Published online Dec 16, 2023. doi: 10.4253/wjge.v15.i12.735
Peer-review started: August 29, 2023
First decision: October 10, 2023
Revised: October 31, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 16, 2023
Processing time: 98 Days and 16.5 Hours
Accurate diagnosis of Helicobacter pylori (H. pylori) infection status is a crucial premise for eradication therapy, as well as evaluation of risk for gastric cancer. Recent progress on imaging enhancement endoscopy (IEE) made it possible to not only detect precancerous lesions and early gastrointestinal cancers but also to predict H. pylori infection in real time. As a novel IEE modality, linked color imaging (LCI) has exhibited its value on diagnosis of lesions of gastric mucosa through emphasizing minor differences of color tone.
To compare the efficacy of LCI for H. pylori active infection vs conventional white light imaging (WLI).
PubMed, Embase, Embase and Cochrane Library were searched up to the end of April 11, 2022. The random-effects model was adopted to calculate the diagnostic efficacy of LCI and WLI. The calculation of sensitivity, specificity, and likelihood ratios were performed; symmetric receiver operator characteristic (SROC) curves and the areas under the SROC curves were computed. Quality of the included studies was chosen to assess using the quality assessment of diagnostic accuracy studies-2 tool.
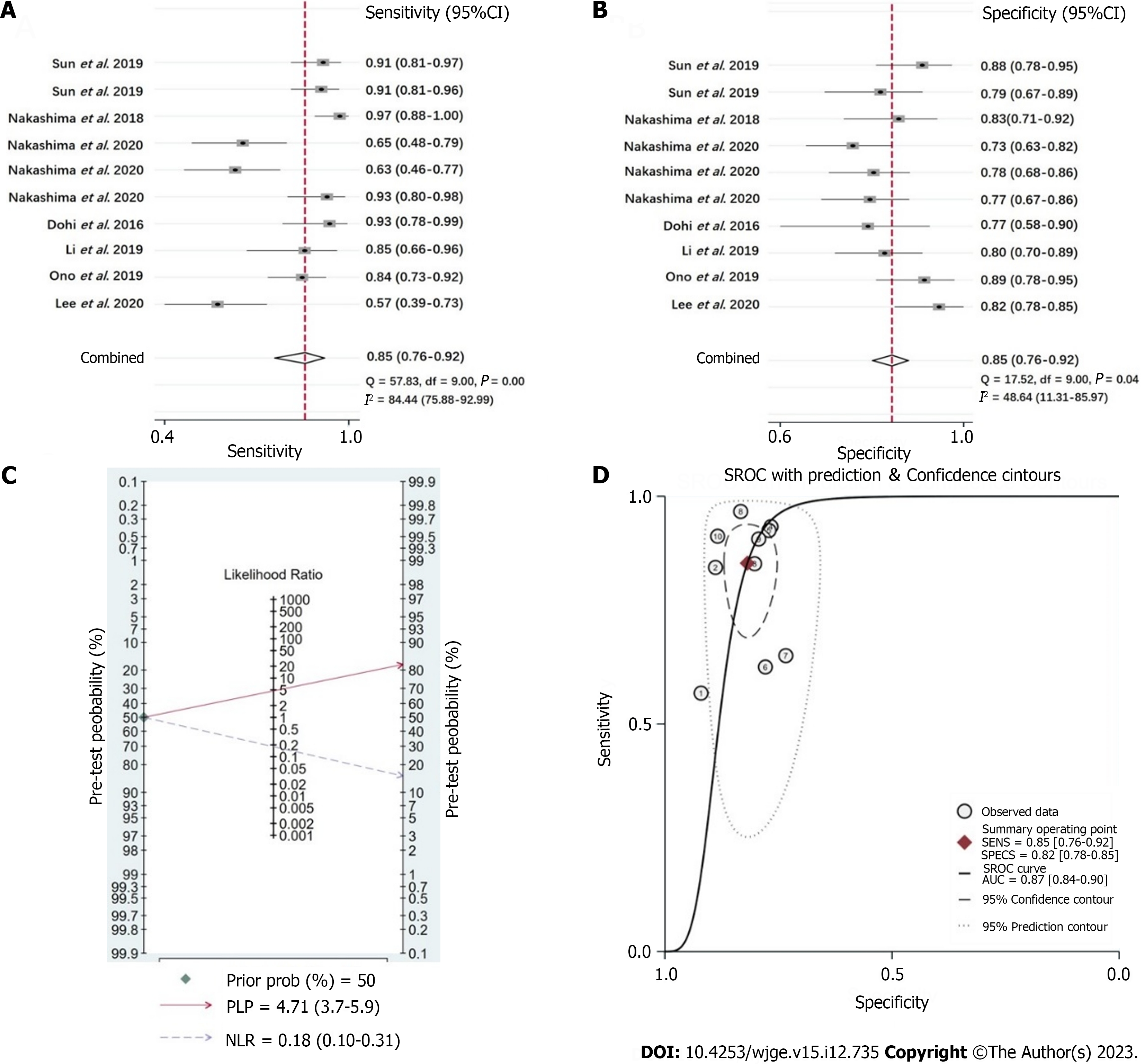
Seven original studies were included in this study. The pooled sensitivity, specificity, positive likelihood rate, and negative likelihood rate of LCI for the diagnosis of H. pylori infection of gastric mucosa were 0.85 [95% confidence interval (CI): 0.76-0.92], 0.82 (95%CI: 0.78-0.85), 4.71 (95%CI: 3.7-5.9), and 0.18 (95%CI: 0.10-0.31) respectively, with diagnostic odds ratio = 26 (95%CI: 13-52), SROC = 0.87 (95%CI: 0.84-0.90), which showed superiority of diagnostic efficacy compared to WLI.
Our results showed LCI can improve efficacy of diagnosis on H. pylori infection, which represents a useful endoscopic evaluation modality for clinical practice.
Core Tip: As a novel imaging enhancement endoscopy modality, linked color imaging (LCI) has exhibited its value on diagnosis of lesions of gastric mucosa through emphasizing minor differences of color tone. In this meta-analysis enrolled seven clinical trials, we showed LCI can improve efficacy of diagnosis on Helicobacter pylori infection compared with white light endoscopy, which represents a useful endoscopic evaluation modality for clinical practice.
- Citation: Zhang Y, Wang JZ, Bai X, Zhang PL, Guo Q. Clinical usefulness of linked color imaging in identifying Helicobacter pylori infection: A systematic review and meta-analysis. World J Gastrointest Endosc 2023; 15(12): 735-744
- URL: https://www.wjgnet.com/1948-5190/full/v15/i12/735.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i12.735
Growing evidences has supported the predominant role of Helicobacter pylori (H. pylori) infection in development of gastric cancer, since World Health Organization designated H. pylori a type 1 carcinogen in 1993. It has been widely accepted that H. pylori infection leads to the progressive way from chronic atrophic gastritis, intestinal metaplasia, to dysplasia[1]. Moreover, prolonged infection with H. pylori cause inflammation, abnormal cell proliferation, release of bacterial virulence factors, and nitrate reduction, all of which contribute to the development of gastric cancer[1]. Recent random controlled trials and meta-analysis have verified that H. pylori eradication therapy appears to reduce new-onset gastric cancer[2-5]. Therefore, from the perspective of clinical practice, it is important to make diagnosis accurately of active H. pylori infection by endoscopic observation with the prevalence of gastroscopy screening in population.
The Kyoto classification of gastritis was advocated in 2013 to evaluate the gastric background mucosa by endoscopic features, eventually to assess the risk of developing gastric cancer[6,7]. Some typical endoscopic findings of gastric mucosa have been literally associated to active H. pylori infection, including diffuse redness, gooseflesh-like nodularity in antrum, and enlarged folds, while regular arrangement of collecting venules presents a sign of non-infection status of H. pylori[8-10]. With the advances of endoscopic techniques, it is feasible to make diagnosis of presence or absence of active H. pylori infection of stomach by using conventional white light imaging (WLI) and imaging enhancement endoscopy (IEE).
Linked color imaging (LCI) is a novel mode of IEE recently launched by FUJIFILM Corporation (Tokyo, Japan), which uses a color tone like WLI by emphasizing minute differences in mucosal colors[11]. In common, mucosal lesions seen in red or white by WLI get redder or whiter under LCI endoscopy, thereby making the lesions more visible during screening. Growing studies have demonstrated that LCI endoscopy can obviously improve the visibility of diffuse redness, map-like redness as well as atrophy and intestinal metaplasia, thus showing the reliability of LCI in recognition of gastritis and early gastric cancer[12-14]. Meanwhile, studies have also conducted to evaluate the diagnostic effect of LCI endoscopy on H. pylori infection status. H. pylori infected mucosa is redder than other uninfected areas due to post-inflammatory congestion and oedema[15]. Compared to WLI, this difference in coloration was amplified by LCI, which may lead to easier identification of lesions suspected of H. pylori infection by the endoscopist, increasing the accuracy of the diagnosis for H. pylori infection. However, the difference between WLI and LCI for H. pylori diagnostic rates remains unknown. Hence in current study, we aim to assess the diagnostic value of LCI for H. pylori active compared to WLI by performing a meta-analysis, to provide evidences for extending the clinical application of LCI endoscopy.
Online English literatures were searched using electronic literature databases including PubMed, Embase, Cochrane and Web of Science. The cut-off time of the articles published was set on April 15, 2022. The keywords used in literature search were “linked color imaging” and “Helicobacter pylori infection” as well as their corresponding abbreviations.
Literature reviews, letters, meeting abstracts, case reports were not included. In addition, duplicated data records were also excluded. In all included studies, the diagnosis of H. pylori active infection under LCI endoscopy was eventually determined by rapid urease test which is the most common test for diagnosis of H. pylori infection. There were no restrictions in terms of the age or sex of study participants.
Data extracted from each study mainly included the following information: First author, year of publication, country, study design, object of research, number of cases, endoscopic system, and test parameters (true positive, false positive, false negative, and true negative). The first and second authors screened the enrolled studies and extracted relevant data. When critical data was not clearly stated, it would be resolved through discussion with the corresponding author.
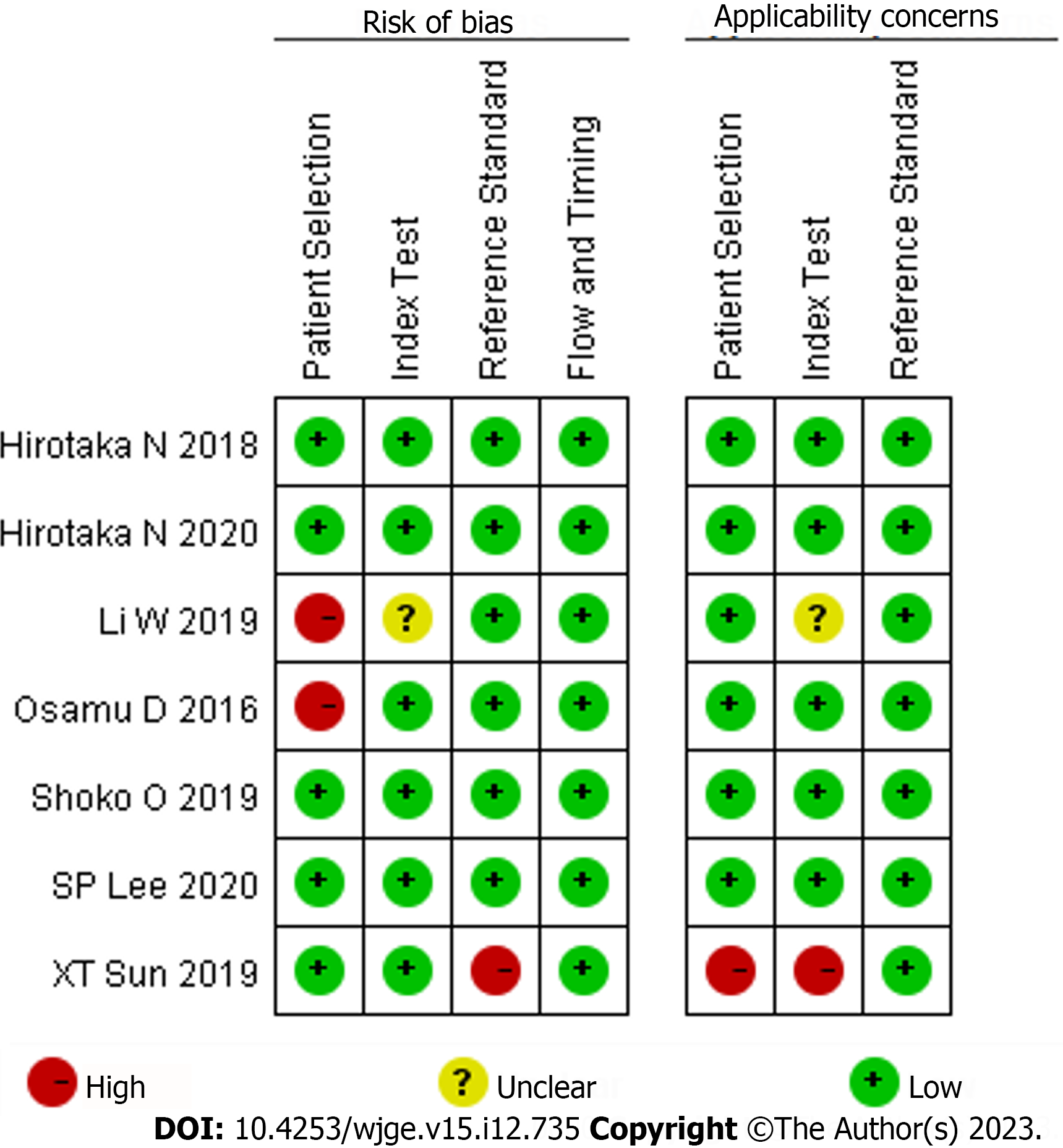
The quality assessment tool of diagnostic tests, the quality assessment of diagnostic accuracy studies-2 was used to evaluate the risk of bias[16]. The scale comprises assessment of risk of bias and applicability. The risk-of-bias assessment is composed of patient selection, evaluated tests, the criterion and patient flow and progress. The applicability assessment included 3 aspects: Patient selection, evaluated tests and the golden criterion. In each aspect, the of bias was defined as “high”, “low”, or “unclear”.
The “midas” command of Stata 15.0 (StataCorp LLC, College Station, TX) was used to fit the two-variable mixed-effect model, and the point estimates of the sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic ratio and their corresponding 95% confidence interval (CI) in each group were combined to draw the comprehensive subject working characteristics [symmetric receiver operator characteristic (SROC)], area under the curve (AUC) and its 95%CI were calculated. The Deek’s funnel plot was used to determine publication bias, and Q statistics and I2 statistics were used to determine whether there was heterogeneity between studies. Levels of 0%-25%, 26%-50%, 51%-75% and more than 75% indicate insignificant, low, moderate, and high heterogeneity respectively. P < 0.05 was considered statistically significant.
We initially searched 94 articles including 25 in PubMed, 16 in Embase, 19 in Cochrane, and 34 in Web of Science. Careful review of the title and abstract and full-text reading were performed independently by two reviewers and the Kappa value was calculated as 0.849. Finally, 7 research articles were selected[17-23] (Table 1). Of them, two studies evaluated the diagnostic effect of LCI by computer-aided diagnosis system (CAD) and artificial intelligence (AI) but not by endoscopist. The specific literature screening process for the included studies is shown in Figure 1. The assessment of bias risk is shown in Figure 2. Of the seven included studies, two were case-free, so there was some bias on patient selection. In addition, in the study performed by Sun et al[21], both measures were tested interchangeably in the same group, and the outcome data were not completely distinguished.
| Ref. | Country | Trial design | Participants (M/F) | Mean age (yr) | Definitive test for H. pylori | Cases of H. pylori infection |
| Lee et al[19], 2020 | United Kingdom | Single center, prospective | 100 (58/42) | 51.2 | RUT | 37 |
| Ono et al[20], 2020 | Japan | Multiple centers, prospective | 127 (66/61) | 62.4 | UBT, serum antibody test | 64 |
| Wang et al[23], 2019 | China | Single center, retrospective | 103 (42/61) | 48.0 | RUT, histological staining | 27 |
| Dohi et al[22], 2016 | Japan | Single center, retrospective | 60 (37/23) | 67.4 | RUT, UBT, serum antibody test | 30 |
| Nakashima et al[17], 2020 | Japan | Single center, prospective | 120 (-) | 57.2 | UBT, serum antibody test | 40 |
| Nakashima et al[18], 2018 | Japan | Single center, prospective | 120 (-) | - | Serum H. pylori, IgG test | 60 |
| Sun et al[21], 2019 | China | RCT | Group A: 127 (66/61) | 47.2 | RUT, histological staining | 64 |
| Group B: 126 (68/58) | 49.7 | 57 |
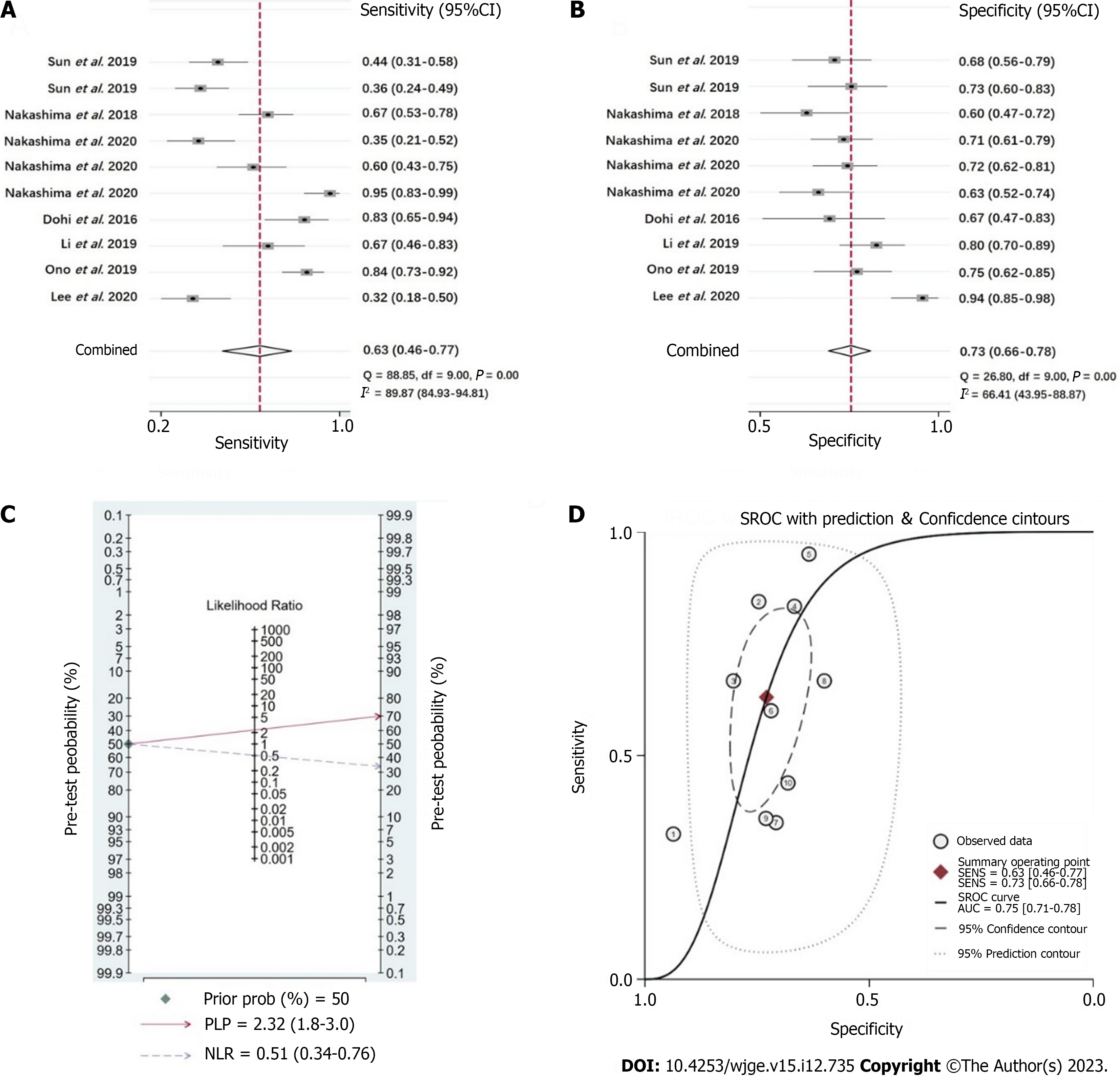
For the overall detection effect on active H. pylori infection in the enrolled studies, WLI endoscopy had a moderate effect of diagnosis with a heterogeneity (I2 = 97) by pooled sensitivity = 0.63 (95%CI: 0.46-0.77) (Figure 3A), pooled specificity = 0.73 (95%CI: 0.66-0.78) (Figure 3B), positive likelihood rate (PLR) = 2.32 (95%CI: 1.8-3.0) (Figure 3C, Supplemen
The sensitivity, specificity, PLR, and NLR of LCI endoscopy for the diagnosis of H. pylori infection of gastric mucosa were 0.85 (95%CI: 0.76-0.92) (Figure 4A), 0.82 (95%CI: 0.78-0.85) (Figure 4B), 4.71 (95%CI: 3.7-5.9) (Figure 4C, Supplemen
Diagnosis of the status of H. pylori infection represents a crucial step in prior to assess the risk of atrophy, intestinal metaplasia and H. pylori associated gastric cancer, according to current consensual strategy on prevention and treatment of gastric cancer. However, the endoscopic diagnosis of H. pylori associated gastritis does not often correspond with the histological findings in clinical practice[24]. Previous studies have disclosed that the accuracy of endoscopic diagnosis of H. pylori infection ranged from 64% to 71% based on the endoscopic appearance alone[19,25]. This moderate accuracy of diagnosis suggests that endoscopy may not be definitive method, but can be important part of comprehensive diagnosis with other invasive or noninvasive tests such as biopsy based rapid urease test or urea breath test.
In the past decades, image enhancement technique upgraded the conventional endoscopy to an indispensable test for diagnosis of gastrointestinal diseases including early malignancies. Emerged researches have demonstrated that various types of IEEs such as blue laser imaging, narrow band imaging and LCI can improve accuracy of diagnosis on H. pylori infection status[20,26-28]. As the latest IEE technique, LCI endoscopy can theoretically highlight the color tone of mucosa thus facilitating the visuality of endoscopic features for active infection of H. pylori, such as diffuse redness, mucosal edema, hemorrhagic spots, enlarged folds, and gooseflesh-like nodularity[29]. Correspondingly, growing evidences have emerged that LCI endoscopy significantly improves recognition of H. pylori associated changes of mucosa to help making diagnosis of H. pylori infection more accurately than conventional WLI endoscopy[18-23].
The combined accuracy of LCI endoscopy on diagnosis of H. pylori active infection concluded by our meta-analysis, is obviously higher than that of conventional WLI endoscopy, which is demonstrated by 0.85 (95%CI: 0.76-0.92) of sensitivity, 0.82 (95%CI: 0.78-0.85) of specificity, 4.71 (95%CI: 3.7-5.9) of PLR, and 0.18 (95%CI: 0.10-0.31) of NLR, with the AUC being 0.87. Although this accuracy is not high enough, it apparently indicates the advantage of LCI endoscopy before patients with suspected H. pylori infection are subjected to invasive tests. Moreover, it has been elucidated that LCI endoscopy not only have good efficacy on diagnosis of current H. pylori infection, but also superior in diagnosis of other abnormalities of H. pylori associated gastritis, such as gastric intestinal metaplasia and atrophy[14,30,31]. Some most recent studies have further demonstrated better effects of LCI endoscopy, in comparison with WLI endoscopy or indigo carmine chromoendoscopy, on identifying featured mucosal appearances after successful H. pylori eradication, thus facilitating to recognize early gastric cancer[32-35]. Therefore, LCI endoscopy is exhibiting the potential as an important alternative modality of endoscopy for gastrointestinal disease screening in future, or at least, as a feasible supplementary method of WLI endoscopy-based screening strategy.
Our analysis had several limitations that may have influence on the results. Firstly, there haven’t been insufficient original studies related to the diagnosis efficacy of LCI on H. pylori infection. The selected studies in our analysis were almost performed in single center, and enrolled relatively small size of patient samples, which restrict further subgroups analysis based on variables. Secondly, these enrolled studies performed different tests to make definite diagnosis of H. pylori infection after LCI endoscopy, such as biopsy based histological staining or rapid urease test, urea breath test, and serological test. Thirdly, two studies of Nakashima et al[17,18] proposed inconsistent diagnosis accuracy of LCI on H. pylori infection, when using AI or CAD instead of endoscopists, that was 96.7% of sensitivity, 83.3% of specificity, 0.95 of AUC for AI, and 62.5% of sensitivity, 92.5% of specificity, 0.82 of AUC for CAD. These problems mentioned above may bring heterogeneity of the analysis and further lead to instability of the results.
Summarily, as a novel technique of image enhancement endoscopy, growing evidences have proved that LCI can significantly improve accuracy of diagnosis on H. pylori infection, as well as H. pylori associated changes of gastric mucosa, including atrophy and gastric intestinal metaplasia. Moreover, by emphasizing the difference of color tone between lesion and surrounding normal mucosa, LCI also shows promising usefulness in detecting early gastric cancer. Combined with current knowledge, it is anticipated to use LCI endoscopy alone for detection of gastric diseases instead of WLI endoscopy in future, while a screening strategy of LCI followed by magnifying IEEs may theoretically have better clinical prospects for early cancer detection.
Diagnosis of Helicobacter pylori (H. pylori) infection is a critical step in assessing the risk of chronic atrophic gastritis, intestinal metaplasia, and H. pylori related gastric cancer. Eradication therapy of H. pylori appears to reduce the incidence of new gastric cancers. Therefore, accurate diagnosis of active H. pylori infection by using endoscopy is essential for the diagnosis and treatment of gastric cancer.
Linked color imaging (LCI) is a novel endoscopic modality recently introduced. Compared to the common white light imaging (WLI), the mucosal lesions in red or white color seen on LCI endoscopy are more visible, which makes it easier to identify early gastric cancer. However, the detection rate of H. pylori with LCI compared to WLI remains to be evaluated.
The diagnostic value of LCI compared with WLI for H. pylori activity was assessed by meta-analysis, to provide evidence for expanding the clinical application of LCI endoscopy.
PubMed, Embase, Embase, and Cochrane Library databases were searched for literature related to LCI and WLI diagnosis of H. pylori. The “midas” command of Stata 15.0 was used to fit the two-variable mixed-effect model. The point estimates of the sensitivity, specificity, likelihood ratio, and diagnostic ratio were combined to draw the comprehensive subject working characteristics [symmetric receiver operator characteristic (SROC)], and area under the curve (AUC) and its 95% confidence interval (CI) were calculated. The Deek’s funnel plot was used to determine publication bias, and Q statistics. I2 statistics were used to determine whether there was heterogeneity between studies.
In this study, 94 articles were initially searched, including 25 in PubMed, 16 in Embase, 19 in Cochrane, and 34 in Web of Science, and 7 research articles were ultimately screened. In WLI diagnosis, the probability of confirming H. pylori infection was 70%. In the case of negative results, the probability of H. pylori infection was 34%. The diagnostic odds ratio (DOR) was 5 (95%CI: 2-9), and SROC was 0.75 (95%CI: 0.71-0.78). In LCI diagnosis, the probability of diagnosis of H. pylori infection was 82%. In the negative case, the probability of H. pylori infection was 15%. The DOR was 26 (95%CI: 13-52) and SROC was 0.87 (95%CI: 0.84-0.90).
LCI improves the diagnostic accuracy of H. pylori infection as well as H. pylori-associated gastric mucosal lesions, which anticipates that LCI alone, rather than WLI, may be applied in the future to screen for gastric disease.
The screening strategy of LCI followed by magnifying image-enhanced endoscopy may theoretically have better clinical perspectives in early cancer diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neri V, Italy; Suzuki H, Japan; Vorobjova T, Estonia S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 2. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 952] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 3. | Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med. 2018;378:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 522] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 4. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 720] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 5. | Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Int Med. 2021;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. |
Kato M.
Endoscopic findings of |
| 7. | Zhou B, Wang Z, Dou Q, Li W, Li Y, Yan Z, Sun P, Zhao B, Li X, Shen F, Zhang B, Guo M. Long-term outcomes of esophageal and gastric cancer patients with cardiovascular and metabolic diseases: A two-center propensity score-matched cohort study. J Transl Int Med. 2023;11:234-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Watanabe K, Nagata N, Nakashima R, Furuhata E, Shimbo T, Kobayakawa M, Sakurai T, Imbe K, Niikura R, Yokoi C, Akiyama J, Uemura N. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19:4374-4379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Mao T, Wang Y, Yin F, Zhao Q, Yang L, Ding X, Tian Z. Association of Endoscopic Features of Gastric Mucosa with Helicobacter pylori Infection in Chinese Patients. Gastroenterol Res Pract. 2016;2016:6539639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Yashima K, Onoyama T, Kurumi H, Takeda Y, Yoshida A, Kawaguchi K, Yamaguchi N, Isomoto H. Current status and future perspective of linked color imaging for gastric cancer screening: a literature review. J Gastroenterol. 2023;58:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Takeda T, Asaoka D, Nojiri S, Nishiyama M, Ikeda A, Yatagai N, Ishizuka K, Hiromoto T, Okubo S, Suzuki M, Nakajima A, Nakatsu Y, Komori H, Akazawa Y, Nakagawa Y, Izumi K, Matsumoto K, Ueyama H, Sasaki H, Shimada Y, Osada T, Hojo M, Kato M, Nagahara A. Linked Color Imaging and the Kyoto Classification of Gastritis: Evaluation of Visibility and Inter-Rater Reliability. Digestion. 2020;101:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Fukuda H, Miura Y, Osawa H, Takezawa T, Ino Y, Okada M, Khurelbaatar T, Lefor AK, Yamamoto H. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J Gastroenterol. 2019;54:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Mizukami K, Ogawa R, Okamoto K, Shuto M, Fukuda K, Sonoda A, Matsunari O, Hirashita Y, Okimoto T, Kodama M, Murakami K. Objective Endoscopic Analysis with Linked Color Imaging regarding Gastric Mucosal Atrophy: A Pilot Study. Gastroenterol Res Pract. 2017;2017:5054237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 554] [Article Influence: 184.7] [Reference Citation Analysis (1)] |
| 16. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10285] [Article Influence: 685.7] [Reference Citation Analysis (3)] |
| 17. | Nakashima H, Kawahira H, Kawachi H, Sakaki N. Endoscopic three-categorical diagnosis of Helicobacter pylori infection using linked color imaging and deep learning: a single-center prospective study (with video). Gastric Cancer. 2020;23:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 18. | Nakashima H, Kawahira H, Kawachi H, Sakaki N. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: a single-center prospective study. Ann Gastroenterol. 2018;31:462-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 19. | Lee SP, Lee J, Kae SH, Jang HJ, Koh DH, Jung JH, Byeon SJ. The role of linked color imaging in endoscopic diagnosis of Helicobacter pylori associated gastritis. Scand J Gastroenterol. 2020;55:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Ono S, Dohi O, Yagi N, Sanomura Y, Tanaka S, Naito Y, Sakamoto N, Kato M. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion. 2020;101:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Sun X, Bi Y, Nong B, Hu D, Sun X, Chen H, Xu Y, Liu Y. Linked color imaging confers benefits in profiling H. pylori infection in the stomach. Endosc Int Open. 2019;7:E885-E892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Dohi O, Yagi N, Onozawa Y, Kimura-Tsuchiya R, Majima A, Kitaichi T, Horii Y, Suzuki K, Tomie A, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;4:E800-E805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Wang L, Lin XC, Li HL, Yang XS, Zhang L, Li X, Bai P, Wang Y, Fan X, Ding YM. Clinical significance and influencing factors of linked color imaging technique in real-time diagnosis of active Helicobacter pylori infection. Chin Med J (Engl). 2019;132:2395-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wang X, Chen JD. Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases. J Transl Int Med. 2023;11:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Okamura T, Iwaya Y, Kitahara K, Suga T, Tanaka E. Accuracy of Endoscopic Diagnosis for Mild Atrophic Gastritis Infected with Helicobacter pylori. Clin Endosc. 2018;51:362-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Dohi O, Majima A, Naito Y, Yoshida T, Ishida T, Azuma Y, Kitae H, Matsumura S, Mizuno N, Yoshida N, Kamada K, Itoh Y. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig Endosc. 2020;32:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Nishikawa Y, Ikeda Y, Murakami H, Hori SI, Hino K, Sasaki C, Nishikawa M. Classification of atrophic mucosal patterns on Blue LASER Imaging for endoscopic diagnosis of Helicobacter pylori-related gastritis: A retrospective, observational study. PLoS One. 2018;13:e0193197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Glover B, Teare J, Patel N. Assessment of Helicobacter pylori status by examination of gastric mucosal patterns: diagnostic accuracy of white-light endoscopy and narrow-band imaging. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1475] [Cited by in RCA: 1257] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 30. | Ono S, Kato M, Tsuda M, Miyamoto S, Abiko S, Shimizu Y, Sakamoto N. Lavender Color in Linked Color Imaging Enables Noninvasive Detection of Gastric Intestinal Metaplasia. Digestion. 2018;98:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Shu X, Wu G, Zhang Y, Wang Y, Zheng Y, Guo Q, Ji R, Zhou Y. Diagnostic value of linked color imaging based on endoscopy for gastric intestinal metaplasia: a systematic review and meta-analysis. Ann Transl Med. 2021;9:506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Kitagawa Y, Suzuki T, Nankinzan R, Ishigaki A, Furukawa K, Sugita O, Hara T, Yamaguchi T. Comparison of endoscopic visibility and miss rate for early gastric cancers after Helicobacter pylori eradication with white-light imaging versus linked color imaging. Dig Endosc. 2020;32:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Majima A, Dohi O, Takayama S, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest Endosc. 2019;90:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Chao H, Yusen Z, Die Y, Yifei LI, Xiaochun Z, Yanqing L. Effects of Traditional Chinese Medicine on the survival of patients with stage I gastric cancer and high-risk factors: a real-world retrospective study. J Tradit Chin Med. 2023;43:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Yasuda T, Yagi N, Omatsu T, Hayashi S, Nakahata Y, Yasuda Y, Obora A, Kojima T, Naito Y, Itoh Y. Benefits of linked color imaging for recognition of early differentiated-type gastric cancer: in comparison with indigo carmine contrast method and blue laser imaging. Surg Endosc. 2021;35:2750-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |