Published online Aug 16, 2025. doi: 10.4253/wjge.v17.i8.107617
Revised: May 6, 2025
Accepted: June 26, 2025
Published online: August 16, 2025
Processing time: 141 Days and 15 Hours
Laparoscopic and endoscopic cooperative surgery (LECS) is a hybrid minimally invasive technique originally developed for treatment of gastric submucosal tumors. Several modifications of LECS—including inverted LECS, non-exposed endoscopic wall-inversion surgery, and closed LECS have evolved over a period of time to address the earlier concerns about peritoneal contamination and tumor seeding. These innovations have led to the application of combined laparoendoscopic techniques to several gastrointestinal (GI) lesions such as the duodenum, colon, and rectum. This minireview explores the evolution, current applications, and future potential of laparoendoscopic surgery in GI diseases.
Core Tip: Laparoscopic and endoscopic cooperative surgery (LECS) is a hybrid procedure combining both the laparoscope and endoscope, classically described for gastric submucosal tumor excision. With the advancing tools and technology, its appli
- Citation: Parikh KS, Kaw P, Kumar A. Laparoendoscopic surgery in gastrointestinal diseases: Status and future perspectives. World J Gastrointest Endosc 2025; 17(8): 107617
- URL: https://www.wjgnet.com/1948-5190/full/v17/i8/107617.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i8.107617
Historically, surgical management of luminal gastrointestinal (GI) tumors was done using an open approach with large incisions. The earliest documentation of minimally invasive surgeries (MIS) was first described by Hippocrates when he used an instrument akin to the present-day endoscope to visualize the rectum (460-375BC)[1]. Major technological advancement in instrumentation occurred after the invention of light bulb by Edison in 1880[2]. Surgical practice transformed gradually over the next few decades with this improvement along with the development of automated carbon dioxide insufflators and thermocoagulation energy devices[3,4]. In 1983, Semm[5] performed the first laparoscopic appendectomy, following which Philipe Mouret, in 1987, performed a laparoscopic cholecystectomy using the first ever endoscope enabled with on-screen video projection[6-8]. Over the years, technology has rapidly advanced and surgery using luminal endoscopy, laparoscopy or robotic platforms for resecting luminal GI tumours can be performed with similar efficiency as open surgery with identical oncological outcomes and similar or even less procedure-related morbidity[9,10].
MIS has revolutionized and set a new standard for surgical practice globally. Superior vision, early post operative recovery, reduced pain and shorter hospital stays are considered the key advantages over traditional open surgery[11]. When this technique was applied to luminal GI tumors, early restoration of GI function with similar oncological results as open surgery could be achieved without any added morbidity[9,10]. Luminal GI tumors have been treated either using the laparoscopic transabdominal or endoscopic intraluminal approach depending on the tumor type, size and stage of the disease. Large bulky non epithelial tumors and locally advanced tumors are usually managed with a laparoscopic approach whereas small and superficial tumors may be managed using either of the two approaches, each having its own advantages and limitations[12]. One key limitation of both techniques when individually used was that the resection was performed without visualization of the opposite side of the lumen wall which would make the resection line arbitrary and imprecise. Occasionally, this would result in excessive or inadequate resection compromising functional or oncological outcomes respectively[13,14]. To overcome this limitation, laparoendoscopic surgery (LES) was proposed, which is a broad category of procedures that utilizes both the modalities collectively either to provide visual assistance while predominantly using one modality or to perform the complete intended procedure using both the modalities in combination[15]. This includes rendezvous assisted procedures like laparoscopy assisted endoscopic resection (LAER), endoscopy assisted laparoscopic resection (EALR) and combined laparoendoscopic procedures [laparoscopic and endoscopic cooperative surgery (LECS)][16]. LECS is a hybrid procedure classically described for gastric submucosal tumor excision. Various modifications have been described to overcome the limitations of the classical technique like risk of abdominal infection, scattering and peritoneal seeding of tumor cells. These innovations have broadened the application of combined laparoendoscopic techniques to other gastrointestinal sites such as the duodenum, colon, and rectum[17]. This minireview explores the evolution, current applications, and future potential of LES in GI diseases.
Diagnostic and therapeutic endoscopy represents the standard of practice for the management of luminal GI pathologies. Traditionally, endoscopy focussed on localization and diagnosis of luminal GI diseases. In the past several decades, rapid innovations and refinement in the technology and equipment have made luminal endoscopic surgery a standard of care for resection of premalignant and early malignant lesions following oncological principles to ensure complete excision[18,19]. The first endoscopic polypectomy was performed by Wolff and Shinya[20] for the treatment of colorectal polyp using a high frequency electric surgical unit. After a decade, “strip biopsy technique” a method of endoscopic mucosal resection (EMR) was proposed in 1984 to enable resection of superficial mucosal lesions using a double channel endoscope and snare[21]. To ensure resection of these superficial mucosal lesions with a 5 mm clear margin surrounding it, Hirao et al[22] proposed the endoscopic resection with local injection of hypertonic saline epinephrine solution technique in 1988. This technique demanded technical skills to avoid perforation of the lumen with the endoscopic needle knife. In the early 1990’s, EMR with a cap fitted pan-endoscope and EMR using multiple band ligation was performed to overcome the risk of perforation[23]. However, EMR was only limited to lesions < 2 cm and those reaching only up to the superficial submucosa. To address this limitation, the insulated tip diathermy knife (IT-Knife) was invented in Japan to perform endoscopic submucosal dissection (ESD). ESD allows en bloc resection of > 2 cm large lesions with precise pathological staging[24]. This opened the gates for third space endoscopic procedures such as submucosal tunnel endoscopic resection or peroral endoscopic tunnel resection for resection of subepithelial tumors and endoscopic full thickness resection (EFTR) for full thickness resection of the luminal wall with closure of the defect using clips or suturing devices. All these techniques are technically demanding and carry some risk of intraoperative or post operative perforation and leaks.
Laparoscopic resection of luminal GI tumors is an established and widely practiced method of surgical treatment that has proven its oncological safety. Laparoscopic resection has been used to manage both early stage and locally advanced luminal tumors arising from the esophagus, gastroduodenal, small bowel and colorectum and also for extraluminal GI diseases. Though laparoscopic resection permits oncological resection of the involved organ along with adequate lymph nodal clearance, this seems appropriate for large and locally advanced malignant lesions[9,10,14]. Resection of small, early stage lesions that are confined to the lumen without transmural involvement or submucosal tumors with limited organ involvement maybe an over kill because it is difficult to estimate the precise location and size of the lesion from the serosal aspect alone using laparoscopy. This may unnecessarily result in too large a resection which may ultimately compromise the long-term quality of life or an inadequate resection with positive margins[14,25,26]. To address these short-comings, the surgeons and the interventional endoscopists collaborated to perform a variety of procedures collectively termed as the rendezvous procedures.
Here, the surgical resections are being carried out predominantly using either endoscopy in LAER or by laparoscopy in EALR while using the other modality for visual assistance to localize the lesion more accurately, avoid full thickness perforation or control of bleeding during the procedure. These hybrid techniques combine the benefits and negate the disadvantages of both modalities individually[16]. Though the rendezvous procedures were better than each individual approach alone, they too lacked the required precision to perform an accurate oncologic resection without overshooting or compromising the margins. The primary intention of using endoscopy or laparoscopy assistance is just for precise localization and visualization of the target lesion[27,28]. These procedures opened gates for the development of an array of procedures, collectively referred to as LECS.
In 2008, Hiki et al[29] from Japan devised the concept of LECS where the incision lines are confirmed endoscopically and accurately determined by the application of an endoscopic mucosal/submucosal incision technique, while the sero
| Various types | |
| Laparoendoscopic rendezvous procedures | LECS |
| Laparoscopy assisted endoscopic resection | Classical LECS |
| Endoscopy assisted laparoscopic resection | Inverted LECS with crown method. nonexposed endoscopic wall-inversion surgery |
| Endoscopy assisted laparoscopic wedge resection | Combination of laparoscopic endoscopic approaches to neoplasia with a non-exposure technique |
| Endoscopy assisted laparoscopic transluminal surgery | Closed LECS |
| Laparoscopic intragastric surgery | |
| Endoscopy assisted laparoscopic intraluminal stapling |
| Ref. | Technique (n) | N | Histology (n) | R0 resection | Conversion | Median hospital stay (days) | Adverse events |
| Gastroesophageal junction tumors | |||||||
| Hoteya et al[35] | LECS | 5 | Leiomyoma (4), GIST (1) | 5 | 0 | 13 | 0 |
| Aoyama et al[36] | LECS (13), NEWS (4), CLEAN-NET (4) | 21 | Leiomyoma (18), GIST (2), ectopic pancreas (1) | 21 | 0 | 9 | 0 |
| Ri et al[37] | LECS | 20 | NR | 20 | 8 | 20 | 4 |
| Gastric tumors | |||||||
| Tsujimoto et al[34] | LECS | 20 | GIST (16), leiomyoma (1), other SMT (3) | 20 | 0 | 12 | 0 |
| Kang et al[33] | LECS | 101 | GIST (78), leiomyoma (13), NET (3), other SMT (7) | 0 | 0 | NR | 2 |
| Ojima et al[38] | LECS (21), endoscopic guided intraluminal gastric surgery (25) | 46 | GIST (46) | 21 | NR | 8 | 1 |
| Mitsui et al[39] | NEWS | 28 | GIST (28) | NR | 2 | NR | 1 |
| Shoji et al[40] | LECS (14), NEWS (26), LWR (31) | 71 | GIST (49), leiomyoma (10), others (12) | NR | NR | NR | 0 |
| Cao et al[41] | LECS (25), endoscopic submucosal dissection (20) | 45 | GIST (45) | 25 | 0 | 4 | 0 |
| Kanehira et al[42] | CLEAN-NET (50), LWR (79) | 69 | GIST (36), leiomyoma (13), schwannoma (11), others (9) | 50 | 0 | 6 | 0 |
| Aoyama et al[43] | LECS (13), NEWS (4), CLEAN-NET (4) | 42 | GIST (24), leiomyoma (5), ectopic pancreas (6), others (7) | 42 | NR | 7 | 1 |
| Ri et al[37] | LECS | 194 | NR | 194 | 2 | 7 | 8 |
| Ref. | Location of lesion (n) | N | Histology (n) | R0 resection | Conversion | Median hospital stay (days) | Adverse events |
| Otowa et al[46] | Above ampulla (3), below ampulla (7) | 10 | Adenocarcinoma (10) | 10 | 0 | NR | 4 |
| Yanagimoto et al[47] | NR | 10 | Adenocarcinoma (6), adenoma (3), NET (1) | 0 | 0 | 9 | 2 |
| Nunobe et al[48] | D1 (63), D2 (128), D3 (15) | 206 | Adenoma (79), adenocarcinoma (70), NET (36), gastrointestinal stromal tumor (7), others (14) | 196 | 11 | 9 | 15 |
| Kanaji et al[49] | D1 (3), D2 (16), D3 (150) | 20 | Adenocarcinoma (16), NET (2), adenoma (1), ectopic pancreas (1) | 19 | 0 | 12 | 2 |
| Ref. | Technique | N | Completion of procedure | R0 resection | Conversion | Median hospital stay (days) | Adverse events |
| Wilhelm et al[50] | Combined laparoscopic and endoscopic resection | 154 | 139 | NR | 7 | 8 | 36 |
| Lee et al[51] | Combined endoscopic and laparoscopic surgery | 65 | 48 | NR | 17 | 1 | 2 |
| Goh et al[52] | Endo-laparoscopic polypectomy | 30 | 22 | NR | 8 | 2 | 4 |
| Schmidt et al[53] | EFTR | 181 | 162 | 139 | NR | NR | 18 |
| Valli et al[54] | EFTR | 60 | 51 | 51 | NR | NR | 4 |
| Tamegai et al[55] | Laparoscopic and endoscopic cooperative surgery | 17 | 17 | 17 | 17 | 7 | 0 |
The key difference between the rendezvous procedures and LECS lies in the extent of integration: Rendezvous procedures focus on assisted visualization and resection, while LECS relies on their concurrent and cooperative use. Various procedures have been described integrating the principles of rendezvous procedures or LECS.
LAER: In this procedure, the interventional endoscopist performs the resection of the lesion on the luminal aspect using EMR or ESD technique while the surgeon assists the procedure using laparoscopy extraluminally to avoid full thickness perforation or controlling bleeding. In instances where inadvertent perforation of the viscus occurs, the surgeon may suture the defect laparoscopically. Additionally, laparoscopy may also aid in performing sentinel lymph node biopsy or perform lymphadenectomy if indicated[56-58].
EALR: In this technique, the laparoscopic surgeon performs a limited or partial resection of the wall of the viscus bearing the target lesion with the assistance of endoscopy to accurately localize the lesion from within the lumen to ensure complete resection with clear margins. The resection may be performed using laparoscopic stapling or using energy source and closure using sutures[16,33,59]. Various procedures performed by using EALR techniques are: (1) Endoscopy assisted laparoscopic wedge resection: The target lesion is localized using endoscopy and under its guidance a laparoscopic wedge resection of the viscus is performed with adequate clear margins taking care of not compromising the calibre of the lumen[60-62]; (2) Endoscopy assisted laparoscopic transluminal surgery: This technique has been described for the management of gastric lesions. After endoscopic localization of the tumor, a laparoscopic gastrotomy is created at the desired site using endoscopic guidance and the target lesion is inverted into the lumen and resected laparoscopically following which the gastrotomy is closed using laparoscopic suturing[63,64]; (3) Laparoscopic intragastric surgery: This technique is used only for gastric lesions. After lesion localization, the laparoscopic camera and working ports are placed through the abdominal and stomach wall into the gastric cavity under endoscopic guidance and the target lesion is removed by partial or full thickness resection and suturing or stapled resection with endoscopic assistance[65-67]; and (4) Endoscopy assisted laparoscopic intraluminal stapling: Another technique described for the management of intragastric lesions, where a single 12 mm laparoscopic port is inserted into the lumen and after endoscopic localization and exposure of the target lesion, it is inverted into the lumen and a laparoscopic stapler is used for resection[68,69].
Laparoscopic and endoscopic rendezvous technique has been used for the simultaneous presence of cholelithiasis and choledocholithiasis where laparoscopic cholecystectomy is performed initially, following which endoscopic retrograde cholangiopancreatography (ERCP) is performed intraoperatively using a guidewire inserted by the surgeon through the cystic duct into the duodenum. This facilitates common bile duct cannulation and mitigates the risk of ERCP induced pancreatitis or pancreatic ductal injury[70,71].
Laparoscopy assisted ERCP has also been performed for biliary intervention in patients who have undergone roux en Y gastric bypass for the treatment of morbid obesity where this procedure is technically challenging due to the presence of altered anatomy. A laparoscopic gastrotomy is made into the remnant stomach, through which the endoscope is inserted for performing ERCP. Alternatively, a trans-jejunal route may be used for the same due to the ease of attaining access for endoscope insertion via the abdominal wall[72,73].
This is a broad terminology that encompasses the ‘classical LECS’ procedure and its subsequent modifications which were proposed to overcome the limitations of the former classical technique.
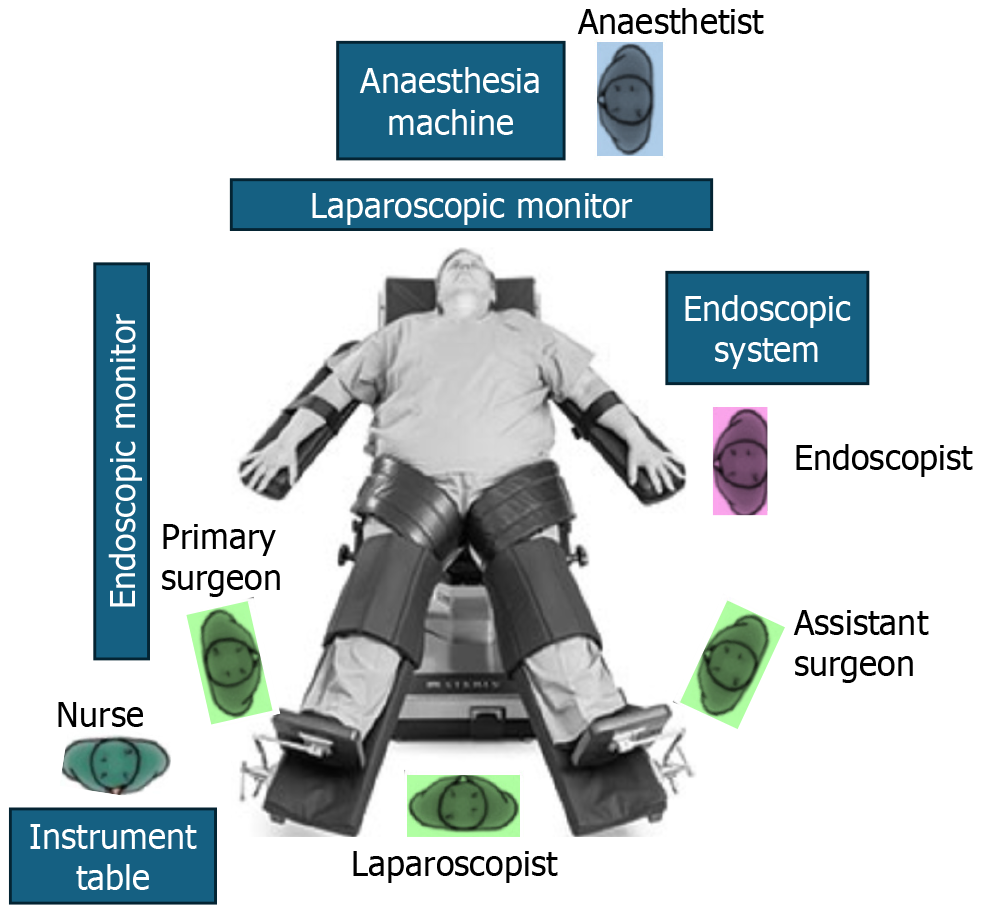
Operative settings, equipment and instruments: The operating team usually comprises a surgeon along with assistants (for camera navigation and retraction) and an interventional endoscopist. These procedures are performed under general anesthesia with the anesthesia unit located at the patient’s head end. The operative set up includes both an endoscopic and a laparoscopic system as depicted in Figure 1.
The endoscopic system includes an endoscope equipped with fine high-resolution vision and advanced apparatus and energy devices for ESD from the luminal aspect, an insulation-tipped diathermic knife and soft coagulation system and an endoscopic over-tube for hassle free repeated scope manipulation, protection of the scope and facilitation of specimen retrieval[44,45,74].
The laparoscopic system includes a camera unit with a high definition 30-degree laparoscope, insufflator, diathermy and ultrasonic energy unit, laparoscopic instruments, and 5 mm and 12 mm laparoscopic ports. Video monitors should be placed appropriately for the surgeon and the endoscopist[45,56].
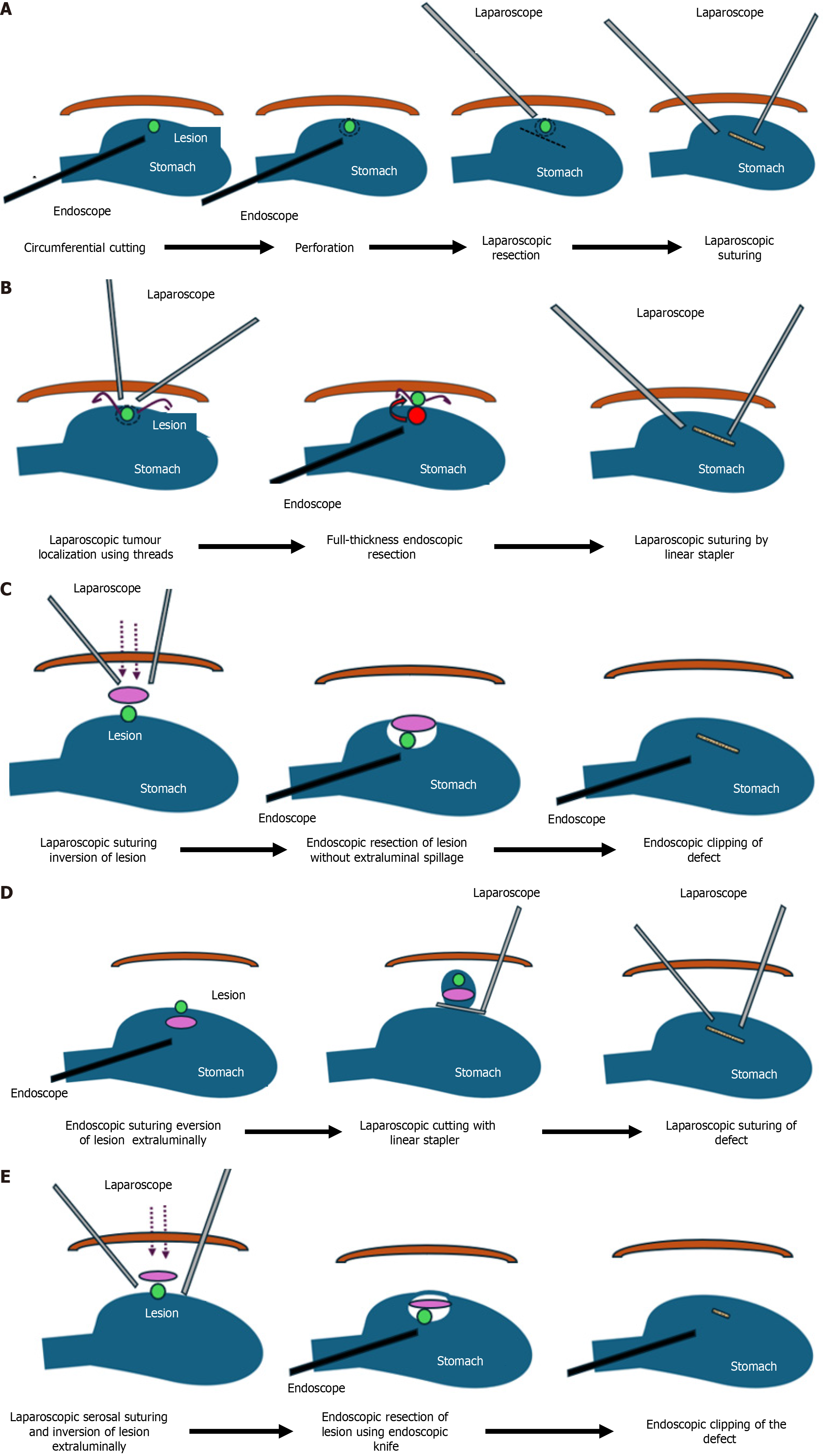
Various techniques of LECS: (1) Classical LECS: Four laparoscopic working ports (3 mm × 5 mm in right upper, left upper and left lower quadrants and 1 mm × 12 mm in right lower quadrant) with a 12 mm infraumbilical camera port are used and 8-10 mmHg pneumoperitoneum is created. An ultrasonically activated device is used for blood vessel preparation around the tumor area in a very limited field. The location of the lesion is confirmed using endoscopy and a mucosal incision line is made around the tumor with a 5 mm margin all around using a needle knife. A mixture of 10% glycerine plus 5% fructose and 0.5% epinephrine are injected into the submucosal layer, following which the mucosa and submucosa are incised with a standard needle knife. The tip of an electrosurgical IT-knife is inserted into the submucosal layer. Then, the marked area is cut circumferentially using the IT-knife. Subsequently, a standard needle knife is pushed toward the serosa and its tip is visualized which is used to perforate the seromuscular layer. This layer is dissected circumferentially using ultrasonic energy source laparoscopically or the IT-knife endoscopically. Once the full thickness resection is complete, the tumor is collected in a specimen bag and the incision line is closed, either using hand sewn suturing or laparoscopic stapling device. Hand sewn closure is preferred at the esophagogastric junction (EGJ) and pyloric end where luminal compromise is of concern[34,75,76]. Figure 2A demonstrates the steps of classical LECS in detail. Care should be taken to avoid excessive preparation of the gastric wall to prevent ischemia at the resected edges or gastric post procedure immobility. Also, opening of the gastric wall may lead to spillage of gastric contents and tumor cells into the peritoneal cavity. Hence this technique carries the risk of intra-abdominal infections and peritoneal dissemination of tumor cells; (2) EFTR with laparoscopic assistance (LECS-related procedure): This procedure was described by Abe et al[77,78] as an LECS-related procedure where the mucosal and submucosal dissection around the tumor was performed endoscopically following which the seromuscular layer dissection was completed from within the lumen using laparoscopic assistance to visualize the resection line and apply counter traction to the gastric wall during endoscopic dissection. Following the full thickness resection, the defect was closed using hand sewn suturing. However, this technique qualifies as a rendezvous procedure rather than a LECS as laparoscopy is used for assistance and not resection; (3) Inverted LECS with crown method: This technique as shown in Figure 2B was initially described and performed for gastric tumors. It begins with localizing the tumor precisely using endoscopy and laparoscopy after which circumferential stitches are placed around the lesion laparoscopically. These stiches are brought out of the abdominal wall using an abdominal closure device to apply traction on the gastric wall surrounding the lesion like a crown. Subsequently, similar to classical LECS, ESD is performed taking appropriate margins around the tumor. The seromuscular layer dissection is completed using endoscopy and laparoscopic ultrasonic energy device and the lesion is inverted into the lumen. After inversion into the lumen, the specimen is extracted per orally and the defect is closed using laparoscopic suturing or stapling device[79]; (4) NEWS: Figure 2C demonstrates the steps of this technique. It involves marking the resection area around the target lesion on the mucosa and serosa using endoscopy and laparoscopy, respectively. Subsequently, sodium hyaluronate and indigo carmine dye is injected into the submucosa following which the seromuscular layer dissection around the tumor is completed using ultrasonic energy device laparoscopically and the seromuscular edges are suture closed after placing a sponge between the undivided submucosa and divided serosa as a spacer to protect the sutures during the ESD phase of resection. The remaining dissection is completed endoscopically using ESD following which the specimen and the sponge are retrieved per orally[32,80]; (5) Combination of CLEAN-NET: In this technique, once the mucosa around the tumor is marked endoscopically, four full thickness sutures are taken laparoscopically to fix the mucosa at the intended resection margin to the serosa underneath under endoscopy guidance. Subsequently, the seromuscular layer is dissected using laparoscopy from the extraluminal aspect beyond the stay sutures and the full thickness specimen is lifted with the help of the stay sutures into the peritoneal cavity maintaining the continuity of the mucosa which acts as a barrier as depicted in Figure 2D. Finally, the full thickness layer of the bowel wall is divided using a laparoscopic stapling device[23,30]. This non-exposure technique, like NEWS, prevents contamination of the peritoneal cavity and peritoneal tumor seedling, but it is relatively technically challenging compared to the other modifications. It is difficult to determine accurate resection lines for larger epithelial neoplasms and tumors located close to the cardia, EGJ or proximal one third of the posterior stomach wall may be difficult to resect using this procedure; and (6) Closed LECS: This technique differs from the NEWS procedure. Here the mucosal markings around the lesion and ESD are performed circumferentially. Subsequently, serosal surface markings are made corresponding to the mucosal markings and a sponge spacer is placed at the center of the serosal resection line. The serosa layer beyond the intended resection edge is sutured over the spacer sponge and the seromuscular layer resection is completed endoscopically. The specimen and the spacer sponge are retrieved per orally in the end as shown in Figure 2E[31].
The emergence of newer robotic endoscopic platforms is poised to play a pivotal role in further refining LES. The robotic platform offers superior vision, enhanced dexterity, precise manipulation with seven degrees of freedom and tremor filtration compared to conventional laparoscopy. With the increase in the availability of the surgical robot across the globe, this approach combined with robotic endoscopes may well be at an advantage with similar or better post-operative outcomes[81]. Third space (TS)-robotic endoscopic cooperative surgery (RECS) has been performed and reported by a few centers for resecting submucosal gastric tumors and polypoidal colorectal tumors. The currently available robotic systems have integrated simulators and virtual reality (VR) based training gear and software that enables teaching and training young surgeons and residents. Simulation and VR training will enhance and simplify the adaptability to this new platform and in turn shorten the learning curve for these procedures. The indications for TS-RECS are the same as LECS and its modifications. More evidence from larger series and prospective studies is awaited to establish its non-inferiority or superiority to LECS[82,83].
With the development of deep learning and convoluted neural network technology, artificial intelligence (AI) has been increasingly applied for diagnosis and surgical management of luminal GI diseases. A variety of computer aided detection, diagnosis and quality assessment software have been integrated into endoscopic systems that facilitate the endoscopist to accurately localize, diagnose and appropriately treat the lesion. Initially developed for white light endoscopy, AI based software have also been developed for confocal laser endomicroscopy and chromoendoscopy to aid in real time diagnosis and guide appropriate management of the lesions in the background of diseased mucosa[84-86]. With the ongoing advances in the field of AI and machine learning (ML) in healthcare and diagnostics, it may be imperative for the surgeons and endoscopists to obtain training and gain experience in this domain in order to use this tool for optimizing treatment outcomes. In the near future, these AI based diagnostic tools may be used to perform LECS or RECS where they can accurately help identify the number of lesions and determine safe and clear resection margins with precision.
Currently, LECS is limited to luminal pathology mainly for benign and early malignant lesions. With the emergence of newer robotic and endoscopic platforms, newer technology integrated with AI and ML, it may be possible to advance its application to resect larger malignant luminal tumors and possibly treat extraluminal GI lesions with precision and perfection in the near future.
| 1. | Hargest R. Five thousand years of minimal access surgery: 3000BC to 1850: early instruments for viewing body cavities. J R Soc Med. 2020;113:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Lau WY, Leow CK, Li AK. History of endoscopic and laparoscopic surgery. World J Surg. 1997;21:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Nagy AG, Poulin EC, Girotti MJ, Litwin DE, Mamazza J. History of laparoscopic surgery. Can J Surg. 1992;35:271-274. [PubMed] |
| 4. | Stellato TA. History of laparoscopic surgery. Surg Clin North Am. 1992;72:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Semm K. Endoscopic appendectomy. Endoscopy. 1983;15:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 723] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Litynski GS. Kurt Semm and the fight against skepticism: endoscopic hemostasis, laparoscopic appendectomy, and Semm's impact on the "laparoscopic revolution". JSLS. 1998;2:309-313. [PubMed] |
| 8. | Périssat J. Laparoscopic surgery: A pioneer's point of view. World J Surg. 1999;23:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU; Korean Laparoendoscopic Gastrointestinal Surgery Study Group. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. 2020;38:3304-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 10. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (1)] |
| 11. | Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1121] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 12. | Ye SP, Zhu WQ, Huang ZX, Liu DN, Wen XQ, Li TY. Role of minimally invasive techniques in gastrointestinal surgery: Current status and future perspectives. World J Gastrointest Surg. 2021;13:941-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Hu J, Or BH, Hu K, Wang ML. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: A multi-center prospective cohort study. Int J Surg. 2016;33 Pt A:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Eisenberg D, Bell R. Intraoperative endoscopy: a requisite tool for laparoscopic resection of unusual gastrointestinal lesions--a case series. J Surg Res. 2009;155:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J Gastroenterol. 2015;21:12482-12497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 18. | Hunt RH. A brief history of endoscopy. Gastroenterology. 2001;121:738-739. [DOI] [Full Text] |
| 19. | Khashab MA, Pasricha PJ. Conquering the third space: challenges and opportunities for diagnostic and therapeutic endoscopy. Gastrointest Endosc. 2013;77:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Wolff WI, Shinya H. Polypectomy via the fiberoptic colonoscope. Removal of neoplasms beyond reach of the sigmoidoscope. N Engl J Med. 1973;288:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 129] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 268] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Inoue H, Endo M, Takeshita K, Yoshino K, Muraoka Y, Yoneshima H. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc. 1992;6:264-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 111] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 25. | Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Laparoscopic surgery for submucosal tumor near the esophagogastric junction. J Laparoendosc Adv Surg Tech A. 2013;23:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic-endoscopic rendezvous resection of gastric tumors. Surg Endosc. 2002;16:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Ridwelski K, Pross M, Schubert S, Wolff S, Günther T, Kahl S, Lippert H. Combined endoscopic intragastral resection of a posterior stromal gastric tumor using an original technique. Surg Endosc. 2002;16:537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 355] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 30. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H, Fujiwara T. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. 2017;20:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 33. | Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013;19:5720-5726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A, Iizuka T, Udagawa H, Kaise M. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Aoyama J, Kawakubo H, Matsuda S, Mayanagi S, Fukuda K, Irino T, Nakamura R, Wada N, Kitagawa Y. Clinical outcomes of laparoscopic and endoscopic cooperative surgery for submucosal tumors on the esophagogastric junction: a retrospective single-center analysis. Gastric Cancer. 2020;23:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 37. | Ri M, Nunobe S, Makuuchi R, Ida S, Kumagai K, Ohashi M, Sano T. Is laparoscopic and endoscopic cooperative surgery (LECS) for gastric subepithelial tumor at the esophagogastric junction safe? Asian J Endosc Surg. 2021;14:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Ojima T, Nakamura M, Nakamori M, Takifuji K, Hayata K, Katsuda M, Takei Y, Yamaue H. Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery. Surg Endosc. 2018;32:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 39. | Mitsui T, Yamashita H, Aikou S, Niimi K, Fujishiro M, Seto Y. Non-exposed endoscopic wall-inversion surgery for gastrointestinal stromal tumor. Transl Gastroenterol Hepatol. 2018;3:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Shoji Y, Takeuchi H, Goto O, Tokizawa K, Nakamura R, Takahashi T, Wada N, Kawakubo H, Yahagi N, Kitagawa Y. Optimal minimally invasive surgical procedure for gastric submucosal tumors. Gastric Cancer. 2018;21:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Cao L, Zheng K, Wang H, Zhao Y, Yang Z, Li W. Laparoscopic and Endoscopic Cooperative Dissection for Small Gastric Gastrointestinal Stromal Tumor without Causing Injury to the Mucosa. Gastroenterol Res Pract. 2019;2019:7376903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Kanehira E, Kanehira AK, Tanida T, Takahashi K, Obana Y, Sasaki K. CLEAN-NET: a modified laparoendoscopic wedge resection of the stomach to minimize the sacrifice of innocent gastric wall. Surg Endosc. 2020;34:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Aoyama J, Goto O, Kawakubo H, Mayanagi S, Fukuda K, Irino T, Nakamura R, Wada N, Takeuchi H, Yahagi N, Kitagawa Y. Clinical outcomes of non-exposed endoscopic wall-inversion surgery for gastric submucosal tumors: long-term follow-up and functional results. Gastric Cancer. 2020;23:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Ichikawa D, Komatsu S, Dohi O, Naito Y, Kosuga T, Kamada K, Okamoto K, Itoh Y, Otsuji E. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol. 2016;22:10424-10431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Garrett KA, Lee SW. Combined Endoscopic and Laparoscopic Surgery. Clin Colon Rectal Surg. 2015;28:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Otowa Y, Kanaji S, Morita Y, Suzuki S, Yamamoto M, Matsuda Y, Matsuda T, Oshikiri T, Nakamura T, Kawara F, Tanaka S, Ishida T, Toyonaga T, Azuma T, Kakeji Y. Safe management of laparoscopic endoscopic cooperative surgery for superficial non-ampullary duodenal epithelial tumors. Endosc Int Open. 2017;5:E1153-E1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Yanagimoto Y, Omori T, Jeong-Ho M, Shinno N, Yamamoto K, Takeuchi Y, Higashino K, Uedo N, Sugimura K, Matsunaga T, Miyata H, Ushigome H, Takahashi Y, Nishimura J, Yasui M, Asukai K, Yamada D, Tomokuni A, Wada H, Takahashi H, Ohue M, Yano M, Sakon M. Feasibility and Safety of a Novel Laparoscopic and Endoscopic Cooperative Surgery Technique for Superficial Duodenal Tumor Resection: How I Do It. J Gastrointest Surg. 2019;23:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Nunobe S, Ri M, Yamazaki K, Uraoka M, Ohata K, Kitazono I, Terashima M, Yamagata Y, Tanabe S, Abe N, Tsuji T, Niimi K, Kawakubo H, Tsukada T, Kitashiro S, Ishizuka N, Hiki N; Society for the Study of Laparoscopic Endoscopic Cooperative Surgery. Safety and feasibility of laparoscopic and endoscopic cooperative surgery for duodenal neoplasm: a retrospective multicenter study. Endoscopy. 2021;53:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Kanaji S, Morita Y, Yamazaki Y, Otowa Y, Takao T, Tanaka S, Urakawa N, Yamamoto M, Matsuda T, Oshikiri T, Nakamura T, Suzuki S, Toyonaga T, Kodama Y, Kakeji Y. Feasibility of laparoscopic endoscopic cooperative surgery for non-ampullary superficial duodenal neoplasms: Single-arm confirmatory trial. Dig Endosc. 2021;33:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Wilhelm D, von Delius S, Weber L, Meining A, Schneider A, Friess H, Schmid RM, Frimberger E, Feussner H. Combined laparoscopic-endoscopic resections of colorectal polyps: 10-year experience and follow-up. Surg Endosc. 2009;23:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Lee SW, Garrett KA, Shin JH, Trencheva K, Sonoda T, Milsom JW. Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum. 2013;56:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Goh C, Burke JP, McNamara DA, Cahill RA, Deasy J. Endolaparoscopic removal of colonic polyps. Colorectal Dis. 2014;16:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 54. | Valli PV, Mertens J, Bauerfeind P. Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD(®)). Surg Endosc. 2018;32:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Tamegai Y, Fukunaga Y, Suzuki S, Lim DNF, Chino A, Saito S, Konishi T, Akiyoshi T, Ueno M, Hiki N, Muto T. Laparoscopic and endoscopic cooperative surgery (LECS) to overcome the limitations of endoscopic resection for colorectal tumors. Endosc Int Open. 2018;6:E1477-E1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Qiu WQ, Zhuang J, Wang M, Liu H, Shen ZY, Xue HB, Shen L, Ge ZZ, Cao H. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. 2013;14:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Acker S, Dishop M, Kobak G, Vue P, Somme S. Laparoscopic-Assisted Endoscopic Resection of a Gastric Leiomyoma. European J Pediatr Surg Rep. 2014;2:003-006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Kato M, Nakajima K, Nishida T, Yamasaki M, Nishida T, Tsutsui S, Ogiyama H, Yamamoto S, Yamada T, Mori M, Doki Y, Hayashi N. Local resection by combined laparoendoscopic surgery for duodenal gastrointestinal stromal tumor. Diagn Ther Endosc. 2011;2011:645609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 60. | Ishikawa K, Inomata M, Etoh T, Shiromizu A, Shiraishi N, Arita T, Kitano S. Long-term outcome of laparoscopic wedge resection for gastric submucosal tumor compared with open wedge resection. Surg Laparosc Endosc Percutan Tech. 2006;16:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 62. | Ohgami M, Otani Y, Kumai K, Kubota T, Kitajima M. [Laparoscopic wedge resection of the stomach for early gastric cancer using a lesion-lifting-method: curative and minimally invasive treatment]. Zentralbl Chir. 1998;123:465-468. [PubMed] |
| 63. | Huguet KL, Rush RM Jr, Tessier DJ, Schlinkert RT, Hinder RA, Grinberg GG, Kendrick ML, Harold KL. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch Surg. 2008;143:587-90; discussion 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, Wakabayashi G. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. 1995;9:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 67. | Schubert D, Kuhn R, Nestler G, Kahl S, Ebert MP, Malfertheiner P, Lippert H, Pross M. Laparoscopic-endoscopic rendezvous resection of upper gastrointestinal tumors. Dig Dis. 2005;23:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surg Laparosc Endosc Percutan Tech. 2011;21:e169-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 69. | Pross M, Wolff S, Nestler G, Schubert D, Kahl S, Lippert H. A technique for endo-organ resection of gastric wall tumors using one intragastric trocar. Endoscopy. 2003;35:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Noel R, Arnelo U, Swahn F. Intraoperative versus postoperative rendezvous endoscopic retrograde cholangiopancreatography to treat common bile duct stones during cholecystectomy. Dig Endosc. 2019;31:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Qian Y, Xie J, Jiang P, Yin Y, Sun Q. Laparoendoscopic rendezvous versus ERCP followed by laparoscopic cholecystectomy for the management of cholecysto-choledocholithiasis: a retrospectively cohort study. Surg Endosc. 2020;34:2483-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 72. | Mohammad B, Richard MN, Pandit A, Zuccala K, Brandwein S. Outcomes of laparoscopic-assisted ERCP in gastric bypass patients at a community hospital center. Surg Endosc. 2020;34:5259-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Dalmonte G, Valente M, Bosi S, Gnocchi A, Marchesi F. Transjejunal Laparoscopic-Assisted ERCP: a Technique to Deal with Choledocholithiasis After Roux-En-Y Reconstruction. Obes Surg. 2019;29:2005-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Aisu Y, Yasukawa D, Kimura Y, Hori T. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J Gastrointest Oncol. 2018;10:381-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Teng TZJ, Ishraq F, Chay AFT, Tay KV. Lap-Endo cooperative surgery (LECS) in gastric GIST: updates and future advances. Surg Endosc. 2023;37:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Kawahira H, Hayashi H, Natsume T, Akai T, Uesato M, Horibe D, Mori M, Hanari N, Aoyama H, Nabeya Y, Shuto K, Matsubara H. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology. 2012;59:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Abe N, Mori T, Takeuchi H, Ueki H, Yanagida O, Masaki T, Sugiyama M, Atomi Y. Successful treatment of early stage gastric cancer by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Gastrointest Endosc. 2008;68:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Mitsui T, Goto O, Shimizu N, Hatao F, Wada I, Niimi K, Asada-Hirayama I, Fujishiro M, Koike K, Seto Y. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Rivero-Moreno Y, Echevarria S, Vidal-Valderrama C, Pianetti L, Cordova-Guilarte J, Navarro-Gonzalez J, Acevedo-Rodríguez J, Dorado-Avila G, Osorio-Romero L, Chavez-Campos C, Acero-Alvarracín K. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus. 2023;15:e42370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 89] [Reference Citation Analysis (0)] |
| 82. | Okamoto K, Kato Y, Yoneyama F, Kimura K, Yamaguchi N, Mizutani F, Jikei K, Kouno H. Robot-assisted laparoscopic partial gastrectomy combined with endoscopy for gastrointestinal stromal tumors with intraluminal growth: a report of two cases. J Surg Case Rep. 2022;2022:rjac416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Okamoto N, Al-Taher M, Mascagni P, Vazquez AG, Takeuchi M, Marescaux J, Diana M, Dallemagne B. Robotic endoscopic cooperative surgery for colorectal tumors: a feasibility study (with video). Surg Endosc. 2022;36:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Roshan A, Byrne MF. Artificial intelligence in colorectal cancer screening. CMAJ. 2022;194:E1481-E1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 85. | Byrne MF, Shahidi N, Rex DK. Will Computer-Aided Detection and Diagnosis Revolutionize Colonoscopy? Gastroenterology. 2017;153:1460-1464.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Antonelli G, Rizkala T, Iacopini F, Hassan C. Current and future implications of artificial intelligence in colonoscopy. Ann Gastroenterol. 2023;36:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/