Published online Feb 16, 2019. doi: 10.4253/wjge.v11.i2.145
Peer-review started: November 5, 2018
First decision: November 28, 2018
Revised: January 9, 2019
Accepted: January 26, 2019
Article in press: January 26, 2019
Published online: February 16, 2019
Processing time: 106 Days and 13 Hours
Duodenal biopsies are commonly obtained during esophagogastroduodenoscopy (EGD) but are very often histopathologically normal. Therefore, a more strategic method for evaluating the duodenal mucosa and avoiding unnecessary biopsies is needed.
To examine the clinical utility of narrow band imaging (NBI) for evaluating duodenal villous morphology.
We performed a prospective cohort study of adult patients at Mayo Clinic Rochester from 2013-2014 who were referred for EGD with duodenal biopsies. A staff endoscopist scored, in real-time, the NBI-based appearance of duodenal villi into one of three categories (normal, partial villous atrophy, or complete villous atrophy), captured ≥ 2 representative duodenal NBI images, and obtained mucosal biopsies therein. Images were then scored by an advanced endoscopist and gastroenterology fellow, and biopsies (gold standard) by a pathologist, in a masked fashion using the same three-category classification. Performing endoscopist, advanced endoscopist, and fellow NBI scores were compared to histopathology to calculate performance characteristics [sensitivity, specificity, positive and negative, negative predictive value (NPV), and accuracy]. Inter-rater agreement was assessed with Cohen’s kappa.
112 patients were included. The most common referring indications were dyspepsia (47%), nausea (23%), and suspected celiac disease (14%). Duodenal histopathology scores were: 84% normal, 11% partial atrophy, and 5% complete atrophy. Performing endoscopist NBI scores were 79% normal, 14% partial atrophy, and 6% complete atrophy compared to 91%, 5%, and 4% and 70%, 24%, and 6% for advanced endoscopist and fellow, respectively. NBI performed favorably for all raters, with a notably high (92%-100%) NPV. NBI score agreement was best between performing endoscopist and fellow (κ = 0.65).
NBI facilitates accurate, non-invasive evaluation of duodenal villi. Its high NPV renders it especially useful for foregoing biopsies of histopathologically normal duodenal mucosa.
Core tip: Duodenal mucosal biopsies are frequently obtained during upper endoscopy to assess villous architecture but are largely negative (i.e., histopathologically normal); thus, a method to better evaluate the duodenal mucosa and avoid unnecessary biopsies is needed. Narrow band imaging (NBI) permits superior inspection of mucosal surfaces via filter separation of conventional white light into only green and blue components. Based on the findings of this prospective study, NBI appears to have excellent diagnostic performance in evaluating duodenal villous morphology and can facilitate targeting of biopsies; its high negative predictive value renders it particularly useful in avoiding biopsies that are likely to reveal histopathologically normal mucosa.
- Citation: Tabibian JH, Perrault JF, Murray JA, Papadakis KA, Enders FT, Gostout CJ. Narrow band imaging evaluation of duodenal villi in patients with and without celiac disease: A prospective study. World J Gastrointest Endosc 2019; 11(2): 145-154
- URL: https://www.wjgnet.com/1948-5190/full/v11/i2/145.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i2.145
The incidence, prevalence, and costs of digestive tract disorders warranting small intestinal mucosal evaluation are substantial and rising in the United States and worldwide[1-5]. Celiac disease alone, for example, occurs in 1 in 140 (i.e., over 2000000) individuals in the United States[1,4] and is associated with thousands of dollars per person-year in increased direct medical costs compared to the general population. It is not an overstatement, therefore, that these disorders, taken together, embody a major gastroenterological (GI) and public health burden.
Esophagogastroduodenoscopy (EGD) with evaluation of the duodenal mucosa is indicated for suspected and known celiac disease as well as other inflammatory and malabsorptive digestive tract disorders. While endoscopic inspection of the duodenal mucosa during EGD is expedient, white light endoscopy (WLE) is not considered (and has been established to not be) sufficiently sensitive to confidently rule out certain mucosal abnormalities, and in particular, those involving duodenal villous morphology[6]. As a result, biopsies of the duodenal mucosa are required for histopathological evaluation; although obtaining and microscopically evaluating duodenal mucosal biopsies is considered the gold standard, it is a time- and resource-intensive approach[7]. Moreover, with anticipated healthcare reform (e.g., bundled payment), it is likely that histopathology costs will ultimately be deducted from EGD reimbursements. This is a problematic prospect considering that a large proportion of duodenal biopsies are histopathologically normal[7,8]. Therefore, methods to avoid unnecessary biopsies would be timely and clinically useful.
Narrow band imaging (NBI) is an ancillary endoscopic imaging modality which offers an enhanced capability to delineate mucosal surfaces and underlying vasculature and is readily available on contemporary endoscopes[9]. Fundamentally underpinning this capability is the principle that depth of light penetration depends on wavelength, i.e., the longer the wavelength of light, the deeper the penetration[10]. NBI technology filters light from the xenon source into green and blue components. Upon illumination of the mucosa, blue light penetrates only superficially, whereas green light penetrates into deeper mucosal layers. This separation of light permits better visualization and more detailed inspection of the mucosa with NBI as compared to WLE alone[9,11-13]. NBI also offers advantages compared to biopsy-based techniques in that it: (1) is non-invasive (i.e., does not add to the risks inherent to EGD); (2) can be rapidly performed by the endoscopists and yield real-time results; (3) does not involve histopathology charges; and (4) allows for wide field inspection and thus may be less prone to sampling error.
With these advantages in mind, and considering the aforementioned unmet clinical needs, we hypothesized that NBI would have high accuracy and clinical utility in the evaluation of duodenal villous morphology. Here, we prospectively and comprehensively examined the performance characteristics of NBI among patients referred for EGD with duodenal mucosal biopsies.
This study was approved by the Mayo Clinic Institutional Review Board (IRB# 13-005715).
Adult (age ≥ 18 years) patients consecutively referred to our outpatient endoscopy center between August 2013 and August 2014 for EGD with an a priori request for duodenal mucosal biopsies were included. This cohort included patients referred for a broad variety of clinical indications, including investigation of celiac disease (suspected or known) as well as other disorders and/or symptoms (Table 1).
| Indication1 | Patients, n (%) | Histopathology, n (%) | ||
| Normal | Atrophy | |||
| Partial | Complete | |||
| Dyspepsia | 47 (42.0) | 44 (93.6) | 2 (4.3) | 1 (2.1) |
| Nausea or vomiting | 26 (23.2) | 25 (96.2) | 1 (3.8)2 | 0 |
| Weight loss | 26 (23.2) | 19 (73.0) | 3 (11.5) | 4 (15.4) |
| Iron-deficiency anemia | 16 (14.3) | 14 (87.5) | 1 (6.3) | 1 (6.3) |
| Diarrhea | 15 (14.0) | 13 (86.7) | 0 | 2 (13.3) |
| Rule out Celiac disease | 15 (14.0) | 13 (86.7) | 1 (6.7) | 1 (6.7) |
| Follow up Celiac disease | 12 (10.7) | 4 (33.3) | 0 | 8 (66.7) |
| Other3 | 3 (2.7) | 2 (66.7) | 1 (33.3) | 0 |
| Total | 112 | 94 (84) | 12 (11) | 6 (5) |
Informed consent was obtained in all patients. Moderate to deep sedation was induced for all procedures with intravenous nurse-administered fentanyl and midazolam or anesthetist-administered propofol. EGD was performed by a senior staff endoscopist (JP, JAM, or KAP) with a diagnostic gastroscope (GIF-H180 or GIF-H190, Olympus America, Center Valley, PA) in the conventional manner with standard accessories. NBI was actuated intraprocedurally by the button on the gastroscope (which electronically places the NBI filter between the RGB filter and the light source) and used to evaluate the duodenal mucosa. At least four biopsies were obtained from the second portion of the duodenum using single-use radial jaw forceps (Boston Scientific, Natick, MA) per hospital standard of practice.
The performing staff endoscopist subjectively scored, in real-time during EGD, the NBI-based appearance of duodenal villi as normal, partial villous atrophy, or complete villous atrophy[14]. These three categories were expected to correspond to a Marsh classification score of 0-2, 3a-3b, and 3c, respectively. The performing endoscopist then captured at least two representative NBI images (one of which had to be either close-up or using near focus) in the second portion of the duodenum and obtained biopsies therein. Duodenal biopsies were sent to the laboratory for staining with hematoxylin and eosin and scored histopathologically (gold standard) by a masked staff GI pathologist using the same three category classification. In cases of heterogeneity in the degree of villous atrophy in the biopsies from a given patient, the most severe score was recorded.
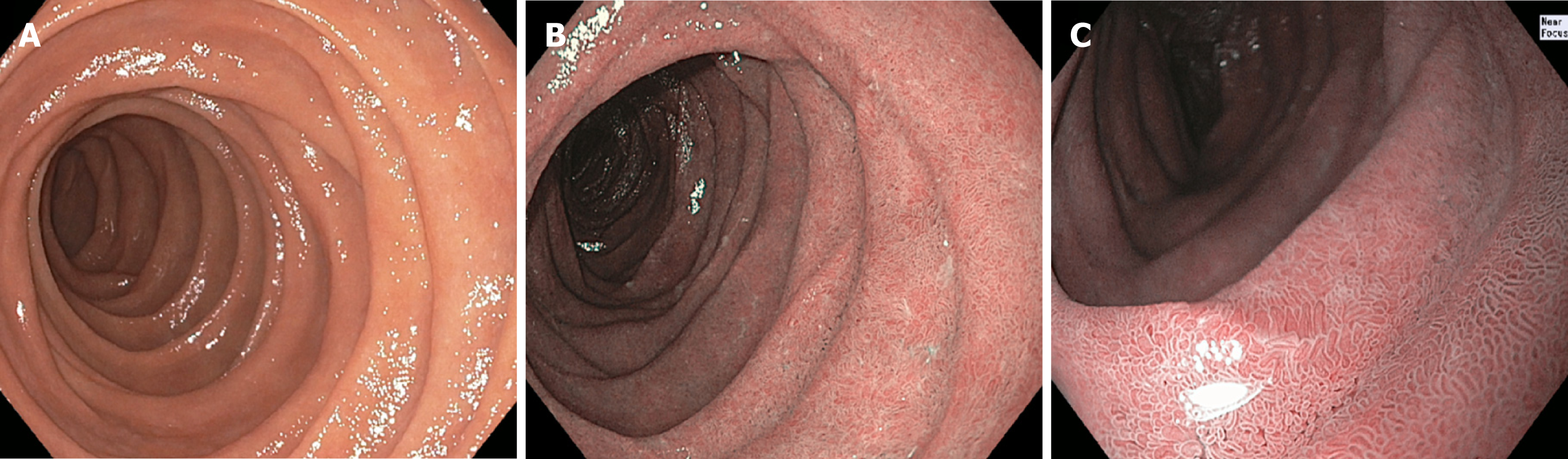
The representative endoscopic NBI images obtained by the performing endoscopist from the second portion of the duodenum were retrospectively reviewed in a masked fashion by an experienced advanced endoscopist (CJG) and a GI fellow (JHT) and classified using the same three category convention (Figure 1). The advanced endoscopist had approximately three decades of clinical experience, and the GI fellow was in his final two years of fellowship during the study and had performed approximately 350 EGDs at the start of the study.
All scores were entered into standardized data collection forms and then aggregated into one dataset for analytical purposes.
The primary outcome of the study was the diagnostic performance of NBI-based duodenal villous morphology scoring compared to histopathological scoring of biopsies from the second portion of the duodenum. The secondary outcome was inter-observer agreement on NBI-based scores.
In addition to NBI-based and histopathological scores of duodenal villi, the following covariates were abstracted from the electronic medical record using a standardized data collection form: Age, sex, indication for EGD, celiac disease status (rule out vs known), changes of peptic duodenitis, duodenal Crohn’s disease, and endoscopic image adequacy (yes or no). Of note, cases wherein NBI evaluation suggested regions of normal villi intermixed with regions of atrophy were recorded (but scored overall as atrophic).
Duodenal villous morphology scores from each of the NBI raters (performing endoscopist, advanced endoscopist, and GI fellow) were compared pairwise to histopathological scores (gold standard) to generate a c-statistic (i.e. area under receiver operating curve for nominal outcomes). In addition, diagnostic performance characteristics of NBI, specifically specificity, sensitivity, positive and negative (NPV) predictive values, and overall accuracy were calculated. NBI scores from each of the three raters were then compared pairwise using Cohen’s kappa to assess inter-observer agreement across a broad range of training and expertise. Analyses were performed using JMP statistical software version 10 (SAS Institute, Cary, NC) with support from the Mayo Clinic Division of Biomedical Statistics and Informatics. All tests were two-tailed, and a P < 0.05 was considered statistically significant.
A total of 112 consecutive patients were included in the study, among whom the median age was 51 years (interquartile range 37-64 years) and 35.2% were male. The most common referring indications included dyspepsia (47%), nausea (23%), and suspected celiac disease (14%), as shown in Table 1.
Among the 112 patients, 94 (84%) had normal duodenal mucosa, 12 (11%) had partial atrophy, and 6 (5%) had complete atrophy based on histopathological evaluation. The highest incidence of abnormal duodenal histopathology was among patients referred for follow up of known celiac disease or for investigation of unexplained weight loss (66.7% and 27.0%, respectively), while the lowest incidence of abnormal histopathology was among those referred for nausea/vomiting (3.8%). Additional details are provided in Table 1.
With respect to NBI-based evaluation, performing endoscopists’ NBI scores were 79% normal, 14% partial atrophy, and 6% complete atrophy as compared to 91%, 5%, and 4% and 70%, 24%, and 6% for advanced endoscopist and GI fellow scores, respectively. As shown in Table 2, NBI scores had excellent agreement with histopathology scores (gold standard). Additional performance characteristics are provided in Table 3; as can be seen, sensitivity was highest for the GI fellow, while specificity was highest for the advanced endoscopist. Overall accuracy was highest for the performing endoscopist and advanced endoscopist (both 93%). Importantly, negative predictive values (NPVs) were particularly high for all three raters, ranging from 93%-100%.
| Histopathology1 | C-statistic | |||
| Normal | Atrophy | |||
| Performing endoscopist | Normal | 87 | 2 | 0.82 |
| Atrophy | 7 | 16 | ||
| Advanced endoscopist | Normal | 94 | 8 | 0.83 |
| Atrophy | 0 | 10 | ||
| GI Fellow | Normal | 76 | 0 | 0.86 |
| Atrophy | 16 | 18 | ||
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| Performing endoscopist | 89 | 93 | 70 | 98 | 93 |
| Advanced endoscopist | 56 | 100 | 100 | 93 | 93 |
| Gastroenterology fellow | 100 | 83 | 53 | 100 | 86 |
Notably, 4 of 7 (57%) and 11 of 16 discordant cases (69%) which were scored as normal by histopathology but non-normal on NBI by the performing endoscopist and GI fellow, respectively, were recorded as having regions of normal villi present intermixed with regions of villous atrophy endoscopically; there were no cases scored as normal by histopathology but non-normal on NBI by the advanced endoscopist (specificity 100%). In addition, it should be mentioned that there were 7 cases which were reported as having normal villous architecture histopathologically but increased intraepithelial lymphocytes (30-70/high power field); of these 7, two were scored as partial villous atrophy by the performing endoscopists, and three were scored as partial villous atrophy by the fellow (all 7 scored as normal by the advanced endoscopist). Whether these cases represented true discordance (i.e., false positive NBI) or early cases of Celiac disease or other duodenopathy is uncertain[15].
There did not appear to be any confounding of NBI scores by the presence of peptic duodenitis (n = 8 cases) or duodenal Crohn’s disease (n = 1 case) with the exception of one case of peptic duodenitis scored as partial atrophy by the GI fellow but normal by the other two raters and by histopathology.
To further understand the performance characteristics of NBI scoring, agreement was calculated between the three NBI raters. Agreement was found to be moderate between performing endoscopist and advanced endoscopist (κ = 0.55), good between performing endoscopist and GI fellow (κ = 0.65), and fair between advanced endoscopist and GI fellow (κ = 0.37). The suboptimal agreement between advanced endoscopist and GI fellow appeared to be a result of the relatively frequent designation of atrophy by the latter compared to more conservative scoring by the advanced endoscopist; this is supported by the high PPV of advanced endoscopist NBI scoring in contrast to the high NPV of GI fellow NBI scoring.
Disorders of the duodenal mucosa affect millions of individuals in the United States and lead to a large but uncertain number of diagnostic tests annually, in particular EGD with endoscopic biopsies and histopathology thereof[4]. In addition to incurring costs, duodenal biopsies are also not without risk, and albeit uncommon, serious complications have been reported[16]. Therefore, a quick, cost-effective, less invasive, and evidence-based technique for evaluating duodenal mucosa would be timely and useful. In this regard, we hypothesized that the inherent properties of NBI would permit clinically useful inspection of mucosal surfaces and specifically duodenal villous morphology. The results herein demonstrate that NBI has excellent diagnostic performance, with its high NPV rendering it particularly useful in avoiding biopsies which are likely to reveal histopathologically normal mucosa.
NBI has an inherently enhanced capability to delineate mucosal surfaces compared to WLE (Figure 2)[10]. In addition, NBI has advantages over tissue biopsies in that it is less invasive, and possibly less prone to sampling error. Moreover, it may be a less costly method of inspecting the duodenal mucosa in populations with low disease prevalence. At our institution alone (Mayo Clinic, Rochester, MN), an average of 8000 patients undergo duodenal mucosal biopsies annually, with some patients requiring two separate biopsy specimen bottles for the bulb and second portion of the duodenum (Mayo Clinic Department of Revenue Recognition. Rochester MN, United States). Each biopsy specimen bottle incurs a charge of approximately $500 for associated processing and histopathological examination (Mayo Clinic Department of Laboratory Medicine and Pathology, Rochester MN, United States). This amounts to over $6000000 annually at our institution, not including the cost of EGD, biopsy forceps, or extra endoscopy suite time and labor needed to obtain and prepare biopsy specimens. It is worth mentioning that anticipated healthcare reforms (e.g., bundled payment or capitation) may lead to these costs being deducted from endoscopist reimbursements, a problematic prospect given that a considerable proportion of duodenal biopsies are performed in low-risk groups who ultimately have normal histopathology results.
The findings of our study extend the findings of earlier, smaller studies and suggest that NBI is sufficiently accurate compared to histopathology to be clinically useful and has favorable inter-observer agreement among individuals with different levels of endoscopic experience. Furthermore, we believe that with brief formal instruction (e.g., training video on scoring of NBI findings), the overall diagnostic performance of NBI, even among GI trainees and junior endoscopists, could be further improved compared to the results seen herein. Given its excellent NPV, it may be particularly useful as an alternative to tissue biopsy in patients with normal appearing duodenal villi by NBI inspection and who have a low pre-test probability of duodenal mucosal pathology, e.g., patients referred for nausea, vomiting, or functional dyspepsia without diarrhea or iron deficiency. Additionally, although not directly studied here, NBI can be used to target duodenal biopsies, thereby facilitating accurate diagnosis (i.e. by decreasing false negatives secondary to sampling error associated with random biopsies and/or in conditions with patchy or ultra-short disease involvement)[17,18]. In a similar vein, an enhanced, real-time ability to recognize villous abnormalities using NBI may identify patients who would benefit from duodenal biopsies but in whom duodenal mucosal disease (e.g., celiac) was not suspected (and thus biopsies were not specifically requested prior to referral for EGD)[6]. These represent currently understudied but potentially valuable applications of NBI.
The present study has several limitations and other features which merit consideration. First, this was a single center study based in an academic, tertiary-care referral setting. Second, we used a simplified classification system for histopathological and NBI scoring; while less detailed than alternative classification systems, this system has been shown to have satisfactory inter-observer agreement and is readily applicable to clinical practice, recognizing though that it may not be sufficiently granular in some scenarios [e.g., cases where the presence of isolated increased epithelial lymphocytes with preserved villous architecture (i.e., Marsh classification 1-2) is regarded a clinically significant finding] (Table 4)[14]. Third, sample size was, nevertheless relatively sizable in that it represents the largest known published cohort of NBI-based duodenal villous inspection with histopathological correlation. Part of the reason why the sample size was limited was that we only included EGDs with an a priori (i.e., “special”) request for duodenal biopsies; in doing so, however, we believe the cohort was enriched for abnormal findings, thus certain diagnostic performance characteristics, e.g., specificity and NPV, may be even higher if applied to all comers (e.g., a non-enriched cohort and/or non-tertiary referral setting). Fourth, we did not have a WLE control arm; though this may have clarified the incremental gain of NBI over conventional WLE, given the latter has been found to be unreliable in accurately assessing villous morphology, we deemed this to generally not be clinically relevant. Fifth, biopsies of the duodenal bulb were not included pro forma as they are not uniformly a part of the practice at our institution and are more susceptible to nonspecific chemical (e.g., peptic) injury. Sixth, magnification endoscopy (i.e., “near focus”) was not specifically studied here, though it likely has the potential to further improve the diagnostic performance of NBI for this application[13,19,20]. Lastly, alternative modalities exist which may similarly help avoid the need for unnecessary duodenal biopsies, such as the water immersion technique (which can be coupled with NBI) and confocal endomicroscopy; however, the former may be less desirable in patients with an unprotected airway while the latter is time-consuming and costly.
| Performing endoscopist | Advanced endoscopist | GI Fellow | |
| Performing endoscopist | - | - | - |
| Advanced endoscopist | 0.55 | - | - |
| GI Fellow | 0.65 | 0.371 | - |
In summary, NBI appears to be a promising tool for non-invasive evaluation of duodenal villous morphology, and in addition, is readily available during routine EGD. Its high NPV makes it especially useful in avoiding biopsies which are likely to reveal histologically normal mucosa. Conversely, it can facilitate targeting of duodenal tissue acquisition so as to avoid false negative biopsies due to sampling error or patchy disease. The use of NBI for the evaluation of duodenal villi may therefore result in improved diagnostic accuracy, avoidance of unnecessary biopsies, and potential cost savings.
Duodenal mucosal biopsies are routinely obtained during upper endoscopy (EGD) but very often are histopathologically normal.
To decrease unnecessary biopsies, a more strategic method for examining the duodenal mucosa is needed.
The primary aim of this study was to examine the clinical utility of narrow band imaging (NBI) for evaluating the morphology.
We performed a prospective cohort study of patients at Mayo Clinic Rochester who were referred for EGD with a request for duodenal biopsies. The performing staff endoscopist scored, in real-time during EGD, the NBI-based appearance of duodenal villi into one of three categories (normal, partial villous atrophy, or complete villous atrophy), captured ≥ 2 representative duodenal NBI images, and obtained duodenal mucosal biopsies. NBI images were then scored by an advanced endoscopist and fellow, and biopsies (gold standard) by a pathologist, in a masked fashion using the same three-category classification. Performing endoscopist, advanced endoscopist, and fellow NBI scores were compared to histopathology scores to calculate performance characteristics [sensitivity, specificity, positive and negative (NPV) predictive values, and accuracy]. Inter-rater agreement was assessed with Cohen’s kappa.
A total of 112 patients were included in the study. The most common referring indications for EGD with duodenal biopsies were dyspepsia (47%), nausea (23%), and suspected celiac disease (14%). Histopathology scores of duodenal biopsies were: 84% normal, 11% partial atrophy, and 5% complete atrophy. Performing endoscopist duodenal NBI scores were 79% normal, 14% partial atrophy, and 6% complete atrophy compared to 91%, 5%, and 4% and 70%, 24%, and 6% for advanced endoscopist and GI fellow, respectively. Diagnostic performance was favorable for all three raters compared to histopathology, and NPV was particularly high (92-100%). NBI score agreement was best between performing endoscopist and fellow (κ = 0.65).
NBI inspection during EGD facilitates accurate, non-invasive evaluation of duodenal villi. It’s particularly high NPV may render it most useful for foregoing biopsies of duodenal mucosa likely to be histopathologically normal.
We believe NBI should routinely be applied to the duodenum during EGD prior to obtaining duodenal biopsies in order to help determine their likely histopathological yield and better target (rather than randomly approach) their acquisition.
| 1. | Choung RS, Ditah IC, Nadeau AM, Rubio-Tapia A, Marietta EV, Brantner TL, Camilleri MJ, Rajkumar SV, Landgren O, Everhart JE, Murray JA. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol. 2015;110:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Green PH, Neugut AI, Naiyer AJ, Edwards ZC, Gabinelle S, Chinburapa V. Economic benefits of increased diagnosis of celiac disease in a national managed care population in the United States. J Insur Med. 2008;40:218-228. [PubMed] |
| 3. | Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2699] [Cited by in RCA: 2583] [Article Influence: 89.1] [Reference Citation Analysis (7)] |
| 4. | Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T, Schmidt M, Shaheen NJ, Sandler RS. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731-1741.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 722] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 5. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1489] [Article Influence: 106.4] [Reference Citation Analysis (1)] |
| 6. | Barada K, Habib RH, Malli A, Hashash JG, Halawi H, Maasri K, Tawil A, Mourad F, Sharara AI, Soweid A, Sukkarieh I, Chakhachiro Z, Jabbour M, Fasano A, Santora D, Arguelles C, Murray JA, Green PH. Prediction of celiac disease at endoscopy. Endoscopy. 2014;46:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Yang JJ, Thanataveerat A, Green PH, Lebwohl B. Cost Effectiveness of Routine Duodenal Biopsy Analysis for Celiac Disease During Endoscopy for Gastroesophageal Reflux. Clin Gastroenterol Hepatol. 2015;13:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288-295. [PubMed] |
| 10. | Ginsberg GG. Seeing the light: enhanced endoscopic imaging to glimpse the Holy Grail. Gastrointest Endosc. 2006;64:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Singh R, Nind G, Tucker G, Nguyen N, Holloway R, Bate J, Shetti M, George B, Tam W. Narrow-band imaging in the evaluation of villous morphology: a feasibility study assessing a simplified classification and observer agreement. Endoscopy. 2010;42:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Banerjee R, Reddy DN. High-resolution narrow-band imaging can identify patchy atrophy in celiac disease: targeted biopsy can increase diagnostic yield. Gastrointest Endosc. 2009;69:984-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Corazza GR, Villanacci V, Zambelli C, Milione M, Luinetti O, Vindigni C, Chioda C, Albarello L, Bartolini D, Donato F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Kakar S, Nehra V, Murray JA, Dayharsh GA, Burgart LJ. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol. 2003;98:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 16. | Taavela J, Popp A, Korponay-Szabo IR, Ene A, Vornanen M, Saavalainen P, Lähdeaho ML, Ruuska T, Laurila K, Parvan A, Anca I, Kurppa K, Mäki M. A Prospective Study on the Usefulness of Duodenal Bulb Biopsies in Celiac Disease Diagnosis in Children: Urging Caution. Am J Gastroenterol. 2016;111:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Mooney PD, Kurien M, Evans KE, Rosario E, Cross SS, Vergani P, Hadjivassiliou M, Murray JA, Sanders DS. Clinical and Immunologic Features of Ultra-Short Celiac Disease. Gastroenterology. 2016;150:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Ravelli A, Villanacci V, Monfredini C, Martinazzi S, Grassi V, Manenti S. How patchy is patchy villous atrophy?: distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol. 2010;105:2103-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | De Luca L, Ricciardiello L, Rocchi MB, Fabi MT, Bianchi ML, de Leone A, Fiori S, Baroncini D. Narrow band imaging with magnification endoscopy for celiac disease: results from a prospective, single-center study. Diagn Ther Endosc. 2013;2013:580526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Penny HA, Mooney PD, Burden M, Patel N, Johnston AJ, Wong SH, Teare J, Sanders DS. High definition endoscopy with or without I-Scan increases the detection of celiac disease during routine endoscopy. Dig Liver Dis. 2016;48:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
STROBE Statement: The authors have read the STROBE checklist, and the manuscript was prepared according to it.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Vynios D, Tseng PH, Chen JQ S- Editor: Dou Y L- Editor: A E- Editor: Tan WW