Published online Sep 16, 2024. doi: 10.4253/wjge.v16.i9.526
Revised: May 29, 2024
Accepted: July 30, 2024
Published online: September 16, 2024
Processing time: 167 Days and 3.9 Hours
Helicobacter pylori (H. pylori) is the most common chronic bacterial infection in humans. The risk of acquiring H. pylori is related to socioeconomic status and living conditions early in life. Treatment regimens must consider local antibiotic resistance patterns. Adventist Health White Memorial Hospital serves a predominantly indigent population in east Los Angeles with a large number of immi
To evaluate the prevalence and resistance of H. pylori and correlate with country of origin.
All gastric biopsies were obtained by a single gastroenterologist at the hospital in a consecutive manner from patients with gastritis from 2017 to 2022 and sent to various labs for evaluation.
Two hundred and sixty-six patients are born in the United States, 450, 171, 70, and 30 patients are immigrants from Mexico, Central and South America (CSA), Asia, and other countries respectively. Overall, 14.65% were found to be infected with H. pylori. Rates of infection in United States-born citizens, immigrants from Mexico, CSA, and Asia are 9.02%, 18.67%, 13.45%, and 11.43% respectively, with Mexican immigrants having a relative risk of 2.3889 [95% confidence interval (CI): 1.4789-3.8588, P = 0.0004] compared to those born in United States. No correlation seen between infection and length of time immigrants were in United States. Relative risk of infection in patients with no proton pump inhibitor use within the past 30 days found to be 1.9276 (95%CI: 1.3562-2.7398, P = 0.0003). Rates of resistance for clarithromycin and levofloxacin are 21.43% and 31.11%.
H. pylori infection appears to be associated with low socioeconomic status and poor living conditions early in life. Clarithromycin and levofloxacin based treatment regimens should be avoided as first line therapy in this region, particularly in patients of Latin American origin.
Core Tip: Gastric biopsies obtained from patients with gastritis in a predominantly indigent immigrant community in Los Angeles were tested for Helicobacter pylori in order to better understand its prevalence and resistance. Immigrants from Mexico found to have a significantly higher risk of infection regardless of length of time in the United States. Infection is significantly higher in those who did not take a proton pump inhibitor. Clarithromycin and levofloxacin should be avoided as first line therapy in this region, particularly in patients of Latin American origin. Infection appears to be associated with low socioeconomic status and poor living conditions early in life.
- Citation: Tabesh A, Antillon RA, Kondradzhyan M, Tan AZ. Prevalence and resistance of Helicobacter pylori in a predominantly Hispanic population. World J Gastrointest Endosc 2024; 16(9): 526-532
- URL: https://www.wjgnet.com/1948-5190/full/v16/i9/526.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i9.526
Helicobacter pylori (H. pylori) is a gram negative, microaerophilic, flagellated bacteria found predominantly in the gastrointestinal tract. This bacterium is also known to change its form from spiral to coccoid which helps it better survive in the gastric environment[1]. It is known to mainly transfer between people from an oro-oral route or a fecal-oral route. Another common source of infection is through vertical transmission which then can create generational infection[2]. H. pylori is classified as a class one carcinogen and is a known culprit associated with duodenal and gastric ulcers, gastritis, gastric adenocarcinoma, and mucosa associated lymphoid tissue lymphoma[2]. H. pylori is the most common infection in the world, reaching 50% prevalence[3]. When observing specific sections of the world, there is around 30%-50% prevalence of infection in developed countries and ranges up to 85%-95% infection prevalence in developing countries[1]. The overall prevalence in the United States is estimated to be around 35% to 40%, which is consistent with the prevalence in other developed countries[4]. In contrast, in Mexico, prevalence has been reported to be 66% and ranges from 48% to 83%[5]. Increasing rates of resistance have mandated the need for local data regarding H. pylori sensitivity to help guide therapy. First line therapies which traditionally include a proton pump inhibitor, clarithromycin and amoxicillin combination have become increasingly ineffective mostly due to clarithromycin resistance[6]. Worldwide, rates of H. pylori antibiotic resistance have been reported to be 47.22% [95% confidence interval (95%CI): 30.50%-75.02%] for metronidazole, 19.74% (95%CI: 5.46%-30.80%) for clarithromycin, 18.94% (95%CI: 14.19%-25.28%) for levofloxacin, 14.67% (95%CI: 2.00%-40.87%) for amoxicillin and 11.70% (95%CI: 0%-50.00%) for tetracycline[7]. In the United States, most recent data find resistance to metronidazole, amoxicillin, and clarithromycin to be 65.4%, 2.1%, and 21.8% respectively[8]. Resistance for levofloxacin and tetracycline has been reported at 57.8% and 2.8% respectively[9]. Adventist Health White Memorial Medical Center (AHWM) is a private, faith based, teaching hospital located in East Los Angeles. It provides health care to more than 2 million people in adensely populated area where most residents live below the federal poverty level. Most patients are of Hispanic background originating from Mexico and Central and South America.
All patients who underwent upper endoscopy on an outpatient basis by a single gastroenterologist at AHWM from 2017 to 2022 were enrolled after signing an informed written consent. Patients who were poor candidates for sedation, suffered from underlying coagulopathy or cirrhosis were excluded. During endoscopy, all patients had 2 sets of biopsies (each set including specimen from the lesser and greater curvature of the gastric antrum and body). One set of specimens was stored in a formalin container and sent to the pathology department where it was processed into histologic slides, to evaluate for H. pylori. This consisted of an H&E slide and H. pylori immunostain [using Ventana anti-H. pylori (SP48) rabbit monoclonal primary antibody] preparation. The second set was placed in a container of Brucella Broth with glycerol and kept on ice. It was then transferred to the microbiology department where it was stored in a -20 refrigerator.
In those patients who were found to be infected with H. pylori, we attempted to grow the organism in the microbiology department at AHWM using the following protocol: (1) Vortex the biopsies in the Brucella Broth with glycerol; (2) Inoculate the campylobacter plate (containing 5% sheep blood, polymyxin B, vancomycin, trimethoprim, and ampho
Our success rate in growing the organism was about 50%. Consequently, we started sending our specimen to ARUP laboratories (Associated Regional and University Pathologists, INC.) in 2019 for culture. After growth, ARUP would send the organism to mayo clinic in Rochester, Minnesota for susceptibility testing. Mayo clinic used broth microdilution. Frequently, the organism was nonviable upon arrival at mayo clinic. Mayo clinic checked for susceptibilities to amoxi
Susceptibility data for the listed antibiotics was documented by the laboratory technician with a written document/form. Once the microbiologic study was completed, the results were provided to the research staff in addition to the corresponding clinician. The data matrix was formulated on “Excel spreadsheet”. The data matrix contains a subject number, ethnicity, sex, and the length of time that the subject has been living in the United States. Collaboration was done between AHWM staff and the research analyst from Loma Linda University. This collaboration brought the completion of the literature review, and the data analysis through Statistical Analysis Software.
Logistic regression model was the primary model used for data analysis. The final model used was “current H. pylori infection” as the dependent variable, with “sex”, “age”, “country of origin”, and “prior proton pump inhibitor (PPI) use” as the independent variables. Before this model was used, missing data analysis was done, linearity analysis for one continuous independent variable was done, correlation analysis was done to rule out any multicollinearity, and outlier analysis and sensitivity analysis on those outliers were done.
Preliminarily, prevalence analysis was performed to observe the prevalence of H. pylori based on patients’ country of origin. Finally, logistic regression of the final model was performed to observe if there were any significant differences of H. pylori infection depending on the region of origin. Analysis was not able to be done for antibiotic resistances because of the low sample size available.
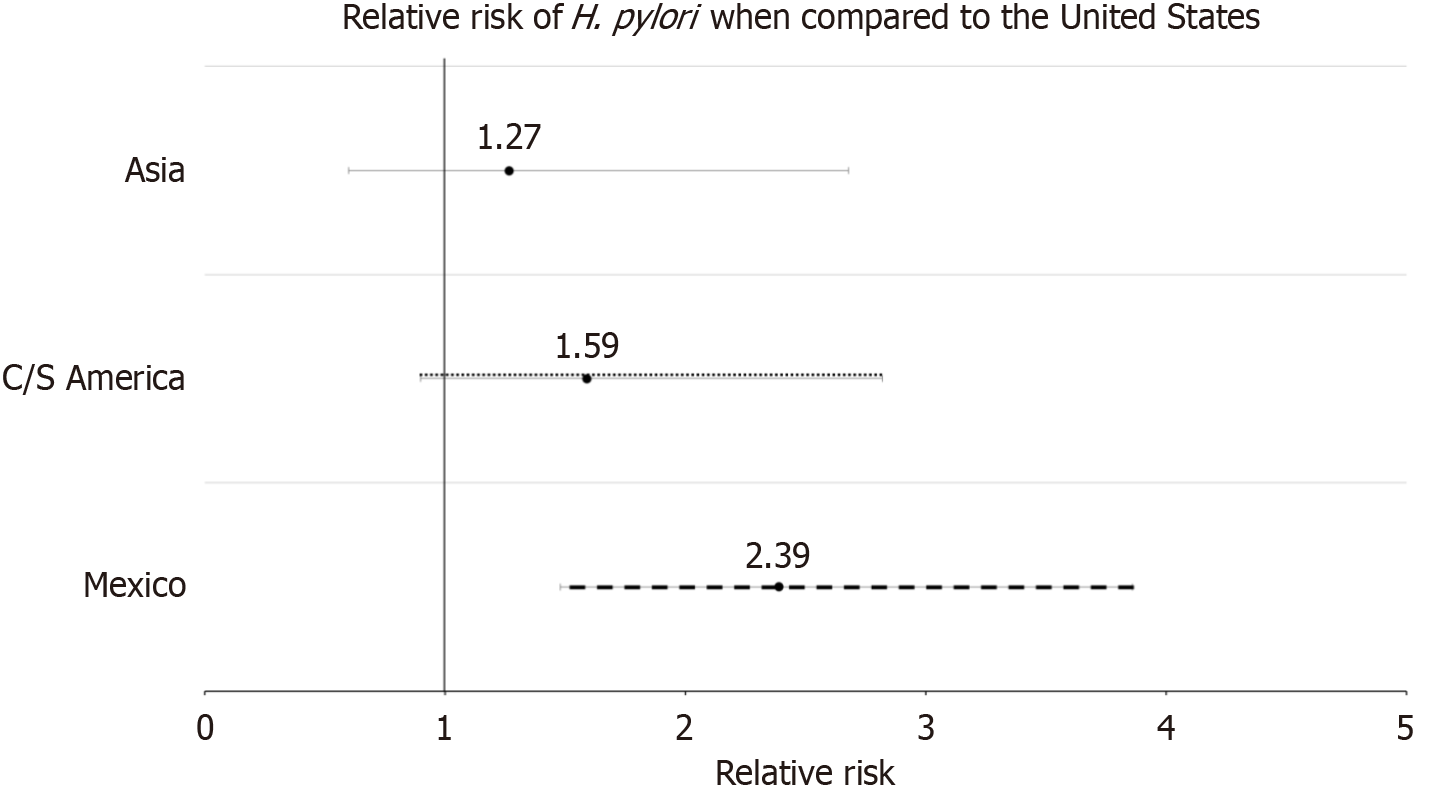
A total of 987 patients were enrolled in the study, but there were 18 observations which were dropped from the risk analysis because of missing data. Baseline demographics are seen in Table 1. Patient’s country of origin was separated into those who were born in the United States, Mexico, South and Central America, Asia, and others. While the overall average age is 57.08 (SD ± 15.20), those who are immigrants have been in the United States for an average of 35.13 years (SD ± 12.03). Almost half of the patients are born in Mexico (46%) while only about 28% are born in the United States (Table 1). Full data was available in 969 subjects which was used for analysis regarding the prevalence of H. pylori, and 18 participants were not included. One hundred and forty-two patients were found to have H. pylori infection (14.65%) (Table 2). Prevalence of H. pylori in patients from various ethnicities is seen in Table 3. While all immigrants have a higher prevalence of infection compared to those born in the United States, those of Mexican origin have the highest prevalence of infection by far. Relative risk of H. pylori infection was found to be 2.3889 in those born in Mexico compared to those born in the United States (1.4789-3.8588, P = 0.0004) (Table 3; Figure 1). Amongst the immigrants, the average number of years in the United States ranged from Asia with the lowest at around 21 years to 38 years in those from Mexico (Table 2). No correlation was seen between the number of years in the United States and the presence of H. pylori infection.
| Variable | n (%) |
| Sex | |
| Male | 323 (32.86) |
| Female | 660 (67.14) |
| Average age | 57.03 (N/A) |
| Average years in the United States | 37.98 (N/A) |
| Ethnicity | |
| United States | 266 (27.51) |
| Mexico | 450 (46.43) |
| Central/South America | 172 (17.68) |
| Asia | 70 (7.14) |
| Other | 12 (1.24) |
| Prior PPI | |
| Yes | 495 (50.15) |
| No | 432 (43.77) |
| Unknown | 60 (6.08) |
| Reasons for EGD | |
| Abdominal pain | 319 (32.29) |
| Abnormal growth/cancer | 33 (3.34) |
| Anemia | 47 (4.76) |
| Dysphagia | 42 (4.25) |
| Early satiety | 6 (0.61) |
| Gastric ulcer | 6 (0.61) |
| Esophagitis | 18 (1.82) |
| GERD | 254 (25.71) |
| Nausea/vomiting | 14 (1.42) |
| Occult blood in stool | 13 (1.32) |
| PEG involvement | 6 (0.61) |
| Weight loss | 56 (5.67) |
| Other | 11 (1.11) |
| None/unknown | 163 (16.50) |
| Country/region of origin | Male | Age, mean ± SD | Years in the United States, mean ± SD | Prior PPI | H. pylori infection |
| United States | 104 (39.10) | 45.69 ± 17.76 | 45.56 ± 17.84 | 113 (42.48) | 22/266 (9.02) |
| Mexico | 137 (30.51) | 62.76 ± 11.03 | 37.95 ± 11.06 | 258 (57.33) | 84/450 (18.67) |
| Central and South America | 44 (26.04) | 60.68 ± 11.45 | 33.21 ± 10.53 | 94 (54.97) | 23/171 (13.45) |
| Asia | 25 (35.71) | 54.89 ± 11.87 | 21.88 ± 11.32 | 26 (37.14) | 8/70 (11.43) |
| Other | 5 (41.67) | 56.92 ± 13.89 | 33.55 ± 15.51 | 4 (33.33) | 3/12 (25.00) |
| Total | 309 (32.56) | 57.08 ± 15.20 | 38.01 ± 14.63 | 495 (51.08) | 142/969 (14.65) |
| Variable | Relative risk (95%CI) | P value |
| Sex (reference male) | ||
| Female | 0.85 (0.61-1.17) | 0.31 |
| Age | 1.00 (0.98-1.01) | 0.50 |
| Country of origin (Reference United States) | ||
| Mexican origin | 2.39 (1.48-3.86) | < 0.01a |
| Central/South American origin | 1.59 (0.90-2.82) | 0.11 |
| Asian origin | 1.27 (0.60-2.68) | 0.54 |
| PPI use | ||
| No prior PPI | 1.93 (1.36-2.74) | < 0.01a |
Those who had not taken a proton pump inhibitor within the past 30 days before the endoscopy had a greater risk of H. pylori infection (1.9276, 1.3562-2.7398, P = 0.0003) (Table 3). Presence of H. pylori did not correlate with patient sex or age. Data regarding the sensitivity of H. pylori is limited due to difficulty in growing the organism. Overall, resistance to amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline are 10.29%, 21.43%, 12.70%, 31.11%, and 0.00% respectively (Table 4). Prevalence of resistance to H. pylori amongst different ethnicities is seen in Table 3. While large differences can be seen among different ethnicities, statistical significance cannot be shown due to an overall small sample size. Evaluation for dual resistance found 27% resistance to clarithromycin and levofloxacin. No other significant findings were seen (Table 5).
| Country/region of origin | Amoxicillin resistance (n = 68) | Clarithromycin resistance (n = 70) | Metronidazole resistance (n = 63) | Levofloxacin resistance (n = 45) | Tetracycline resistance (n = 58) |
| United States | 3/11 (27.27) | 1/11 (9.09) | 1/10 (10.00) | 0/6 (0.00) | 0/9 (0.00) |
| Mexico | 4/45 (8.89) | 12/46 (26.09) | 6/43 (13.95) | 11/31 (35.48) | 0/38 (0.00) |
| Central and South America | 0/9 (0.00) | 2/9 (22.22) | 1/8 (12.50) | 3/6 (50.00) | 0/7 (0.00) |
| Asia | 0/1 (0.00) | 0/2 (0.00) | 0/0 (0.00) | 0/1 (0.00) | 0/3 (0.00) |
| Other | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) | 0/1 (0.00) | 0/1 (0.00) |
| Total | 7/68 (10.29) | 15/70 (21.43) | 8/63 (12.70) | 14/45 (31.11) | 0/58 (0.00) |
| Antibiotic pair | Dual resistance |
| Amoxicillin + clarithromycin (n = 50) | 1 (2) |
| Amoxicillin + metronidazole (n = 48) | 0 (0) |
| Amoxicillin + levofloxacin (n = 32) | 1 (3) |
| Amoxicillin + tetracycline (n = 50) | 0 (0) |
| Clarithromycin + metronidazole (n = 44) | 3 (7) |
| Clarithromycin + levofloxacin (n = 26) | 7 (27) |
| Clarithromycin + tetracycline (n = 46) | 0 (0) |
| Metronidazole + levofloxacin (n = 26) | 3 (12) |
| Metronidazole + tetracycline (n = 44) | 0 (0) |
| Levofloxacin + tetracycline (n = 28) | 0 (0) |
Prevalence of H. pylori in our patient population is around 15%, which is considerably lower than 35% which has been reported in other national studies. This discrepancy is most likely due to the unique socioeconomic status and ethnicity of our patient population. Even though the majority of our patients are poor and underinsured, patients enrolled in the study all have some insurance coverage, mostly some type of Medi-Cal (equivalent to Medicaid nationally). Furthermore, the majority of our patients originate from Mexico with very few African Americans compared to the overall national prevalence. All immigrants have a higher prevalence of H. pylori compared to those born in the states. However, this is only statistically significant in those born in Mexico in whom H. pylori is more than twice as prevalent as those born in the United States (19% vs 9%, P = 0.0004). This is despite the fact that those born in Mexico have been in the United States an average of 38 years. Based on our data, immigrants from Mexico moved to the United States around the age of 25 and most likely became infected before that time.
There is high resistance to clarithromycin and levofloxacin (> 15%) in our patient population. These two antibiotics should be avoided as first line therapy for H. pylori. This is particularly true in those who are immigrants from Mexico and South/Central America. While overall resistance to amoxicillin is around 10%, those born in the United States have a much higher resistance of 27%. In comparison, prevalence of H. pylori infection in Latin American countries has been reported to be around 60%, ranging from 30% to 90% depending on the socioeconomic status of the population. Studies from Mexico have shown resistance of H. pylori to metronidazole and amoxicillin to be 59% and 3% respectively[11]. Overall, in Latin American countries, H. pylori resistance to metronidazole, amoxicillin, clarithromycin, fluoroquinolones and tetracycline have been reported to be 56%, 4%, 12%, 15%, and 7% respectively[11]. This again highlights the importance of having regional data for choosing optimal antibiotic regimen for treatment of H. pylori. Our study suggests that H. pylori is associated with decreased use of PPI. This is consistent with some prior studies which show an inverse correlation between gastroesophageal reflux disease, which is the most common reason for taking PPI, and presence of H. pylori infection[12]. It has been proposed that corpus-dominant gastritis induced by H. pylori decreases acid secretion via local inflammation and increased levels of cytokines, such as tumor necrosis factor alpha and interleukin-1-beta[13]. These changes can eventually lead to hypochlorhydria and gastric atrophy with decreased acid secretion. Need for PPI should not have any impact on the decision to eradicate this potentially carcinogenic organism. Our inability to grow the organism effectively from the very beginning of the study greatly limits our data regarding its sensitivity. Furthermore, the need to send organisms to different laboratories during the study required us to compare MIC’s to different antibiotics from different laboratories with slight variances.
Our results seem to confirm a well-documented relationship between H. pylori infection and low socioeconomic status and poor living conditions early in life when most people seem to acquire this infection. Furthermore, our findings suggest that at least in our patient population, ethnicity may be a considerable factor when choosing the optimal antibiotic regimen for treating H. pylori. Negative correlation between use of PPI’s and prevalence of H. pylori reinforces the theory that H. pylori lead to decreased gastric acid secretion. Larger studies are needed to confirm these findings.
We would like to thank Hector Montero, the staff at White Memorial GI lab, Preoperative Services and Microbiology Department led by Fulgentius Wong (Chot) without whom this project would have never been completed. Authors have no relevant financial relationships to disclose.
| 1. | Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 2. | Burkitt MD, Duckworth CA, Williams JM, Pritchard DM. Helicobacter pylori-induced gastric pathology: insights from in vivo and ex vivo models. Dis Model Mech. 2017;10:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2199] [Article Influence: 244.3] [Reference Citation Analysis (3)] |
| 4. | Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, Sulka A, Swaminathan B, Taylor T, Hoekstra M, Griffin P, Smoot D, Peek R, Metz DC, Bloom PB, Goldschmidt S, Parsonnet J, Triadafilopoulos G, Perez-Perez GI, Vakil N, Ernst P, Czinn S, Dunne D, Gold BD. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Alarid-Escudero F, Enns EA, MacLehose RF, Parsonnet J, Torres J, Kuntz KM. Force of infection of Helicobacter pylori in Mexico: evidence from a national survey using a hierarchical Bayesian model. Epidemiol Infect. 2018;146:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 748] [Article Influence: 46.8] [Reference Citation Analysis (1)] |
| 7. | Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J Methodol. 2015;5:164-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 8. | Mégraud F, Graham DY, Howden CW, Trevino E, Weissfeld A, Hunt B, Smith N, Leifke E, Chey WD. Rates of Antimicrobial Resistance in Helicobacter pylori Isolates From Clinical Trial Patients Across the US and Europe. Am J Gastroenterol. 2023;118:269-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Hulten KG, Lamberth LB, Kalfus IN, Graham DY. National and Regional US Antibiotic Resistance to Helicobacter pylori: Lessons From a Clinical Trial. Gastroenterology. 2021;161:342-344.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277-283. [PubMed] |
| 11. | Camargo MC, García A, Riquelme A, Otero W, Camargo CA, Hernandez-García T, Candia R, Bruce MG, Rabkin CS. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Straubinger RK, Greiter A, McDonough SP, Gerold A, Scanziani E, Soldati S, Dailidiene D, Dailide G, Berg DE, Simpson KW. Quantitative evaluation of inflammatory and immune responses in the early stages of chronic Helicobacter pylori infection. Infect Immun. 2003;71:2693-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |