Published online Jan 16, 2024. doi: 10.4253/wjge.v16.i1.11
Peer-review started: August 17, 2023
First decision: September 13, 2023
Revised: September 27, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: January 16, 2024
Processing time: 150 Days and 21.7 Hours
Many studies have addressed safety and effectiveness of non-anaesthesiologist propofol sedation (NAPS) for gastrointestinal (GI) endoscopy Target controlled infusion (TCI) is claimed to provide an optimal sedation regimen by avoiding under- or oversedation.
To assess safety and performance of propofol TCI sedation in comparison with nurse-administered bolus-sedation.
Fouty-five patients undergoing endoscopy under TCI propofol sedation were prospectively included from November 2016 to May 2017 and compared to 87 patients retrospectively included that underwent endoscopy with NAPS. Patients were matched for age and endoscopic procedure. We recorded time of sedation and endoscopy, dosage of medication and adverse events.
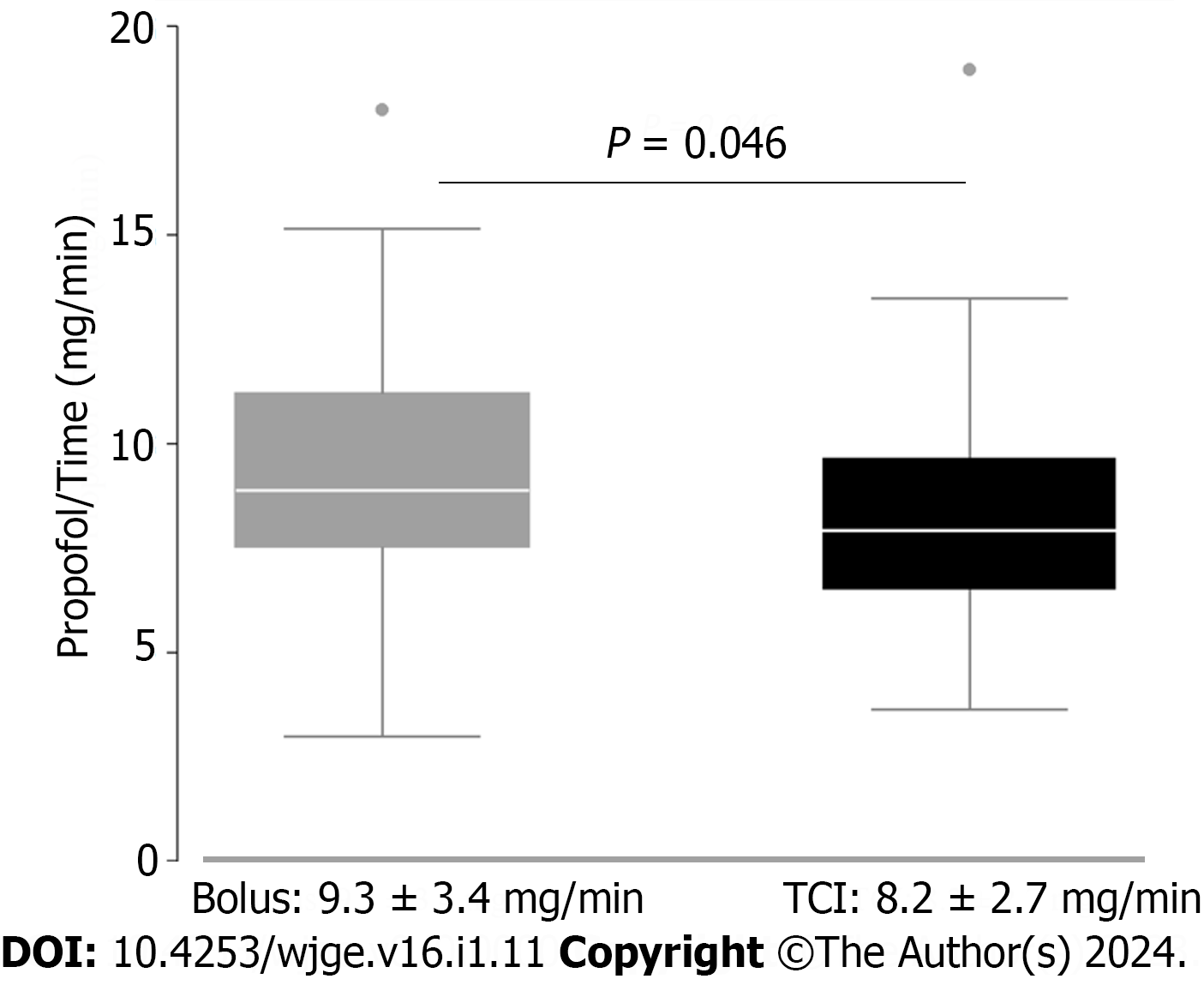
There was a significant reduction in dose per time of propofol administered in the TCI group, compared to the NAPS group (8.2 ± 2.7 mg/min vs 9.3 ± 3.4 mg/min; P = 0.046). The time needed to provide adequate sedation levels was slightly but significantly lower in the control group (5.3 ± 2.7 min vs 7.7 ± 3.3 min; P < 0.001), nonetheless the total endoscopy time was similar in both groups. No differences between TCI and bolus-sedation was observed for mean total-dosage of propofol rate as well as adverse events.
This study indicates that sedation using TCI for GI endoscopy reduces the dose of propofol necessary per minute of endoscopy. This may translate into less adverse events. However, further and randomized trials need to confirm this trend.
Core Tip: First, target controlled infusion (TCI) is claimed to provide an optimal sedation regimen. Secondly, little is known about the differences of time of sedation and propofol dosage between nurse-administered intermittent bolus propofol sedation and TCI. Thirdly, sedation using TCI for gastrointestinal (GI) endoscopy reduces the dose of propofol necessary per minute of endoscopy (8.2 ± 2.7 mg/min vs 9.3 ± 3.4 mg/min; P = 0.046). Fourthly, sedation using TCI for GI endoscopy could have an impact on propofol total dosage on prolonged endoscopy procedures. Fifthly, this may translate into less adverse events and higher safety when using TCI in prolonged procedures.
- Citation: Sarraj R, Theiler L, Vakilzadeh N, Krupka N, Wiest R. Propofol sedation in routine endoscopy: A case series comparing target controlled infusion vs manually controlled bolus concept. World J Gastrointest Endosc 2024; 16(1): 11-17
- URL: https://www.wjgnet.com/1948-5190/full/v16/i1/11.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i1.11
Many studies have addressed the safety and effectiveness of non-anaesthesiologist propofol sedation (NAPS) for gastrointestinal (GI) endoscopy[1-5]. A high dose of propofol has been recognized as an independent risk factor for sedation-related complications[6]. For the safe use of propofol during endoscopic procedures performed by non-anaesthesiologists, controlled comparisons between different methods of propofol administration are still needed.
One of the most frequent methods of propofol sedation in GI endoscopy is manual administration of boluses. This method may be sub optimal during long-lasting endoscopies[7]. Target Controlled Infusion (TCI) is a delivery system with an infusion mode that uses pharmacokinetic models (based on age, sex, height, weight and dosing history in the individual patient) to calculate infusion rates required to reach and maintain a desired target concentration in the target tissue of the brain. Ultimately, the system is claimed to provide a calculated optimal sedation regimen hence avoiding under- or oversedation[8-10].
The aim of the present study was to assess safety and performance, in terms of time of sedation and dosage of propofol during TCI sedation in comparison with nurse-administered intermittent bolus propofol sedation.
Forty-five consecutive patients undergoing endoscopy under TCI propofol sedation were prospectively included from November 2016 to May 2017. These were compared to a historic cohort of sex and age-matched patients that underwent endoscopy with bolus-sedation (n = 80). These comparator patients were matched for type endoscopic procedure. Exclusion criteria were age under 18 years; pregnant and lactating women; American Society of Anaesthesiologists class IV; allergy to propofol, fentanyl, or benzodiazepine; and anticipated difficult airway.
Hospital faculty experienced endoscopists performed all endoscopic procedures in Table 1. Physical monitoring included heart rate, peripheral arterial oxygen saturation, and non-invasive blood pressure being monitored and recorded continuously with a bedside monitor. Blood pressure was recorded every 2 min. All patients received oxygen 2 L/min via nasal cannula throughout the procedure.
| Endoscopy | Bolus (n = 80) | TCI (n = 45) |
| Gastroscopy | 7 (8.7) | 3 (6.6) |
| Colonoscopy | 18 (22.5) | 9 (20.0) |
| Gastro/Colo | 30 (37.5) | 18 (40.0) |
| EUS | 20 (25.0) | 12 (26.7) |
| ERCP | 5 (6.3) | 3 (6.7) |
Propofol was administered intravenously by using the Module Dependable Process Station TCI system (Fresenius Kabi, Bad Homburg, German) using the pharmacokinetic parameter set according to the Schnider model. The initial setting of the target blood concentration of propofol was set at 2.0 mg/mL. The predicted brain tissue concentration of propofol at each time point was calculated automatically and was shown on the monitor of the TCI pump. The primary plasma target concentration was set at 1.5 g/mL with the possibility to increase the target by 0.3 g/mL every two minutes to a maximum of 3.5 g/mL. this adjustment was made upon the patients response based on the Observers Assessment of Alertness/Sedation (OAA/S) score[11].
Historic comparator sedation protocol: Manual sedation was following the "20/2 rule"[12] with an induction bolus dose of 0.5-1.0 mg/kg of propofol (Disoprivan 1%) followed by titration of maximum 20 mg every 2 min. Low doses of fentanyl bolus (25-100 g) could be added at the discretion of the endoscopist in both sedation regimens.
Once patient lost verbal command and eyelash reflex (OAA/S scores < 2) endoscopy was started. The induction period was defined as the time from the start of propofol infusion to insertion of the endoscope. The procedure time was defined as the time of the first endoscope insertion until endoscope removal.
Adverse events were defined as hypoxemia (peripheral oxygen saturation less than 90 %), hypotension (drop of mean arterial pressure below 60 mmHg), bradycardia (drop heart rate below 50 beats per minute for more than 1 min), and tachycardia (rise of heart rate above 110 beats per minute for more than 1 min). If hypoxemia occurred during the sedation, we performed chin lift on the patient and increased the oxygen dose.
The primary endpoint of the study was the consumption of propofol (mg) during endoscopy evaluated as dose (mg) per time (min).
Secondary endpoints include time of induction, total sedation time and safety regarding adverse events during sedation. The primary hypothesis stated that the use of TCI sedation would decrease the use of propofol over time and therefore be associated with a safer sedation.
All statistical analyses were performed with Stata 12.0. Results are presented as mean ± (SD). Differences between groups were calculated with Student’s t-test, Wilcoxon rank sum test and Chi2 test whenever appropriate. A value of P < 0.05 was regarded as significant.
All patients successfully underwent smooth procedures and no severe adverse event occurred. The demographic characteristics of the study participants did not show significant differences between the TCI group and the control group with respect to sex (female: 57% vs 43%; P = 0.67) and median age (55.9 vs 56.2; P = 0.17).
Endoscopy characteristics are shown in Table 1 and did not differ significantly between groups (P = 0.55).
The average total propofol consumption did not significantly differ between the groups (378.6 ± 213.1 mg vs 340.07 ± 150.07 mg; P = 0.59). However, there was a significant reduction in dose per time of propofol administered in the TCI group, compared to the bolus group (8.2 ± 2.7 mg/min vs 9.3 ± 3.4 mg/min; P = 0.046, Figure 1).
The time needed to provide proper sedation level was slightly but statistically significantly lower in the control group (5.3 ± 2.7 min vs 7.7 ± 3.3 min; P < 0.01). Nonetheless, the total endoscopy time was not different (42.3 ± 19.3 min vs 43.5 ± 18.2 min; P = 0.57).
There were no significant differences in the number of interventions utilizing fentanyl (71.2% vs 73.3% P = 0.8). However, average dose of fentanyl being used was significantly less in the TCI as compared to the control group (59.14 ± 28.37ug vs 36.67 ± 16.52 g; P 0.01).
No difference between bolus-sedation and TCI was observed for the rate of adverse events (26% vs 24%; P = 0.95, Table 2).
| Bolus (n = 80) | TCI (n = 45) | P value | |
| Total adverse events | 21 (26.0) | 11 (24.0) | 0.95 |
| Hypoxemia | 3 (14.4) | 4 (36.4) | |
| Hypotension | 10 (47.6) | 5 (45.4) | |
| Bradycardia | 4 (19.0) | 2 (18.2) | |
| Tachycardia | 4 (19.0) | 0 |
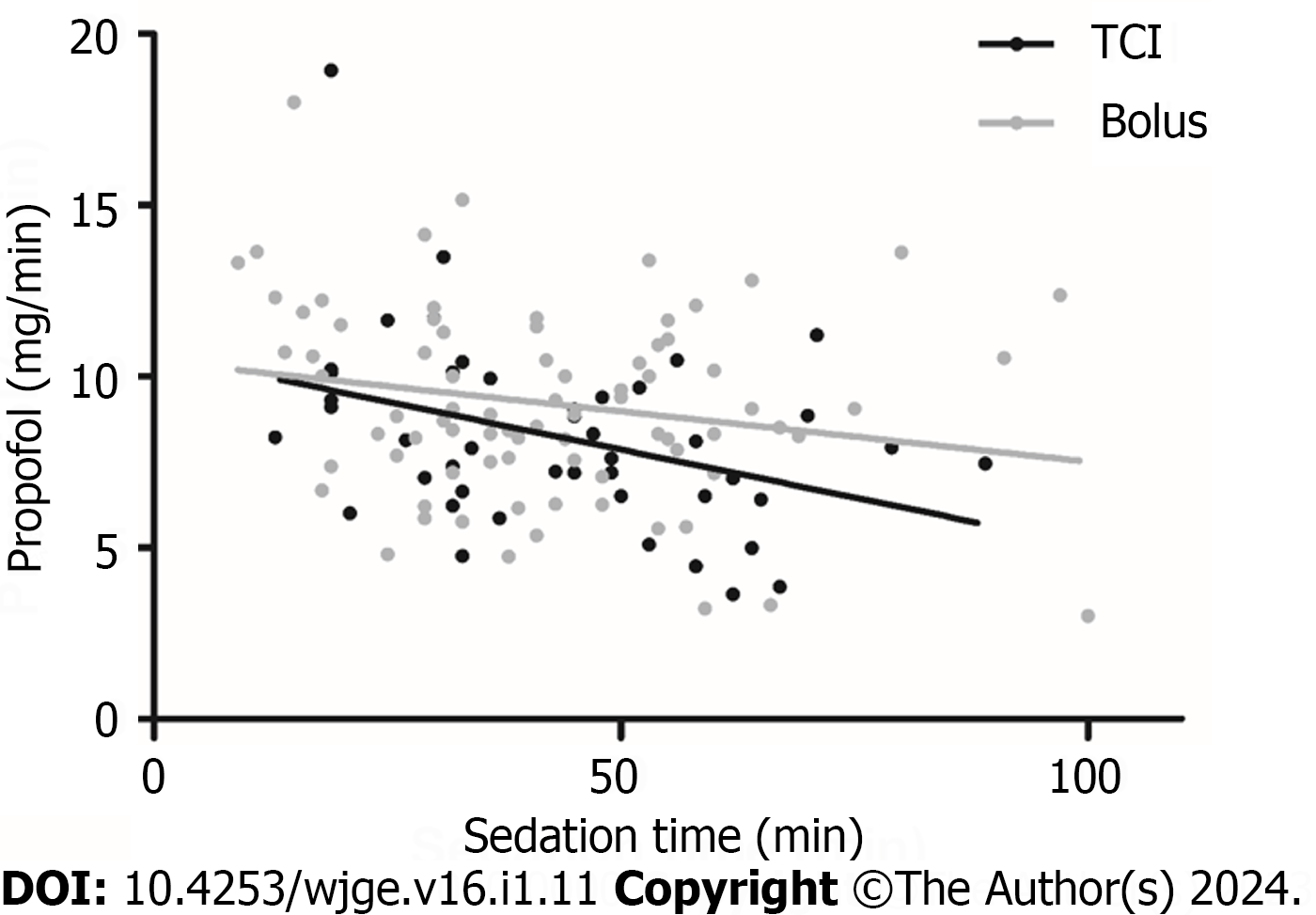
We ran a subgroup analysis with either short (less than 30 min) or long (more than 1 h) exams, for which results are shown in Tables 3 and 4. We found no significant reduction of dosage per time in favour of the TCI group looking at longer exams (8.94 ± 3.21 mg/min vs 6.82 ± 2.44 mg/min; P = 0.08, Figure 2). A reduction in total propofol dose in favour of the TCI group was observed looking at longer exams; this did not reach statistical significance (656.15 ± 291.42 vs 484.67 ± 200.02; P = 0.27).
| Variable (mean ± SD) | Bolus (n = 21) | TCI (n = 10) | Delta | P value |
| Induction time (min) | 4.81 ± 1.67 | 5.8 ± 1.81 | 0.99 | 0.11 |
| Total time (min) | 20.62 ± 6.49 | 21 ± 4.71 | 0.38 | 0.65 |
| Total dose (mg) | 198.1 ± 69.19 | 204.4 ± 74.23 | 6.3 | 0.81 |
| Dose/time (mg/min) | 10.14 ± 3.25 | 9.87 ± 3.58 | 0.27 | 0.58 |
| Variable (mean ± SD) | Bolus (n = 13) | TCI (n = 9) | Delta | P value |
| Induction time (min) | 6.08 ± 5.15 | 9.44 ± 3.50 | 3.36 | < 0.01 |
| Total time (min) | 73.30 ± 14.29 | 69.89 ± 8.95 | 3.41 | 0.89 |
| Total dose (mg) | 656.15 ± 291.42 | 484.67 ± 200.02 | 71.48 | 0.27 |
| Dose/time (mg/min) | 8.94 ± 3.21 | 6.82 ± 2.44 | 2.12 | 0.08 |
Propofol has been widely accepted as an ideal agent for endoscopy sedation because of the rapid onset of action and short recovery time[1,2]. However, propofol may cause cardiorespiratory inhibition necessitating providing of cardiorespiratory support with a ventilator until propofol is metabolized because there are no antagonists available. Thus, it is necessary to keep a balance between adequate sedation depth and minimized adverse effects. Intermittent bolus and continuous infusion are both alternatives for administration of propofol. However, the great variation in individual responses to propofol may be an important concern regarding safety during endoscopies[13].
During time-consuming endoscopic procedures, it may be difficult to obtain the optimal titration of drugs without increasing the risks of severe hypoxia, prolonged sedation and patient discharge after procedure[14].
Among different systems available for propofol administration, TCI uses a pharmacokinetic model to achieve and maintain a selected target plasma propofol concentration, through variation of the infusion rate, with a good predictive performance[8]. Previous studies on the use of TCI-based propofol administration demonstrated its feasibility and help in avoiding over- or under-sedation GI endoscopy[9,10]. Specifically, TCI-administered propofol sedation has been reported to achieve higher endoscopists satisfaction score, faster recovery of patients and more stable hemodynamic and respiratory conditions during endoscopy than manual infusion regimens particularly in hands of unexperienced training anaesthesiologists[15-17].
TCI-based propofol sedation has been evaluated in large series of various endoscopic procedures demonstrating safety and benefits[18,19].
The results of our study indicate that sedation using TCI for GI endoscopy reduces the dose of propofol necessary per minute of endoscopy. All procedures were carried out successfully and both methods of sedation were associated with adequate clinical sedation levels.
The occurrence of adverse events (around 25% in both groups) may seem high. However, we used very sensitive and conservative cut-offs to define adverse events, most of which were not severe or even life threatening. It is important to emphasie that our cohort didn’t include any patients who would have increased risk for and/or require per se a higher dosage of propofol known as confounding factors such as: Primary Sclerosing Cholangitis, IV Drug users, bad experience in pervious endoscopy, patients with severe pain syndromes and/or being on opiates.
We also ran a subgroup analysis with either short (less than 30 min) or long (more than 1 h) exams, expecting to find better results with longer endoscopy procedure.
The analysis is therefore based on fewer results and the results did not reach statistical significance, but we found a trend tend towards reduction of dosage per time (2.12 mg/min) in favour of the TCI group. It may also be interesting to note that a reduction of total propofol dose of approximatively 170 mg in favour of the TCI group was found, even if this difference did not reach statistical significance because of the large variance.
Another advantage that was stated by the nursing staff is the convenience of the pump, allowing for more time and focus for the endoscopy nurse to help with the procedure if necessary, as well as the fewer manual interactions of the syringes, which reduces the risk of contamination.
Interestingly, significantly less fentanyl was used in the TCI group. This could be interpreted as a relative underuse of propofol in the bolus group, where the total amount of propofol would have been expected to be higher compared to the TCI group. It seemed that in the bolus group, at least some of the propofol was substituted by fentanyl.
In conclusion, our study indicates that sedation using TCI for GI endoscopy reduces the dose of propofol necessary per minute of endoscopy and this could have an impact especially on prolonged endoscopy procedures. This may also translate into less adverse events and higher safety when using TCI in prolonged procedures. However, further studies on large scale with prospective randomized-controlled design are needed to standardize sedation with propofol. With proper education, TCI sedation could then be implemented in routine endoscopy procedures.
Non-anaesthesiologist propofol sedation (NAPS) for gastrointestinal (GI) endoscopy is safe and effective. Target controlled infusion (TCI) is claimed to provide an optimal sedation regimen by avoiding under or over-sedation.
Little is known about the differences of time of sedation and propofol dosage between nurse-administered intermittent bolus propofol sedation and TCI.
The aim of this study is to assess safety and performance of propofol TCI sedation in comparison with nurse-administered bolus-sedation.
Forty-five patients undergoing endoscopy under TCI propofol sedation were prospectively included from November 2016 to May 2017 and compared to 87 patients retrospectively included that underwent endoscopy with NAPS.
Sedation using TCI for GI endoscopy reduces the dose of propofol necessary per minute of endoscopy (8.2 ± 2.7 mg/min vs 9.3 ± 3.4 mg/min; P = 0.046). Time needed to provide adequate sedation levels was lower in the control group. No differences between TCI and bolus-sedation was observed for mean total-dosage of propofol rate as well as adverse events.
Sedation using TCI for GI endoscopy reduces the dose of propofol necessary per minute of endoscopy.
Sedation using TCI for GI endoscopy could have an impact on propofol total dosage especially on prolonged endoscopy procedures. This may also translate into less adverse events and higher safety when using TCI in prolonged procedures.
| 1. | Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB, Heuss LT, Peter S, Beglinger C, Sinnott JA, Welton T, Rofail M, Subei I, Sleven R, Jordan P, Goff J, Gerstenberger PD, Munnings H, Tagle M, Sipe BW, Wehrmann T, Di Palma JA, Occhipinti KE, Barbi E, Riphaus A, Amann ST, Tohda G, McClellan T, Thueson C, Morse J, Meah N. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229-37; quiz 1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Dumonceau JM, Riphaus A, Schreiber F, Vilmann P, Beilenhoff U, Aparicio JR, Vargo JJ, Manolaraki M, Wientjes C, Rácz I, Hassan C, Paspatis G. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline--Updated June 2015. Endoscopy. 2015;47:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Cohen LB, Dubovsky AN, Aisenberg J, Miller KM. Propofol for endoscopic sedation: A protocol for safe and effective administration by the gastroenterologist. Gastrointest Endosc. 2003;58:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | American Association for Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates, Vargo JJ, DeLegge MH, Feld AD, Gerstenberger PD, Kwo PY, Lightdale JR, Nuccio S, Rex DK, Schiller LR. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc. 2012;76:e1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Heuss LT, Froehlich F, Beglinger C. Nonanesthesiologist-administered propofol sedation: from the exception to standard practice. Sedation and monitoring trends over 20 years. Endoscopy. 2012;44:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol. 2008;43:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Newson C, Joshi GP, Victory R, White PF. Comparison of propofol administration techniques for sedation during monitored anesthesia care. Anesth Analg. 1995;81:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 8. | Schnider TW, Minto CF, Struys MM, Absalom AR. The Safety of Target-Controlled Infusions. Anesth Analg. 2016;122:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Fanti L, Agostoni M, Arcidiacono PG, Albertin A, Strini G, Carrara S, Guslandi M, Torri G, Testoni PA. Target-controlled infusion during monitored anesthesia care in patients undergoing EUS: propofol alone versus midazolam plus propofol. A prospective double-blind randomised controlled trial. Dig Liver Dis. 2007;39:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Struys MM, De Smet T, Glen JI, Vereecke HE, Absalom AR, Schnider TW. The History of Target-Controlled Infusion. Anesth Analg. 2016;122:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | De Oliveira GS Jr, Kendall MC, Marcus RJ, McCarthy RJ. The relationship between the Bispectral Index (BIS) and the Observer Alertness of Sedation Scale (OASS) scores during propofol sedation with and without ketamine: a randomized, double blinded, placebo controlled clinical trial. J Clin Monit Comput. 2016;30:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Zaluardo MP, Krayer S, Brunner T, Walder B, Bauerfeind P, Hartmeier S, Ammann P, Weilenmann D, Jacob AL, Franzen D. Empfehlungen und Standards für die Analgosedierung durch Nicht-Anästhesisten. Swiss Medical Forum. 2016;. [DOI] [Full Text] |
| 13. | Cohen LB. Endoscopy: Can computer-aided personalized sedation bridge troubled waters? Nat Rev Gastroenterol Hepatol. 2011;8:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Agostoni M, Fanti L, Gemma M, Pasculli N, Beretta L, Testoni PA. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc. 2011;74:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Barawi M, Gress F. Conscious sedation: is there a need for improvement? Gastrointest Endosc. 2000;51:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Wang JF, Li B, Yang YG, Fan XH, Li JB, Deng XM. Target-Controlled Infusion of Propofol in Training Anesthesiology Residents in Colonoscopy Sedation: A Prospective Randomized Crossover Trial. Med Sci Monit. 2016;22:206-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Chiang MH, Wu SC, You CH, Wu KL, Chiu YC, Ma CW, Kao CW, Lin KC, Chen KH, Wang PC, Chou AK. Target-controlled infusion vs. manually controlled infusion of propofol with alfentanil for bidirectional endoscopy: a randomized controlled trial. Endoscopy. 2013;45:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Gotoda T, Okada H, Hori K, Kawahara Y, Iwamuro M, Abe M, Kono Y, Miura K, Kanzaki H, Kita M, Kawano S, Yamamoto K. Propofol sedation with a target-controlled infusion pump and bispectral index monitoring system in elderly patients during a complex upper endoscopy procedure. Gastrointest Endosc. 2016;83:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 19. | Lin YJ, Wang YC, Huang HH, Huang CH, Liao MX, Lin PL. Target-controlled propofol infusion with or without bispectral index monitoring of sedation during advanced gastrointestinal endoscopy. J Gastroenterol Hepatol. 2020;35:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece; Sun SY, China S-Editor: Lin C L-Editor: A P-Editor: Cai YX