©The Author(s) 2026.
World J Gastrointest Endosc. Jan 16, 2026; 18(1): 113788
Published online Jan 16, 2026. doi: 10.4253/wjge.v18.i1.113788
Published online Jan 16, 2026. doi: 10.4253/wjge.v18.i1.113788
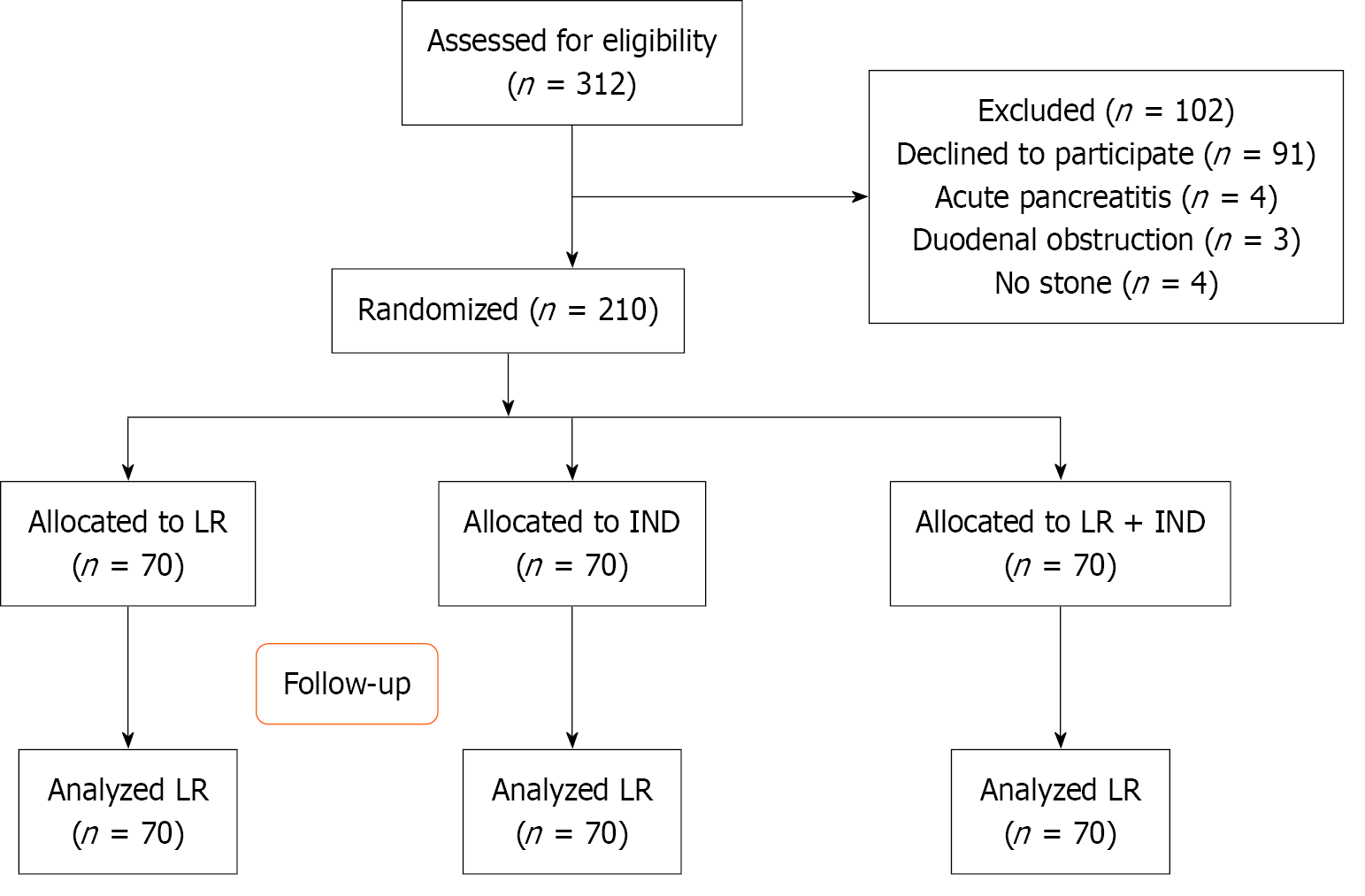
Figure 1 Flow diagram with enrollment and outcomes.
IND: Indomethacin; LR: Lactated Ringer’s solution.
Figure 2 The incidence of the primary outcome of post-endoscopic retrograde cholangiopancreatography pancreatitis and secondary outcome readmission rates.
A: The incidence of the primary outcome of post-endoscopic retrograde cholangiopancreatography pancreatitis. The difference between lactated ringer’s solution (LR) and LR + indomethacin (IND) was significant (P = 0.04); B: The incidence of secondary outcome readmission rates. Bars from left to right indicate the LR, IND, and LR + IND groups. The difference between LR and LR + IND was significant (P = 0.03). IND: Indomethacin; LR: Lactated Ringer’s solution; PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
- Citation: Amalou K, Benboudiaf N, Medkour MT, Belghanem F, Chetroub H, Rekab R, Belloula A, Bouaouina F, Saidani K. Lactated Ringer’s solution in combination with indomethacin for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A prospective, randomized trial. World J Gastrointest Endosc 2026; 18(1): 113788
- URL: https://www.wjgnet.com/1948-5190/full/v18/i1/113788.htm
- DOI: https://dx.doi.org/10.4253/wjge.v18.i1.113788