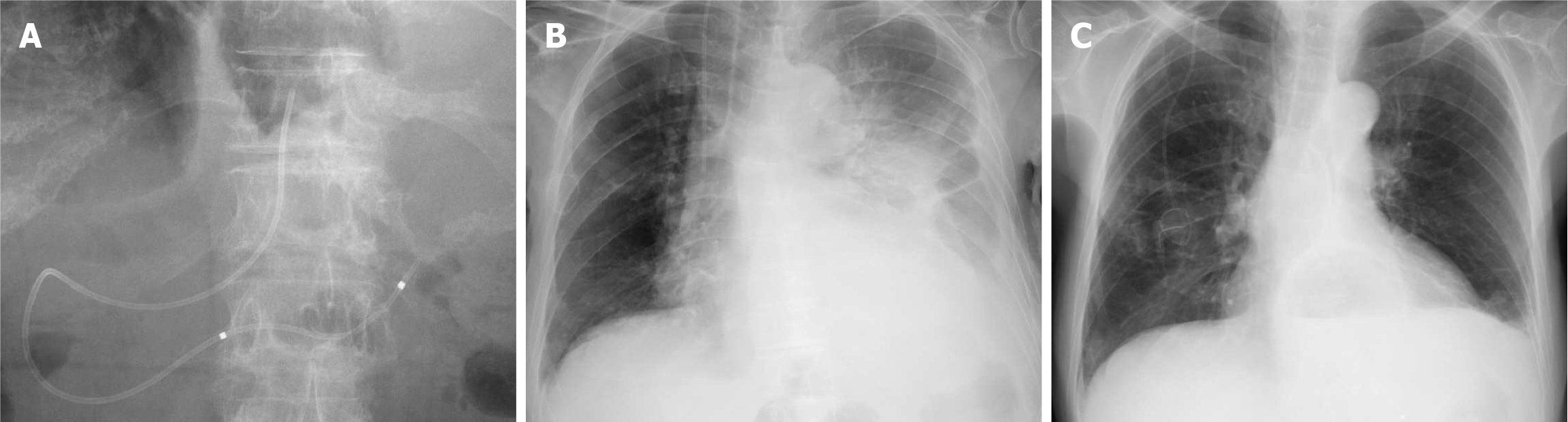
On day 11, internalization of the ENPD tube was performed by upper gastrointestinal endoscopy (Figure 5A). The endoscope was advanced into the stomach, and the ENPD tube was cut inside the stomach using a Loop Cutter (Olympus, Tokyo, Japan), allowing free drainage of the clear pancreatic fluid.
On day 14 of hospitalization, the patient was discharged. A chest radiograph obtained 16 days post-discharge (26 days after the second ENPD placement) showed a marked reduction in left pleural effusion (Figure 5B and C). Subsequently, chemotherapy was resumed.
PFL is characterized by the extravasation of pancreatic juice due to the disruption of the pancreatic duct or cysts. Common causes include surgical interventions, pancreatitis, and trauma. PFL associated with pancreatic cancer is rare. Ota et al[6] reported a case in which PFL developed a few days after the placement of a fully covered self-expandable metallic stent (SEMS) for obstructive jaundice caused by pancreatic head cancer. They hypothesized that compression of the main pancreatic duct orifice by the SEMS led to pancreatic duct disruption. In the present case, PFL developed during chemotherapy for pancreatic cancer at the time of mild tumor progression. Cancer progression was presumed to result in pancreatic ductal stenosis and disruption, leading to a PFL.
The treatment of PFL typically begins with conservative management, including fasting and intravenous fluid administration. If conservative treatment fails, endoscopic intervention is the next step, followed by percutaneous drainage or surgery if necessary.
Endoscopic treatment of PFL includes transpapillary drainage via ERCP and transmural drainage via EUS[2]. Transpapillary drainage is the first-line approach because it involves placing a drainage tube in an existing pancreatic duct, which reduces the risk of peritonitis or perforation compared with transmural drainage[7]. However, passing a catheter through a stenotic pancreatic duct to the leakage site can be challenging. Additionally, transpapillary procedures carry the risk of post-ERCP pancreatitis[8].
EUS-guided transmural drainage has advanced in recent years and is considered an alternative for cases where transpapillary access is difficult[9,10]. Given that this technique involves creating a new fistula, the risk of peritonitis or gastrointestinal perforation is higher than that of transpapillary drainage[11]. However, when the fluid collection is of sufficient size, high technical success rates have been reported[12].
When endoscopic drainage is technically unfeasible, percutaneous drainage or surgery may be considered. However, percutaneous drainage is known to negatively affect the patient’s quality of life and carries a higher risk of developing a non-healing fistula[13].
At our institution, we primarily choose ERCP for patients meeting any of the following criteria: (1) Imaging suggests communication between the main pancreatic duct and the pancreatic fluid leakage, and the leakage site appears to be accessible via the duct; (2) Placement of a drainage tube distal to a main pancreatic duct stricture is expected to be effective even if the tip does not directly reach the leakage site; (3) Communication with the pancreatic duct cannot be confirmed on imaging, but the location and size of the PFL make EUS-guided puncture technically challenging; and (4) PFL is suspected, but the diagnosis is unclear, and it is desirable to perform diagnostic pancreatography concurrently.
In cases not meeting the above criteria—or even if they do—when the leakage is large and EUS-guided drainage is considered safer and technically easier, EUS-guided drainage may be considered as the initial approach. If both ERCP and EUS-guided drainage fail, percutaneous drainage is considered.
In the present case, although pancreatic fluid leakage distal to the main pancreatic duct stricture was suspected, its communication with the duct could not be clearly confirmed on CT. Therefore, ERCP was selected as the first-line approach for both diagnostic and therapeutic purposes. Additionally, as the leakage site was small and considered difficult to puncture, and due to the presence of a severe hiatal hernia, endoscopic ultrasound-guided transmural drainage was unfeasible.
Dilators or balloons are commonly used to expand a stenotic pancreatic duct during drainage. Dilators include non-electrified mechanical dilators, balloon dilators, and electrosurgical dilators[14-16]. In our case, during the initial ERCP, the guidewire was successfully advanced to the leakage site; however, placement of a drainage tube could not be achieved. As the distal pancreatic duct was nearly obliterated, a guidewire was inserted through an extremely fine tract traversing the tumor tissue. This tract was rigid within the tumor, making dilation with conventional devices, e.g., mechanical and balloon dilators, particularly difficult, prompting the consideration of a drill dilator. However, it was initially unclear whether the contrast extravasation site was a true or newly created leakage point. Therefore, during the first ERCP, an ENPD tube was placed in the main pancreatic duct on the papillary side of the stenosis. Follow-up CT confirmed that contrast accumulation was limited to the preexisting leakage site. During the second ERCP, the drill dilator successfully expanded the tract, allowing ENPD placement at the leakage site. The drill dilator has a drill-shaped design that advances via rotational force[17]. Shiomi et al[18] reported a case in which a drill dilator was effective in dilating the main pancreatic duct stricture caused by pancreatic stones in acute pancreatitis. Hara et al[19] described the use of a drill dilator for pancreatic duct dilation during EUS-guided pancreatic duct drainage. In the present case, the stricture was not located within the benign tissue, as described in those reports, but rather within a narrow tract traversing the cancerous pancreatic tissue. Nevertheless, even such a rigid, tumor-involved tract can be successfully dilated using a drill dilator.
Our case also involved a left pleural effusion secondary to the PFL. Pancreatic disease-related pleural effusions can be classified into two types[20]. The first type is reactive pleural effusion, which manifests with normal pleural fluid amylase levels[21]. The second type is pancreatic pleural effusion, characterized by direct leakage of pancreatic juice into the pleural cavity, resulting in large effusions with markedly elevated amylase levels, often exceeding 1000 U/L[22]. Although chronic pancreatitis is the most common cause, pancreatic malignancy-associated pleural effusions have been reported in a few cases[23-26]. In our case, the pleural fluid amylase levels were only mildly elevated, and ERCP did not reveal contrast leakage into the pleural cavity, suggesting that the effusion was reactive. As the PFL improved, the inflammation subsided, leading to resolution of the pleural effusion.
We encountered a case of PFL with reactive left pleural effusion associated with pancreatic cancer. The guidewire traversed a relatively long distance through the tumor to reach the leakage site. Expanding this rigid tract was challenging with conventional dilators but was successfully achieved using a drill dilator. Placement of a drainage tube led to the resolution of both the PFL and reactive pleural effusion. Effective drainage of the PFL improves the patient’s quality of life and allows for the continuation of cancer therapy. In conclusion, when conventional or balloon dilators fail to expand the stenotic tract, a drill dilator may facilitate successful passage through the stricture.
| 1. | Nahm CB, Connor SJ, Samra JS, Mittal A. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol. 2018;11:105-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Larsen M, Kozarek R. Management of pancreatic ductal leaks and fistulae. J Gastroenterol Hepatol. 2014;29:1360-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Kao LS, Bulger EM, Parks DL, Byrd GF, Jurkovich GJ. Predictors of morbidity after traumatic pancreatic injury. J Trauma. 2003;55:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Razjouyan H, Maranki JL. Endoscopic Retrograde Cholangiopancreatography for the Management of Pancreatic Duct Leaks and Fistulas. Gastrointest Endosc Clin N Am. 2024;34:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | TNM Classification of Malignant Tumours, 8th ed. Edited by James D. Brierley, Mary K. Gospodarowicz, Christian Wittekind. Wiley-Blackwell, 2017. |
| 6. | Ota S, Shiomi H, Nakano R, Nishimura T, Enomoto H, Iijima H. A case of delayed pancreatic fistula after covered self-expandable metallic stent deployment for pancreatic head cancer. Clin J Gastroenterol. 2023;16:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Tanaka T, Kuroki T, Kitasato A, Adachi T, Ono S, Hirabaru M, Matsushima H, Takatsuki M, Eguchi S. Endoscopic transpapillary pancreatic stenting for internal pancreatic fistula with the disruption of the pancreatic ductal system. Pancreatology. 2013;13:621-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 798] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 9. | Tyberg A, Sharaiha RZ, Kedia P, Kumta N, Gaidhane M, Artifon E, Giovannini M, Kahaleh M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Nakai Y. Technical tips for endoscopic ultrasound-guided pancreatic duct access and drainage. Int J Gastrointest Interv. 2020;9:154-159. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Will U, Fueldner F, Buechner T, Meyer F. Endoscopic Ultrasonography-Guided Drainage of the Pancreatic Duct (EUS-PD)-Indications and Results with a Literature Review. J Clin Med. 2024;13:7709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Bhakta D, de Latour R, Khanna L. Management of pancreatic fluid collections. Transl Gastroenterol Hepatol. 2022;7:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Mohan BP, Shakhatreh M, Dugyala S, Geedigunta V, Gadalay A, Pahal P, Ponnada S, Nagaraj K, Asokkumar R, Adler DG. EUS versus percutaneous management of postoperative pancreatic fluid collection: A systematic review and meta-analysis. Endosc Ultrasound. 2019;8:298-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Imoto A, Ogura T, Higuchi K. Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: Techniques and Literature Review of Transmural Stenting. Clin Endosc. 2020;53:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Honjo M, Itoi T, Tsuchiya T, Tanaka R, Tonozuka R, Mukai S, Sofuni A, Nagakawa Y, Iwasaki H, Kanai T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc Ultrasound. 2018;7:376-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Ogura T, Nishioka N, Yamada M, Ueshima K, Higuchi K. Endoscopic ultrasound-guided pancreatic duct drainage using a fine-gauge balloon catheter. Endoscopy. 2019;51:E145-E146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Nakano S, Minaga K, Matsuyama K, Matsumoto H, Uenoyama Y, Yamashita Y. Successful dilation of a hard biliary stricture associated with primary sclerosing cholangitis using a novel drill dilator. Endosc Int Open. 2023;11:E568-E570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Shiomi H, Nakano R, Ota S, Nishimura T, Enomoto H, Iijima H. Recanalization using a novel drill-shaped dilator for a severe pancreatic duct stricture and impacted pancreatic duct stone. Endoscopy. 2023;55:E351-E353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Hara K, Okuno N, Haba S, Kuwahara T, Kuraishi Y, Yanaidani T, Fumihara D, Yamada M, Yasuda T, Ishikawa S. Utility of a novel drill dilator for easier EUS-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci. 2022;29:e91-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Ranjan P, Bansal R, Sachdeva M, Kumar M. Pancreaticopleural fistula: Report of two cases and review of literature. Journal of Digestive Endoscopy. 2013;04:123-127. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Branca P, Rodriguez RM, Rogers JT, Ayo DS, Moyers JP, Light RW. Routine measurement of pleural fluid amylase is not indicated. Arch Intern Med. 2001;161:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Aswani Y, Hira P. Pancreaticopleural fistula: a review. JOP. 2015;16:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 23. | Sankarankutty M, Baird JL, Dowse JA, Morris JS. Adenocarcinoma of the pancreas with massive pleural effusion. Br J Clin Pract. 1978;32:294-297. [PubMed] |
| 24. | England DW, Kurrein F, Jones EL, Windsor CW. Pancreatic pleural effusion associated with oncocytic carcinoma of the pancreas. Postgrad Med J. 1988;64:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Shimaoka S, Tashiro K, Matsuda A, Nioh T, Niihara T, Ohi H, Yamasuji T, Nishimata Y, Nishimata H, Suenaga T, Kajiya Y. Minute carcinoma of the pancreas presenting as pancreatic pleural effusion. J Gastroenterol. 2003;38:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Shiraishi J, Yugawa K, Nagata S, Maeda T. Pancreatic-pleural fistula from tail pseudocyst in a patient with pancreatic head cancer: a case report. Int Cancer Conf J. 2022;11:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/