Published online Aug 16, 2025. doi: 10.4253/wjge.v17.i8.104238
Revised: March 26, 2025
Accepted: July 2, 2025
Published online: August 16, 2025
Processing time: 237 Days and 9.4 Hours
Refractory esophageal strictures (ES) are defined an anatomical restriction with
To evaluate the efficacy and safety of the most recent adjuvant treatments, with the aim of avoiding or, at least, postponing surgery.
Intralesional steroids or mitomycin C injections with antiproliferative and anti-fibroblastic properties have been attempted, but have been abandoned because of systemic adsorption, local complications, or lack of efficacy. Self-expanding metal stents are generally designed for the palliation of neoplastic strictures in adults and rarely employed in pediatrics because of the high risk of complications, in terms of stent migration, local pain and perforation. Our group developed a customized dynamic esophageal stent to stabilize esophageal patency and promote continuous dilatation determined by the food passage between the stent and the REES wall, but it requires an appropriate diameter for placement.
Recently peroral endoscopic tunneling for restoration of the esophagus has been employed to treat esophageal obstructions exploiting the submucosal space. Re-absorbable self-expanding stents (like SX-ELLA Stent Esophageal Degradable BD-BD stent) and energy-delivering surgical devices (HARMONIC ACE™ + 7 Laparoscope) have also been proposed.
After an overview about the historically applied adjuvant therapies, we aim to update the common knowledge with our recent experience of these new minimally invasive options for pediatric REES and refractory ES in three exemplary cases, focusing on their mid-term effectiveness and safety for the purpose of maintain the patency after standard endoscopic dilations and avoiding or, at least, postponing an invasive replacement surgery.
Core Tip: The management of refractory and recurrent esophageal strictures is particularly challenging in pediatric age. Reiterative endoscopic dilations and replacement surgery are the standard treatment but burdened by high procedural risks and non-negligible economic and social costs. Several adjuvant strategies have proven inadequate as real alternatives to surgery. Here we report our experience with the most innovative adjuvant techniques for the treatment of recurrent esopha
- Citation: Imondi C, Bartoli ME, Torroni F, Faraci S, Caldaro T, De Angelis P, Balassone V. Innovative endoscopic alternatives for the conservative management of recurrent/refractory esophageal strictures in children: A case series. World J Gastrointest Endosc 2025; 17(8): 104238
- URL: https://www.wjgnet.com/1948-5190/full/v17/i8/104238.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i8.104238
Esophageal strictures (ES) in children have typically a benign origin and may be congenital (10%-15%) or acquired[1]. European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines define refractory ES (RES) as an anatomical restriction without active inflammation resulting in dysphagia after a minimum of five seriated dilatations in a 4-weeks interval. Recurrent ES (REES) refer to the inability to maintain a satisfactory luminal diameter for four weeks once an age-appropriate feeding diameter has been achieved[2,3]. Seriated endoscopic dilations (ED) with semirigid Savary-Giliard bougies or balloon dilators are the reference maintenance of ES in pediatric age[4].
In case of REES, reiterative dilations increase the risk of complications and may cause psychological problems in children with high costs for families and health systems. In addition, a therapeutic plan should be customized to the RES etiology and morphology (in terms of length, aspect, number, and level) and different conservative strategies should be chosen accordingly. A relevant role in the exacerbation of the stricture is also played by gastro-esophageal reflux disease (GERD), promoting inflammation and stricture worsening. Surgical approach is burdened with high mortality and morbidity (such as vague nervous injury, bleeding, fatal mediastinitis or iatrogenic fistulas) determining prolonged hospitalization and delayed oral refeeding in such fragile patients with associated comorbidities.
Therefore, several adjuvant treatments aiming to reduce fibroblastic activity in scar tissue and minimize the damage to the injured esophageal wall have been described, but they are burdened by lack of efficacy and not negligible side effects[5-7]. Recently peroral endoscopic tunneling for restoration of the esophagus (POETRE) has been proposed to treat esophageal obstructions exploiting the submucosal space. Resorbable self-expanding stents (like SX-ELLA Stent Eso
Despite the absence of specific controlled trials, different non-surgical adjuvant treatments have been evaluated in clinical practice for REES. Mitomycin C (MMC) is an antibiotic and an antineoplastic with anti-fibroblastic properties. A pro
There are no reports of adverse effects of topic MMC, but the application is technically challenging: The drug should be targeted precisely to the stricture, protecting the surrounding mucosa from its potentially harmful cytostatic effect. Due to the risk of dysplastic transformation of the exposed esophageal mucosa, a careful endoscopic follow-up with multiple biopsies should be planned after MMC application[6,10].
Another concern is the absence of standardized drug concentrations and dosing intervals: The concentration of 0.4 mg/mL is the most widely used with a median interval of application of 4 weeks[11]. Long-term follow-up and prospective studies are needed to better define optimal application technique, dosage, concentration, duration and number of MMC applications both for caustic. REES and for those of different etiology[6]. The use of corticosteroids, both systemically and intralesional, has also been described. Intralesional triamcinolone acetonide (TAC) injection, with a concentration of 10-40 mg/mL and a volume ranging between 0.5 mL and 2.8 mL, seems to reduce collagen synthesis, fibrosis and chronic cicatricial processes[12].
The efficacy of intralesional steroid injection (ISI) remains however unclear, since the most encouraging results come from uncontrolled studies. Furthermore, ISI is likely to be more effective in strictures with high degree of active inflammation, than in long standing fibrotic ones[13], but data on REES histological inflammatory activity are lacking. Bicakci et al[14] demonstrated persistent dysphagia relief after a median of five combined sessions with TAC injection and balloon dilation in 10 children with REES after caustic ingestion. Nijhawan et al[15] showed a significantly improved periodic dilation index (number of dilatations per month) and dysphagia score from pre- to post intervention period in 11 patients with corrosive REES.
Recently, contrasting outcomes emerged from a systematic review addressing the safety and effectiveness of ISI in addition to ED in young REES children, demonstrating an overall complication rate of 7%, with a greater incidence of local complications compared to systemic ones. A reduction of needed dilatations was seen after ISI, compared to the number of ED performed before the intervention (5.2 vs 1.3), but further prospective and comparative studies are needed for a more comprehensive evaluation of the effectiveness of this technique[16].
The rationale of esophageal stenting for REES is to provide continuous radially oriented pressure for a long period allowing the esophagus to maintain lumen patency and simultaneously relaxing the stricture. Remodeling of scar tissue may occur while the stent is in place, which may result in persistent luminal patency and reduced risk of REES[6]. Three main types of esophageal stents are available now: Self-expanding metallic stents (SEMS), self-expanding plastic stents (SEPS) and biodegradable stents (BDS)[17,18].
Metallic esophageal stents were initially approved for the palliative management of malignant dysphagia in adults, subsequently the technique was also adopted in pediatric age for the temporary management of REES in esophageal atresia using SEPS, with encouraging results. Since uncovered metal stents are not indicated for benign REES, self-expanding silicone-coated stents, or SEPS, were designed to increase patency and prevent ingrowth of tissue from a hyperplastic reaction.
In 2003 Broto et al[19] reported the first experience with siliconated polypropylene stents (Poliflex/Rüsch) in a series of 10 pediatric patients with severe REES (9 ES after caustic ingestion, and 1 ES resulting from EA), with complete recovery of dysphagia in 5/10 patients and requiring for stent replacement after a follow-up ranging from 4 months to 19 months in 3/10 patients. There was no mortality, and the morbidity associated with the procedure was limited to late distal esophagitis managed with proton pump inhibitors (PPI). Unfortunately, the only SEPS still available in Western coun
Fully covered SEMS have a long-term clinical success rate of 35%-45% for benign refractory ES (BRES). However, migration rate is high also for fully covered SEMS (25%-35%), and adverse events are seen in 20%-25% of patients[21]. A non-negligible issue was the inadequacy in size of stents for pediatric patients, which led to the production of customized stents. The first esophageal customized dynamic stent was described in 2011 in our center and consists of a silicone stent with an external diameter of 7.9 mm or 12.7 mm built coaxially on a nasogastric tube, which guarantees its correct position, fixed to the nostril via a silicone transversal bar. The 2 ends of the dynamic stent are designed to allow the passage of food between the stent and the esophageal wall, favoring the esophageal plasticity and preventing stricture recurrence. The dynamic stent is kept in place for a minimum period of 40 days. From 1988 to 2010, 79 children with ES underwent ED and custom-stent placement, reporting an effectiveness rate of 88.6% (70 of 79 patients).
There was one stent-related major complication: A fistula between the esophagus and an anomalous subclavian artery presented with massive hemorrhage in a patient with a post-surgical stricture after esophago-jejunoplasty for esophageal atresia. The complication was surgically treated and likely occurred because of erosion of the stent into the esophageal wall. Due to the possible presence of anatomical variants, such as an aberrant right subclavian artery or a right aortic arch, it is therefore mandatory to perform a thoracic computed tomography with intravenous contrast or magnetic resonance imaging for all patients being evaluated for possible stent placement. Other reported complications include stent misplacement or migration, gagging, retching, transient chest pain, nausea, vomiting, gastroesophageal reflux, ulcerative esophagitis, inability to remove the stent, tracheal compression, and esophageal perforation[5,22-24]. Endo
In 2006 Hordijk et al[27] investigated the efficacy of EIT to alleviate dysphagia in 24 adults with anastomotic REES after transhiatal esophageal resection. Subjects were treated with a single-session EIT after 1 ED. After 2 years of follow-up, more than 85% of patients were still dysphagia-free[26]. A follow-up randomized study by the same authors, including 62 patients with no previously treated anastomotic strictures, however, found no differences between ED and EIT, con
Muto et al[29] found that EIT resulted in significantly higher patency rates than repeated ED in the management of anastomotic REES at 6 months and 12 months follow-up, also reporting a satisfactory safety profile with only two conservatively managed iatrogenic perforations. Regarding the application of EIT in pediatric age, there are still limited and exclusively retrospective data: Manfredi et al[30] performed EIT in 58 pediatric ES (36 REES and 22 non-refractory strictures, respectively). In the refractory group, 61% of patients met treatment success criteria significantly reducing the median number of annual dilations, while in the non-refractory strictures group, 100% of patients met the success criteria. The overall rate of adverse events was 5.3% (7/133), with 3 major (2.3%) and 4 minor (3%) events. Although further prospective longitudinal studies are needed to validate this treatment and standardize its different applications, EIT shows promise as an additional therapeutic option for pediatric REES and can be considered before surgical resection even in the most severe cases, in expert hands.
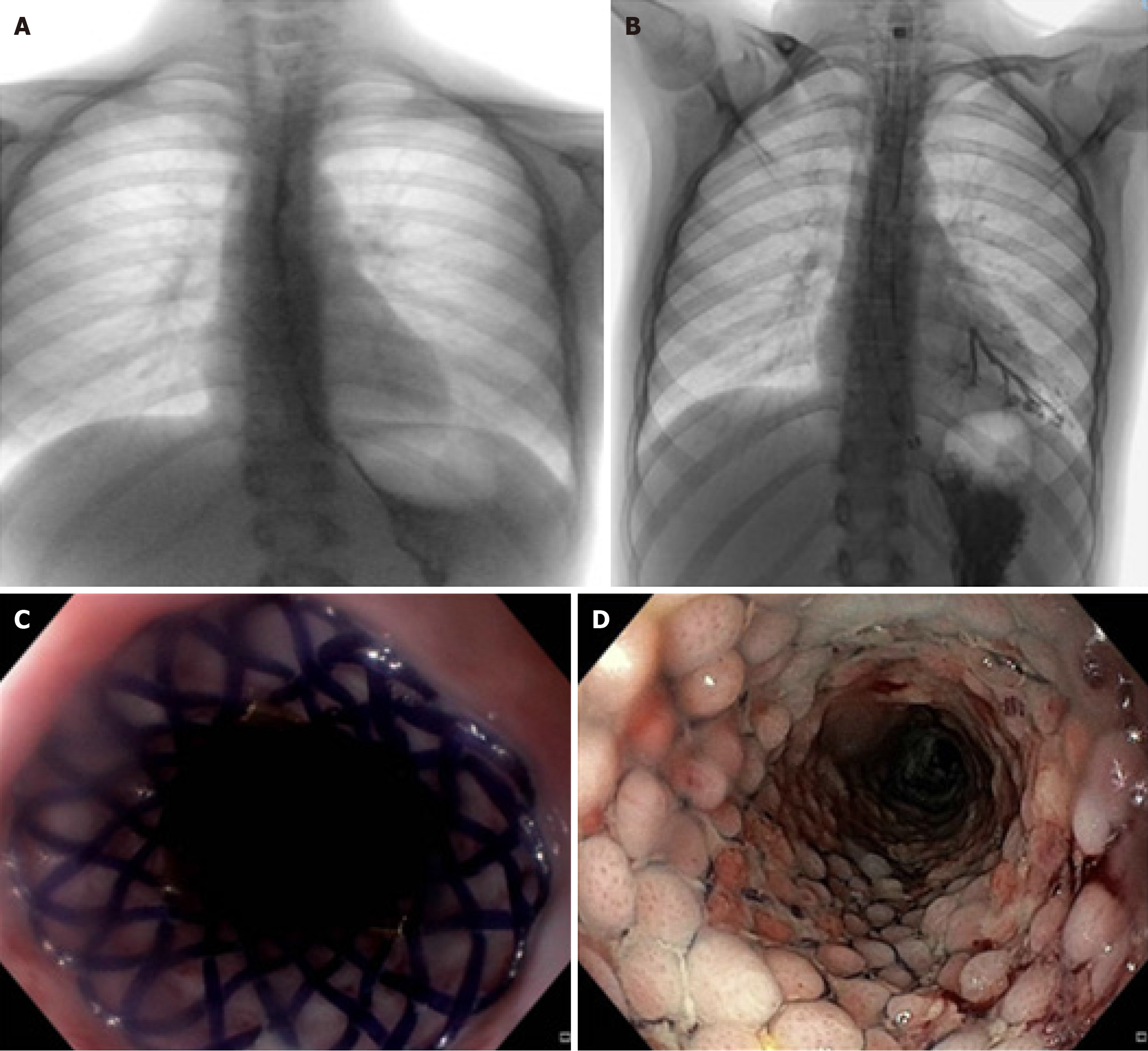
Case 1 BDS (Ella stent) in the management of a long corrosive res in an adolescent: A 12-year-old female was referred to our institution from another country due to a severe corrosive REES after caustic ingestion about 3 months before. At the admission, she presented severe dysphagia and drooling and a weight loss of 5 kg. Both the esophagogram and the endoscopy showed a diffusely irregular esophageal lumen with 2 major narrowing one extending for 15 mm immediately below the cricopharynx and another extending for 25 mm in distal esophagus, with a residual lumen of approximately 3 mm (Figure 1A).
After 6 close-range EDs using semirigid Savary-Giliard bougies, we placed a dynamic stent large 10.5 mm and long 20 cm with no immediate complications. The esophagogram performed after 24 hours excluded esophageal leakage, and the patient initially tolerated soft foods and was discharged. Ten days later, she was re-admitted for fever and worsening dysphagia: An esophagogram showed a regular passage of contrast medium between the esophageal wall and the dynamic stent but an extraluminal spread of soluble contrast and Chest X-ray demonstrated a broncho-esophageal fistula.
A conservative management with vacuum assisted therapy (salem sunk tube) parenteral nutrition and wide-spread IV antibiotics was attempted but failed because of the position and the characteristics of the esophageal fistula (Figure 1B). After a multidisciplinary consultation we decided to conservatively manage the esophageal perforation by removing the dynamic stent and positioning of SX-ELLA BDS.
Although being aware of the length (extended from 25 cm to 33 cm from the upper dental arch-upper dental arch) and tortuosity of the stenosis, which are unfavorable prognostic factors for the successful outcome of conservative treatment, we chose the BDS as a bridging therapy for a more definitive esophageal replacement surgery, until a good nutritional status was restored. Furthermore, the failure of the dynamic stent treatment was also contributed by the concomitant severe GERD, which increased the fragility of the distal esophageal mucosa predisposing to perforation; so, the choice of BDS was also motivated by the aim of theoretically promote distal re-epithelialization and conservative resolution of the breach, using a stent with a lower risk of migration.
Under general anesthesia, in supine position and X-ray guidance, we removed the dynamic stent and introduced a standard gastroscope. The esophagus presented with rigid and pale mucosa, with no macroscopic signs of esophagitis and two small fistulas in distal esophagus at 25 cm and 33 cm from the UDA, respectively. Skin radiopaque markers of the interested esophageal tract and a guidewire were left in place; than a SX-ELLA BDS 20 mm × 80 mm was released to cover the esophageal section under X-ray guidance (Figure 1C). Finally, salem sunk tube with continuous suction was initially left in place.
Seven days after the stent placement, an esophagogram confirmed the resolution of esophageal leakage and fistula so the aspiration tube was removed. Progressive refeeding with soft diet was started and the patient was discharged a week later (Figure 1D). Thanks to the BDS placement, she had about 5 months of good health before developing dysphagia again. The patient then required three monthly dilations until a second BDS was placed, with gradually resumption of oral feeding and recovery of an adequate nutritional status. Unfortunately, due to the length (85 mm) and tortuosity of the stenosis, even after removing the second stent the patient again manifested dysphagia, so in agreement with the family we planned an esophagocoloplasty.
Case 2 submucosal recanalization in a girl with REES after button-battery ingestion: A girl with a history of accidental button-battery ingestion at the age of 2 years, was referred to our attention since March 2016 for REES management. Because of battery’s impaction, she underwent cervicotomy and esophagotomy in another hospital and developed a tracheo esophageal fistula treated conservatively and resulted in a ES. After repeated ED sessions, a dynamic esophageal stent was placed from March to June 2016, obtaining only a transient improvement in stricture caliber.
In January 2020, after 4 years of unsuccessful dilations and no improvement of dysphagic symptoms, the family accepted a surgical resection of the stricture with esophago-esophageal anastomosis. The surgery was technically challenging due to the tenacious tracheo-esophageal adhesions and again complicated by anastomotic leakage and then recurrence of RES with pre-stenotic pseudodiverticulum.
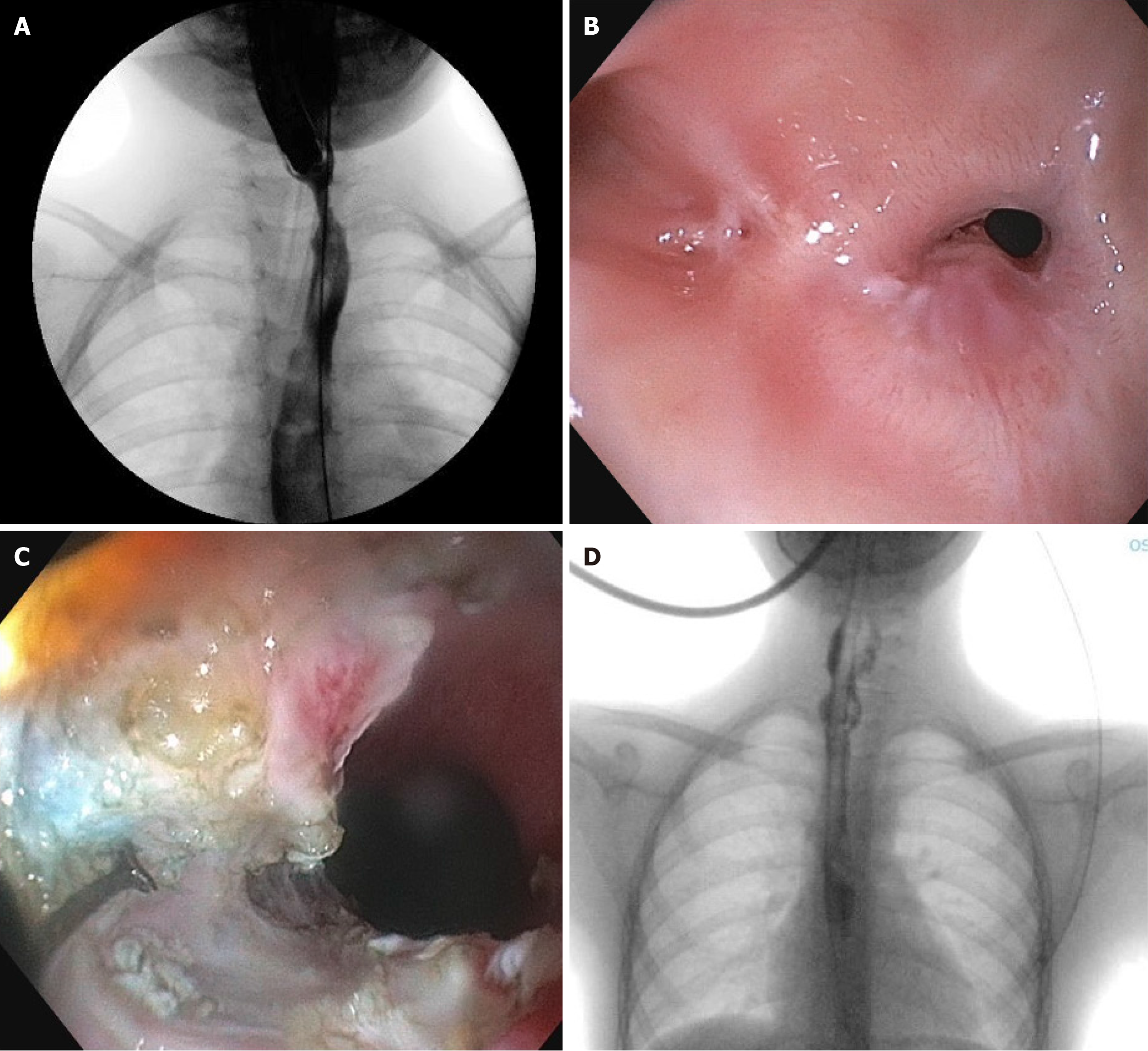
From March 2020 to June 2023, she underwent monthly EDs and various adjuvant treatments were attempted, including 4-quadrant injection of triamcinolone, 4 needle-knife EITs and 1 cutting-balloon incision of the anastomotic scar tissue but we could obtain only a transient improvement of the stricture’s caliber (Figure 2A). After multidisciplinary consultation and given the family’s refusal for an esophageal replacement surgery, in June 2023 (when she was 10-year-old) we opted for a further conservative attempt, performing a submucosal REES recanalization via endoscopic septostomy of the known pre-stenotic pseudodiverticulum, which we describe below.
Under general anesthesia, in supine position, a standard gastroscope (Olympus GIF-H190) was introduced. At 13 cm from UDA, the anastomotic stricture was found with a caliber of approximately 3 mm, surmounted by the known pseudodiverticulum (Figure 2B). By submucosal infiltration with saline and indigo carmine, a mucosal and common fibromuscular wall section was performed. By alternating insulation-tipped knife and triangle-tip-knife, we achieved to obtain a complete flattening of the fibromuscular septum until the esophageal lumen was recanalized, allowing the passage of the standard gastroscope and therefore solid food (Figure 2C). Finally, we injected 40 mg of TAC into the residual septum. At the end of the procedure, X-ray confirmed absence of leakage and a Blake 20 Ch drainage was positioned through the stricture instead nasogastric tube.
After the procedure no major complications occurred. In first post-operative day an esophagogram excluded any leakage (Figure 2D), so the girl started refeeding with a well-tolerated soft diet. She was discharged two days after the procedure tolerating a mild diet. Unfortunately, the family also refused the placement of an adjuvant stent to keep open the recanalized tract, so the patient developed REES again four months after the procedure. Today she’s still requiring regular EDs every two months, due to the persistent family’s refusal of a replacement surgery. However, thanks to both seriated EDs and POETRE, we obtained a REES shortening, allowing the periodic EDs to be more effective and leng
Case 3 pseudodiverticulum septotomy in a child with a previous history of long-gap esophageal atresia: A 4-year-old girl from another country who underwent surgical repair of a long-gap esophageal atresia in the context of VACTERL association, came to our attention reporting vomiting and dysphagia for solids and liquids. The patient underwent cer
In our hospital an esophagogram was firstly performed, revealing a large diverticulum of the pre-anastomotic esophagus. The endoscopic examination confirmed an eccentric dilatation (pseudodiverticulum) of the esophageal tract overlying an anastomotic annular narrowing. An extrinsic pulsatile compression close to the pseudodiverticulum was determined by pulmonary vessels. CT angiography excluded significant associated vascular anomalies. In order to avoid surgery in a child who had already undergone many surgeries, we opted for endoscopic treatment of esophageal pre-anastomotic pseudodiverticulum by sectioning the common wall on the opposite side to the ab-extrinsic pulsatile compression, with a 5 mm Just Right Stapler next to the GIFXP190N ultra-thin gastroscope.
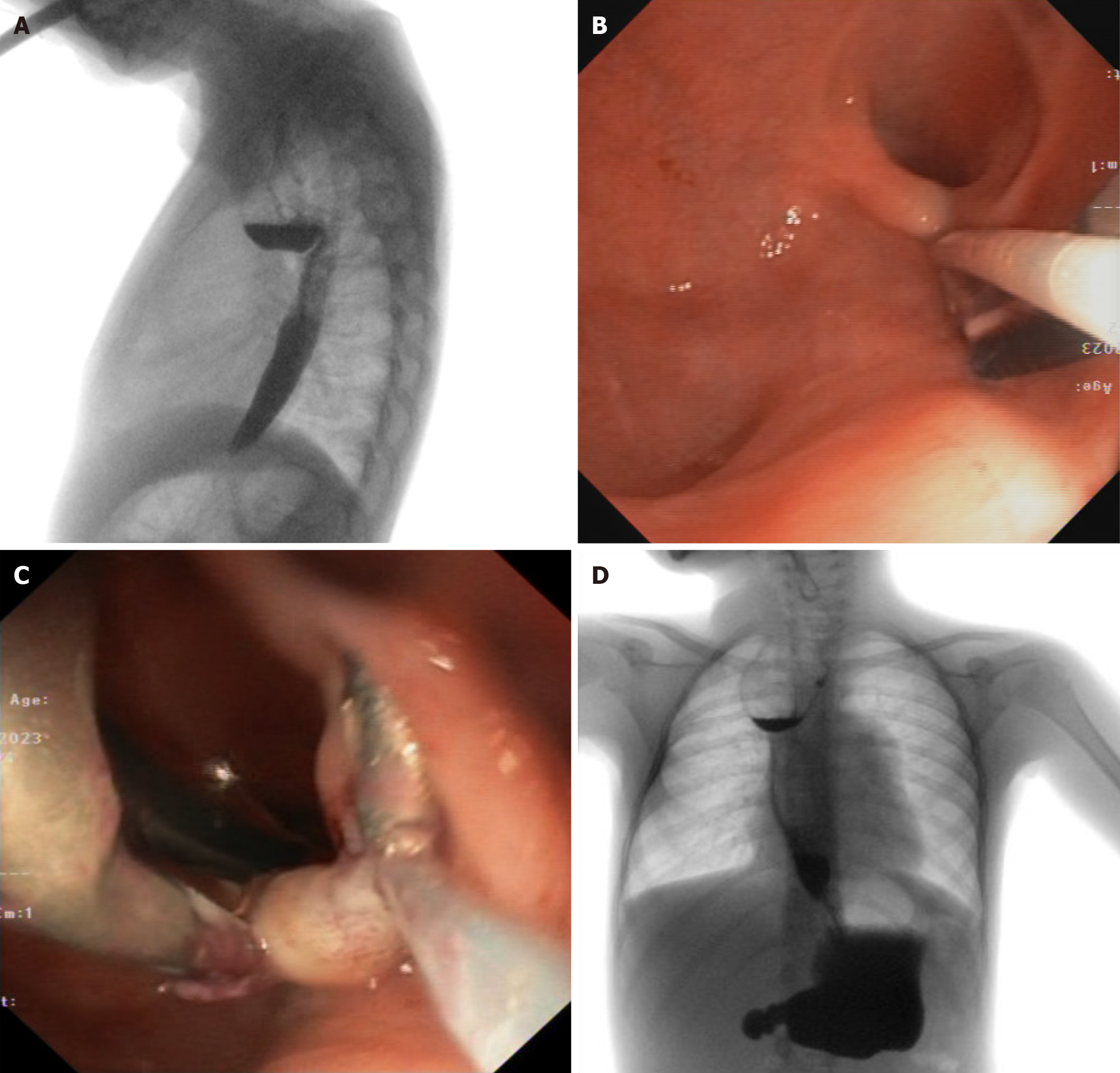
In the absence of procedure-related complications, the child was discharged on a well-tolerated soft diet. One year after the procedure, however, an esophagogram and then an endoscopic check-up was performed, revealing improvement in clearance of the oral contrast medium compared to the previous examination, but persistence of the known pseudodiverticulum, with a small fibrous spur adjacent to external pulsatile compression (Figure 3A). The child had only mild dysphagia for solids and linear growth was adequate. After multidisciplinary discussion, we opted again for a conservative management of the persistent pseudodiverticulum to improve the patient's quality of life by resolving the emetic symptoms and prevent the possible recurrence of anastomotic stricture by reducing predisposing factors to local inflammation (such as food stagnation in the pre-anastomotic diverticulum).
Under general anesthesia, in supine position with hyperextension of the head, an ultra-thin gastroscope (Olympus GIF-XP190) was introduced. The fibrous septum of the known pseudodiverticulum was then mobilized with foreign body forceps to exclude the presence of interposed structures of the mediastinum. A clip with a surgical loop for externalized countertraction (Figure 3B) was placed before proceeding with the section of the diverticulum using a 5 mm Harmonic Advanced Cutting and Energy™ laparoscope introduced by mouth parallel to the thin gastroscope (Figure 3C). Antibiotic prophylaxis and antiemetic therapy were finally administered.
After the procedure no major complications occurred. In first post-operative day an esophagogram excluded any leakage, so clear liquids and, after 24 hours, a well-tolerated soft diet were started. She was discharged three days after the procedure with 1 mg/kg PPI therapy. PPI therapy was discontinued after 3-months and she is still tolerating a soft diet six months after the procedure (Figure 3D). After six months, the patient returned to her home country and was lost to endoscopic follow-up. However, an esophagogram was recently performed showing a good passage of the marked meal and a substantial stability of the known residual pseudodiverticulum. An endoscopic evaluation will be needed to establish the need for further treatments.
Mini-invasive treatment of ES in pediatric age is challenging both for the peculiar comorbidities of these patients and the issues about the efficacy and safety of these techniques due to the lack of randomized controlled trials in children. In this article we aimed to analyze the possible mini-invasive techniques in order to avoid or, at least, delay a surgical approach which often risks being demolitive with high health and psychological costs for children and their families.
ED with bougies or balloons remain the mainstream maintenance treatment for RES but there is no consensus either about the degree or duration of the effective dilations. Over the last 20 years several adjuvant treatments with the dual purpose of both maintaining the REES caliber after dilation and further gaining caliber through the continuous dilatory action of customized dynamic stents or by exploiting the peri-stenotic tissues have emerged, including the stricture’s submucosal recanalization and the treatment of REES complications, such as pre-stenotic diverticula.
The constant radial thrust exerted by custom dynamic stents is profitable in gradually gaining caliber after initial ED treatment, but it is difficult to manage in cases where the esophageal wall is extremely fragile, exposing it to a greater risk of perforation. In the specific case of our first patient, the concomitance of a long erosive RES extended from the proximal to the distal third of the esophagus including cardia and severe GERD after scarring retraction and probably shortening of the esophagus, could have led to a particular wall fragility in the distal tract, favoring at the same time the formation of the fistula under the continuous radial thrust of the dynamic stent and the migration of the stent itself.
After successful conservative management of the fistula, to both maintain the acquired patency and promote the re-epithelialization of the fragile distal mucosa, we used a BDSs, made from a biodegradable polymer which is slowly adsorbed and is particularly attractive in children since stent removal is not needed. Furthermore, by releasing drugs or anti-fibrotic agents during the scaffold degradation process BDSs could theoretically induce different cell growth on the other sides of the same stent, encouraging re-endothelialization on the luminal side and at the same time limiting the proliferation of smooth muscle cells on the abluminal side[31,32].
Currently, the only available BDS, the Ella BD stent (Ella CS, Hradec Králové, Czech Republic) maintains its integrity and radial force for 6-8 weeks and then disintegrates over a period of 8-12 weeks[33]. Available results are modest and discordant, demonstrating a significant symptoms improvement and lower dilations frequency in the first 12 weeks, but a high risk of recurrence after a longer follow-up (> 3-6 months). Long-term relief of dysphagia varies between 30% and 40% of patients. Although there is a significant reduction in migration risk (15.3%), adverse events, including bleeding and severe retrosternal pain, are not infrequent (20%-25% of patients)[21,28].
In a multicenter randomized controlled trial, individuals who were administered BDS experienced a significantly lower rate of emergency department visits in contrast to those who underwent dilation after a three-month follow-up period. This indicates that the benefits concerning safety and efficacy linked to repeated dilations and the timely introduction of BDS into the BRES treatment protocol were only short-lived (P < 0.001). At the six-month follow-up, the results for both groups were similar. No notable differences were observed in the occurrence of adverse events or in the improvement of dysphagia scores between the two groups[34].
The effectiveness of BDS varies from over one-third to a quarter of cases, similar to other stent types, according to a review by Gkolfakis et al[35]. Although they might happen more frequently, adverse events are usually mild. The validity of these findings is still questionable, though, given the variety of the included studies and the lack of high-quality, randomized studies. A recent systematic review, following PRISMA guidelines and based on a prospectively registered protocol, evaluated the clinical and technical efficacy of the SX-ELLA BDS in 246 adult patients undergoing treatment for BRES[36]. The review also examined variations in dysphagia scores, rates of stent migration, and overall safety before and after the insertion of the BDS. The findings indicated a moderate clinical success rate of 41.9% and a high technical success rate of 97.2%. Additionally, a pooled analysis revealed a 1.8-point decrease in dysphagia scores post-BDS insertion (95% confidence interval: 1.68-1.91). Re-intervention was needed in 89 patients, accounting for 36.2% of the total, whilst stent migration was observed in 6.5% of the patients. There were 37 significant clinical side effects associated with BDS insertion (15.0%)[36].
Limited to pediatric series, Awolaran et al[37] did not find a favorable outcome in three cases of severe REES (2 following caustic ingestion and 1 after long-gap esophageal atresia repair), as BDS insertion led only to a temporary dysphagia improvement. All patients continued to experience recurring symptoms as they did prior to the stent placement, and the frequency of dilations remained consistent. In addition, stenting resulted in mucosal hyperplasia that contributed to symptoms recurrence.
BDSs are a promising tool for the conservative management of BRES. However, their superiority compared to other available therapeutic options remains to be demonstrated with clearcut impressive outcomes. On the other hand, although complications associated to BDS are usually minor, severe complications also occur at relatively high rates. More evidence, especially from well-designed randomized controlled trials are needed to elucidate the proper clinical settings for their use also in pediatric age[35].
Finally, the biodegradability of BDSs is an advantage because it does not need to be removed, but at the same time a limit, because it maintains its integrity and radial force for 6-8 weeks and then disintegrates in a period of 8-12 weeks. This makes BDSs a temporary solution to maintain esophageal patency after ED, but carries a risk of REES recurrence that requires the simultaneous formulation of a different long-term program. Furthermore, it would also be desirable to have a customized production for pediatric age.
Like BDSs releasing anti-fibrotic agents, another adjuvant strategy which could prevent RES recurrence by promoting esophageal epithelialization is emerging. In fact, corrosive agents may cause full-thickness injury of the esophageal wall, resulting in a remodeling of the extracellular matrix until normal esophageal tissue is replaced by connective tissue, with scar retraction and stiffening of the esophagus. The concomitant destruction of stromal stem cells in the basement membrane of the esophagus leads to loss of its regenerative capacity. The employment of mesenchymal stem cells (MSCs) to promote esophageal remodeling founds on their own multipotent nature, which can differentiate into specific cell lineages and home in sites of injury, restoring damaged tissue.
Kantarcioglu et al[38] first described the use of intravenous MSCs to promote esophageal wall repair in a murine model of severe caustic esophagitis, with histopathologic confirmation of both the homing behavior and differentiation pro
Oral administration of MSCs gel would be a promising treatment for the prevention of post-ESD stricture[39]. These results provide evidence for clinical application of MSCs in esophageal diseases in humans. Nevertheless, time course and dose dependent studies with different types of stem cells and application modalities are necessary to establish the real potential of MSCs in promoting tissue repair and preventing RES recurrence, after conservative treatment. In the second case we reported a modified application of the POETRE technique for the submucosal recanalization of a short anastomotic REES, by sectioning the fibromuscular septum of the pre-stenotic pseudodiverticulum. This technique was described by Wagh et al[40] and Wagh et al[41] in adults with complete esophageal obstruction: A dual-endoscope simul
In our experience short-term efficacy of this procedure was very satisfactory, with rapid resumption of soft diet feeding and no procedure-related complications. REES recurrence after a successful recanalization highlights the importance of stenting as an adjuvant therapy to stabilize RES after dilatation and prevent REES development. Prevention of RES recurrence also involves reducing risk factors predisposing to local inflammation, such as food stagnation. In fact, increasing the volume of the esophagus above a stenosis is useful for obtaining greater pressure on the stenosis itself. By facilitating the passage of food, it is easier to maintain patency in a similar way to the functioning of the dynamic stent.
To exemplify this concept, we described the use of a 5 mm Harmonic Advanced Cutting and Energy™ laparoscope in the conservative treatment of a pre-anastomotic pseudodiverticulum after surgical correction of EA. In last decades this instrument has become the treatment of choice in adult patients with Zenker’s diverticulum: Using high-frequency vibration, the harmonic scalpel cuts and coagulates simultaneously with minimal spread to adjacent tissues[42].
Furthermore, compared to monopolar and bipolar diathermy, there is better visibility in the surgical field due to less bleeding, smoke, and charring. As the harmonic scalpel does not require electrical energy to be transferred through the patient, no stray energy is present to produce shocks or burns elsewhere other than the surgical site[43].
Baldwin et al[44] described endoscopic stapled diverticulotomy in 51 patients with hypopharyngeal diverticulum: Normal swallowing was resumed within 36 hours of surgery in 73% of them and 93% by 72 hours. Two patients required nasogastric feeding for 5-7 days. The procedure was characterized by low morbidity, short hospital stays and early return to normal feeding. Tarifi et al[45] in 2022 reported the case of a 64 year-old man who came to attention for an oropha
In 2020 Kwek et al[43] compared tonsillectomy safety and patient satisfaction outcomes between Harmonic ACE® and monopolar diathermy in an adult population: The operative time was comparable, but in the Harmonic scalpel arm there was less intraoperative bleeding, lower risks of delayed hemorrhage and readmission. Post-operative analgesia scores were similar. In addition, in the Harmonic ACE® group there was an earlier return to normal diet and activity.
Limitations of this endoscopic technique are few: Incomplete exposure of the common septum and diverticulum because of unfavorable anatomy (prominent upper incisors, limited mouth opening, inability to extend the neck, too-distal diverticulum or a < 2 cm hypopharyngeal diverticulum) makes it difficult or impossible to properly position the diverticuloscope[46]. Moreover, diverticulectomy requires a meticulous closure that is time-consuming and has the potential to leak, form fistulas and become infected[47].
This report has some limitations: Firstly, we selected only three exemplary cases from our recent experience in the management of REES in pediatric age. Furthermore, etiology and characteristics of reported REES was remarkable different. In the absence of multi-centric studies with rigorous design and a larger sample, the long-term effectiveness of different strategies is difficult to be compared.
Our experience with the most recent mini-invasive adjuvant treatments for REES and RES aims to underline the continuous effort of pediatricians into providing a valid alternative to a demolitive surgery. In our opinion, this report has the merit of drawing attention to the lack of standardization that still characterize the management of benign REES in long life expectancy-individuals and therefore promote systematic reviews on the topic. In pediatric age, devices designed for adults are often used; it would be desirable for the future to create custom-made products for children as well. New generation of anti-fibroblastic agents and tissue-remodeling factors may represent the awaited answer in the prevention of recurrence once a proper caliber for age has been laboriously obtained.
| 1. | Sag E, Bahadir A, Imamoglu M, Sag S, Reis GP, Erduran E, Cakir M. Acquired noncaustic esophageal strictures in children. Clin Exp Pediatr. 2020;63:447-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Krishnan U, Mousa H, Dall'Oglio L, Homaira N, Rosen R, Faure C, Gottrand F. ESPGHAN-NASPGHAN Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children With Esophageal Atresia-Tracheoesophageal Fistula. J Pediatr Gastroenterol Nutr. 2016;63:550-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 3. | Vandenplas Y. Management of Benign Esophageal Strictures in Children. Pediatr Gastroenterol Hepatol Nutr. 2017;20:211-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Alberca de Las Parras F, Navalón Rubio M, Egea Valenzuela J. Management of refractory esophageal stenosis in the pediatric age. Rev Esp Enferm Dig. 2016;108:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Dall'Oglio L, Caldaro T, Foschia F, Faraci S, Federici di Abriola G, Rea F, Romeo E, Torroni F, Angelino G, De Angelis P. Endoscopic management of esophageal stenosis in children: New and traditional treatments. World J Gastrointest Endosc. 2016;8:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 6. | Tambucci R, Angelino G, De Angelis P, Torroni F, Caldaro T, Balassone V, Contini AC, Romeo E, Rea F, Faraci S, Federici di Abriola G, Dall'Oglio L. Anastomotic Strictures after Esophageal Atresia Repair: Incidence, Investigations, and Management, Including Treatment of Refractory and Recurrent Strictures. Front Pediatr. 2017;5:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | García-Cano J, Muñoz-Sánchez M, Morillas-Ariño J. Stenting of strictures close to the upper esophageal sphincter with the Polyflex stent. World J Gastrointest Endosc. 2009;1:65-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (2)] |
| 8. | Ghobrial CM, Eskander AE. Prospective study of the effect of topical application of Mitomycin C in refractory pediatric caustic esophageal strictures. Surg Endosc. 2018;32:4932-4938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Chapuy L, Pomerleau M, Faure C. Topical mitomycin-C application in recurrent esophageal strictures after surgical repair of esophageal atresia. J Pediatr Gastroenterol Nutr. 2014;59:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Berger M, Ure B, Lacher M. Mitomycin C in the therapy of recurrent esophageal strictures: hype or hope? Eur J Pediatr Surg. 2012;22:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Rustagi T, Aslanian HR, Laine L. Treatment of Refractory Gastrointestinal Strictures With Mitomycin C: A Systematic Review. J Clin Gastroenterol. 2015;49:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kochhar R, Poornachandra KS. Intralesional steroid injection therapy in the management of resistant gastrointestinal strictures. World J Gastrointest Endosc. 2010;2:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 13. | Szapáry L, Tinusz B, Farkas N, Márta K, Szakó L, Meczker Á, Hágendorn R, Bajor J, Vincze Á, Gyöngyi Z, Mikó A, Csupor D, Hegyi P, Erőss B. Intralesional steroid is beneficial in benign refractory esophageal strictures: A meta-analysis. World J Gastroenterol. 2018;24:2311-2319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Bicakci U, Tander B, Deveci G, Rizalar R, Ariturk E, Bernay F. Minimally invasive management of children with caustic ingestion: less pain for patients. Pediatr Surg Int. 2010;26:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Nijhawan S, Udawat HP, Nagar P. Aggressive bougie dilatation and intralesional steroids is effective in refractory benign esophageal strictures secondary to corrosive ingestion. Dis Esophagus. 2016;29:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | van Hal ARL, Pulvirenti R, den Hartog FPJ, Vlot J. The Safety of Intralesional Steroid Injections in Young Children and Their Effectiveness in Anastomotic Esophageal Strictures-A Meta-Analysis and Systematic Review. Front Pediatr. 2021;9:825030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | ASGE Technology Committee; Varadarajulu S, Banerjee S, Barth B, Desilets D, Kaul V, Kethu S, Pedrosa M, Pfau P, Tokar J, Wang A, Song LM, Rodriguez S. Enteral stents. Gastrointest Endosc. 2011;74:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Lorenzo-Zúñiga V, Moreno-de-Vega V, Marín I, Boix J. Biodegradable stents in gastrointestinal endoscopy. World J Gastroenterol. 2014;20:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Broto J, Asensio M, Vernet JM. Results of a new technique in the treatment of severe esophageal stenosis in children: poliflex stents. J Pediatr Gastroenterol Nutr. 2003;37:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell À, Garcia-Pagán JC, Dumonceau JM, Conio M, de Ceglie A, Skowronek J, Nordsmark M, Seufferlein T, Van Gossum A, Hassan C, Repici A, Bruno MJ. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 21. | Fuccio L, Hassan C, Frazzoni L, Miglio R, Repici A. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy. 2016;48:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Caldaro T, Torroni F, De Angelis P, Federici di Abriola G, Foschia F, Rea F, Romeo E, Dall'Oglio L. Dynamic esophageal stents. Dis Esophagus. 2013;26:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Foschia F, De Angelis P, Torroni F, Romeo E, Caldaro T, di Abriola GF, Pane A, Fiorenza MS, De Peppo F, Dall'Oglio L. Custom dynamic stent for esophageal strictures in children. J Pediatr Surg. 2011;46:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lévesque D, Baird R, Laberge JM. Refractory strictures post-esophageal atresia repair: what are the alternatives? Dis Esophagus. 2013;26:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Samanta J, Dhaka N, Sinha SK, Kochhar R. Endoscopic incisional therapy for benign esophageal strictures: Technique and results. World J Gastrointest Endosc. 2015;7:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Hordijk ML, van Hooft JE, Hansen BE, Fockens P, Kuipers EJ. A randomized comparison of electrocautery incision with Savary bougienage for relief of anastomotic gastroesophageal strictures. Gastrointest Endosc. 2009;70:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc. 2006;63:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Siersema PD. How to Approach a Patient With Refractory or Recurrent Benign Esophageal Stricture. Gastroenterology. 2019;156:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Muto M, Ezoe Y, Yano T, Aoyama I, Yoda Y, Minashi K, Morita S, Horimatsu T, Miyamoto S, Ohtsu A, Chiba T. Usefulness of endoscopic radial incision and cutting method for refractory esophagogastric anastomotic stricture (with video). Gastrointest Endosc. 2012;75:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Manfredi MA, Clark SJ, Medford S, Staffa SJ, Ngo PD, Hamilton TE, Smithers CJ, Jennings RW. Endoscopic Electrocautery Incisional Therapy as a Treatment for Refractory Benign Pediatric Esophageal Strictures. J Pediatr Gastroenterol Nutr. 2018;67:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Intervent Radiol. 2006;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Ramcharitar S, Vaina S, Serruys PW. The next generation of drug-eluting stents: what's on the horizon? Am J Cardiovasc Drugs. 2007;7:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | ASGE Technology Committee; Tokar JL, Banerjee S, Barth BA, Desilets DJ, Kaul V, Kethi SR, Pedrosa MC, Pfau PR, Pleskow DK, Varadarajulu S, Wang A, Song LM, Rodriguez SA. Drug-eluting/biodegradable stents. Gastrointest Endosc. 2011;74:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Walter D, van den Berg MW, Hirdes MM, Vleggaar FP, Repici A, Deprez PH, Viedma BL, Lovat LB, Weusten BL, Bisschops R, Haidry R, Ferrara E, Sanborn KJ, O'Leary EE, van Hooft JE, Siersema PD. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial. Endoscopy. 2018;50:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Gkolfakis P, Siersema PD, Tziatzios G, Triantafyllou K, Papanikolaou IS. Biodegradable esophageal stents for the treatment of refractory benign esophageal strictures. Ann Gastroenterol. 2020;33:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Kailla E, Rezai F, Kansci AK, Akande O, Gossage J. SX-ELLA biodegradable stent for benign oesophageal strictures: a systematic review and proportion meta-analysis. Surg Endosc. 2023;37:2476-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Awolaran O, McGuirk S, Arul GS. Biodegradable Stents in the Management of Refractory Esophageal Strictures in Children. J Laparoendosc Adv Surg Tech A. 2020;30:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Kantarcioglu M, Caliskan B, Demirci H, Karacalioglu O, Kekilli M, Polat Z, Gunal A, Akinci M, Uysal C, Eksert S, Gurel H, Celebi G, Avcu F, Ural AU, Bagci S. The efficacy of mesenchymal stem cell transplantation in caustic esophagus injury: an experimental study. Stem Cells Int. 2014;2014:939674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Mizushima T, Ohnishi S, Hosono H, Yamahara K, Tsuda M, Shimizu Y, Kato M, Asaka M, Sakamoto N. Oral administration of conditioned medium obtained from mesenchymal stem cell culture prevents subsequent stricture formation after esophageal submucosal dissection in pigs. Gastrointest Endosc. 2017;86:542-552.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Wagh MS, Yang D, Chavalitdhamrong D, Draganov PV. Per-oral endoscopic tunneling for restoration of the esophagus (POETRE). Gastrointest Endosc. 2014;80:330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Wagh MS, Draganov PV. Per-oral endoscopic tunneling for restoration of the esophagus: a novel endoscopic submucosal dissection technique for therapy of complete esophageal obstruction. Gastrointest Endosc. 2017;85:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | May JT 4th, Padhya TA, McCaffrey TV. Endoscopic repair of Zenker's diverticulum by harmonic scalpel. Am J Otolaryngol. 2011;32:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Kwek WMJ, Chua SMCA, Xu SH, Tan THL, Huang XY, Loh I, Lee TS. Randomized controlled study comparing tonsillectomy safety and patient satisfaction outcomes between HARMONIC ACE® + shears and monopolar diathermy in an adult population - A pilot study. Am J Otolaryngol. 2020;41:102568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Baldwin DL, Toma AG. Endoscopic stapled diverticulotomy: a real advance in the treatment of hypopharyngeal diverticulum. Clin Otolaryngol Allied Sci. 1998;23:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Tarifi AA, Al-Qutiesh BH, Badran KH, Al-Mallah HH, Medina JE. Transoral Endoscopic Resection of Oropharyngeal Pedunculated Giant Fibrolipoma Using Harmonic Scalpel: A Case Report. Ear Nose Throat J. 2024;103:NP700-NP704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Philippsen LP, Weisberger EC, Whiteman TS, Schmidt JL. Endoscopic stapled diverticulotomy: treatment of choice for Zenker's diverticulum. Laryngoscope. 2000;110:1283-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Cook RD, Huang PC, Richstmeier WJ, Scher RL. Endoscopic staple-assisted esophagodiverticulostomy: an excellent treatment of choice for Zenker's diverticulum. Laryngoscope. 2000;110:2020-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/