Published online Jan 28, 2017. doi: 10.4254/wjh.v9.i3.139
Peer-review started: April 26, 2016
First decision: July 20, 2016
Revised: October 25, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: January 28, 2017
Processing time: 270 Days and 23.9 Hours
To examine if liver transplant recipients with high-risk non-alcoholic steatohepatitis (NASH) are at increased risk for pre-transplant portal venous thrombosis.
Data on all liver transplants in the United States from February 2002 through September 2014 were analyzed. Recipients were sorted into three distinct groups: High-risk (age > 60, body mass index > 30 kg/m2, hypertension and diabetes), low-risk and non-NASH cirrhosis. Multivariable logistic regression models were constructed.
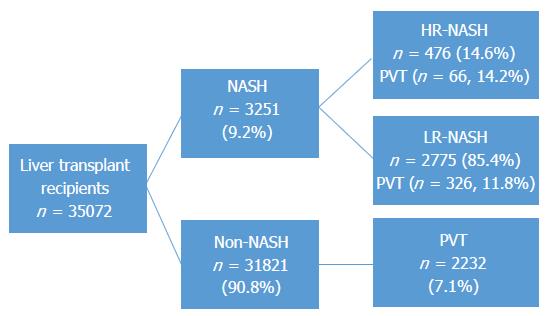
Thirty-five thousand and seventy-two candidates underwent liver transplantation and of those organ recipients, 465 were transplanted for high-risk and 2775 for low-risk NASH. Two thousand six hundred and twenty-six (7.5%) recipients had pre-transplant portal vein thrombosis; 66 (14.2%) of the high-risk NASH group had portal vein thrombosis vs 328 (11.8%) of the low-risk NASH group. In general, all NASH recipients were less likely to be male or African American and more likely to be obese. In adjusted multivariable regression analyses, high-risk recipients had the greatest risk of pre-transplant portal vein thrombosis with OR = 2.11 (95%CI: 1.60-2.76, P < 0.001) when referenced to the non-NASH group.
Liver transplant candidates with high-risk NASH are at the greatest risk for portal vein thrombosis development prior to transplantation. These candidates may benefit from interventions to decrease their likelihood of clot formation and resultant downstream hepatic decompensating events. Prospective study is needed.
Core tip: Non-alcoholic steatohepatitis (NASH) is increasing in prevalence and is expected to be the leading indication for liver transplantation in the foreseeable future. There is a growing body of evidence supporting the clinical importance of a thrombophilic state in patients with NASH. In NASH patients, the most severe hypercoagulable environment is found in patients with NASH cirrhosis. High-risk NASH patients (concomitant age > 60 years, obesity, diabetes and hypertension) have inferior post transplantation outcomes, however, how this group’s risk of clotting compares to other etiologies of liver disease is unknown. In a retrospective nationwide United States based cohort, we provide further evidence of coagulation derangement in NASH and identify a new high-risk subtype in the high-risk NASH population. Whether or not this high-risk group may benefit from preventative anticoagulation remains unknown.
- Citation: Stine JG, Argo CK, Pelletier SJ, Maluf DG, Caldwell SH, Northup PG. Advanced non-alcoholic steatohepatitis cirrhosis: A high-risk population for pre-liver transplant portal vein thrombosis. World J Hepatol 2017; 9(3): 139-146
- URL: https://www.wjgnet.com/1948-5182/full/v9/i3/139.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i3.139
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of hepatic disorders ranging from simple steatosis with or without mild inflammation to non-alcoholic steatohepatitis (NASH) which is diagnosed by the presence of inflammation, cellular injury with hepatocyte ballooning and accumulation of Mallory-Denk bodies and in the most advanced cases, fibrosis[1]. NAFLD is increasing in prevalence in Western society[2] with rates approaching 20%-30%[3] and more importantly, NASH is projected to become the number one indication for liver transplantation in the foreseeable future[4], especially in light of the new all oral direct acting antiviral treatment regimens for hepatitis C virus (HCV), and it is the most rapidly growing indication for simultaneous liver-kidney transplantation[5]. High-risk NASH (HR-NASH) is a subtype of NASH defined by the presence of the following: Age > 60 years, body mass index (BMI) > 30 kg/m2, hypertension and diabetes[6,7]. In general, liver transplant recipients with NASH have similar liver graft and overall one-, three- and five-year survival rates when compared to other etiologies[4-6,8,9]. Outcomes for HR-NASH recipients are less promising with single center experiences showing significantly lower one-[7] and five-year survival rates[6]. While the exact explanation for this remains relatively unexplored, post-transplant cardiovascular events[8], some of which are attributable to macrovascular arterial thrombosis[7,9], and chronic renal dysfunction[10] are more common in NASH patients and these could contribute to lesser outcomes.
Venous thromboembolism (VTE) including portal vein thrombosis (PVT) is a common affliction in patients with cirrhosis[11]. Incidence rates of PVT are reported to be as high as 16%[12] and 30-d mortality is increased in patients with pulmonary embolism (PE) or deep vein thrombosis (DVT)[11]. In a matched retrospective case-control study of 414 patients, Di Minno et al[13] found that NAFLD was associated with VTE (PE or DVT) with an OR of 1.8 on adjusted multivariable analysis controlling for additional VTE and NAFLD risk factors. While the presence of PVT may mirror the degree of liver disease burden, it is nonetheless associated with adverse outcomes including increased pre- and post-liver transplant mortality and impaired quality of life, as well as technical challenges during the transplant procedure[14,15]. We have previously shown that liver transplant recipients with NASH are predisposed to pre-transplant PVT[16]. Patients with NASH and metabolic syndrome, which encompasses many of the features of the HR-NASH definition, are known to have increased degrees of fibrosis[17], and presumably increased thrombotic risk. To date, there is a lack of data investigating the relationship between pre-transplant PVT and HR-NASH. We aim to explore this potential association and hypothesize that liver transplant recipients with HR-NASH are at increased risk for PVT when compared directly to other NASH patients and all other etiologies of liver disease.
Data on all transplants in the United States during the model for end-stage liver disease (MELD era) through September 2014 were reviewed from the Organ Procurement and Transplantation Network (OPTN) with permission from the United Network for Organ Sharing (UNOS). Status 1a, multi-organ, living donor, re-transplants, pediatric recipients, donation after cardiac death, recipients with pre-transplantation transjugular intrahepatic portosystemic shunts and malignancy (hepatocellular carcinoma, hepatoblastoma, cholangiocarcinoma) were excluded. Recipients with cryptogenic cirrhosis were also excluded due to the potential for misclassification of NASH. The conclusions of the model were not significantly changed with the exclusion of the cryptogenic recipients. Recipients were sorted into two distinct groups: Those with NASH and those without NASH (all other etiologies except cryptogenic cirrhosis, which was excluded due to the potential for misclassification of NASH). The NASH group was then subdivided into HR and low-risk (LR) subgroups. HR-NASH was based on the standard definition used in previous large-scale single center experiences and was defined as the presence of all of the following: Age > 60 years, BMI > 30 kg/m2, and pre-transplantation hypertension and diabetes[6,7]. Recipient characteristics (age at listing and at transplantation, ethnicity, gender, BMI, diabetes), severity of liver disease based on native laboratory MELD score at allocation, laboratory values [international normalized ratio (INR), bilirubin, creatinine, albumin], and clinically relevant manifestations of portal hypertension (ascites and hepatic encephalopathy) were reviewed in each of the three groups to compare baseline covariates.
Separate analyses were performed comparing recipients with NASH to non-NASH controls and comparing HR-NASH to LR-NASH. In the UNOS data set, PVT is categorized as “Present”, “Not present”, or “Unknown” and the data are based upon direct surgical evaluation of the veins at the time of hepatectomy. The degree of clot burden is not specified in the dataset nor is the chronicity. Based on previously validated methodology and due to the potential for misclassification bias[16], 831 recipients with “Unknown” PVT status were excluded. In general, univariate comparisons of the excluded cases to the included cohort with known PVT status did not reveal any baseline differences with the exception that the included cohort had a greater percentage of patients with cholestatic liver disease (10.2% vs 5.5%, P < 0.001). It was felt by the study team that this was not a significant clinical factor in the analysis. The dataset also does not contain information on treatment of PVT or testing for thrombophilia.
Recipients were statistically evaluated in multiple factors including demographics, medical comorbidities, waiting list and transplantation characteristics. Univariate comparisons were performed using the Student-t test, Wilcoxon sign rank test, χ2 test, or Fisher exact test as appropriate. Multivariable models were constructed using logistic regression and analysis of maximum likelihood estimates to test the primary hypothesis that patients with HR-NASH are at increased risk for the development of PVT and to assess statistical associations and risk factors for the development of PVT. Individual covariates were included in the multivariable model if they were statistically significant to P < 0.20 in univariate analysis, have been shown in the literature to be important or were deemed to be clinically important by the study team[18,19]. In separate models, individual components of the HR-NASH definition (hypertension, age, BMI and diabetes) were entered into the model as individual variables to ensure one of these did not dominate. Final variables included in the regression model included HR-NASH, LR-NASH, individual laboratory values at transplant (creatinine, bilirubin, INR, albumin, sodium), HCV, cholestatic liver disease, male gender, African American race, Hispanic race, encephalopathy (which was dichotomized into those with severe encephalopathy with score > 2), ascites (similarly dichotomized), pre-transplant dialysis treatment and autoimmune liver disease. No data imputation was performed. All statistical tests for significance were two sided and a significance level P less than or equal to 0.05 was considered statistically significant. All data set manipulation and statistical analyses were performed using SAS (version 9.4, Cary, NC). No transplants involving prisoners were included in this analysis. Institutional review board approval was not required for this study as the UNOS/OPTN dataset is de-identified.
Thirty-five thousand and seventy-two candidates underwent liver transplantation and of those organ recipients, 3240 (9.2%) were transplanted for NASH of which 465 met criteria for HR-NASH (1.3%) and 2775 for LR-NASH (7.9%). Two thousand six hundred and twenty-six (7.5%) recipients had pre-transplant PVT, of which 394 (12.2%) were in the NASH group (Figure 1). The prevalence of PVT was not significantly different between HR-NASH and LR-NASH (n = 66, 14.2% vs n = 328, 11.8%, P = 0.145). In general, NASH recipients were older, more likely to be female, less likely to be African American or Hispanic, had higher BMI values and were more likely to have diabetes, hypertension and renal dysfunction (Table 1). Severity of liver disease, while statistically significantly different, was not deemed to be clinically significantly different (e.g., MELD at listing of 20.0, 95%CI: 19.8-20.3 for NASH vs 19.6, 95%CI: 19.5-19.7 for non-NASH). The leading indication for transplantation in the non-NASH group was chronic HCV (46.6%) while alcoholic liver disease was the second leading indication (19.0%).
| NASH (n = 3240) | Other etiologies (n = 31832) | P value | |
| Recipient characteristics | |||
| Age at listing, mean years (95%CI) | 57.6 (57.3-57.9) | 52.2 (52.1-52.3) | < 0.001 |
| Age at transplant, mean years (95%CI) | 58.1 (57.8-58.4) | 52.7 (52.6-52.8) | < 0.001 |
| Male gender | 1747 (52.9) | 22099 (67.3) | < 0.001 |
| African American race | 65 (2.0) | 3.559 (10.9) | < 0.001 |
| Hispanic race | 348 (10.5) | 4009 (12.2) | 0.005 |
| BMI at transplant, kg/m2, mean (95%CI) | 32.3 (32.0-32.5) | 27.8 (27.7-27.9) | < 0.001 |
| Hypertension requiring medical treatment | 160 (33.2) | 2155 (19.2) | < 0.001 |
| Diabetes | 1698 (51.4) | 6211 (18.9) | < 0.001 |
| Portal vein thrombosis | 394 (12.2) | 2232 (7.1) | < 0.001 |
| Etiology of liver disease | |||
| Alcohol alone | 6236 (19.0) | ||
| Autoimmune disease | 1.202 (3.7) | ||
| Cholestatic disease | 3638 (11.1) | ||
| Hepatitis B | 986 (3.0) | ||
| Hepatitis C | 15298 (46.6) | ||
| Other | 4472 (14.0) | ||
| Severity of liver disease | |||
| MELD score at listing, mean (95%CI) | 20.0 (19.8-20.3) | 19.6 (19.5-19.7) | 0.014 |
| MELD score at transplantation, mean (95%CI) | 23.5 (23.2-23.8) | 22.8 (22.7-22.9) | < 0.001 |
| Laboratory values | |||
| Serum bilirubin, mg/dL, mean (95%CI) | 7.4 (7.1-7.8) | 9.1 (9.0-9.2) | < 0.001 |
| INR, mean (95%CI) | 1.92 (1.90-1.95) | 1.93 (1.92-1.94) | NS |
| Serum albumin, g/dL, mean (95%CI) | 3.0 (3.0-3.1) | 3.0 (2.9-3.0) | 0.002 |
| Creatinine, g/dL, mean (95%CI) | 1.81 (1.76-1.86) | 1.65 (1.63-1.67) | < 0.001 |
| On dialysis at transplantation | 488 (10.6) | 4135 (12.2) | < 0.001 |
| Portal hypertension manifestations | |||
| Moderate-severe ascites at transplant | 1210 (36.7) | 10782 (32.9) | < 0.001 |
| Moderate-severe hepatic encephalopathy at transplant | 375 (11.7) | 3708 (11.3) | NS |
When comparing HR-NASH recipients to LR-NASH recipients, several differences were noted (Table 2). As expected by definition, HR-NASH recipients were older both at listing (64.0 years, 95%CI: 63.8-64.3 vs 56.7 years, 95%CI: 56.2-56.9, P < 0.001) and at transplantation (64.5 years, 95%CI: 64.2-64.7 vs 57.0 years, 95%CI: 56.8-57.4, P < 0.001). BMI values were greater for HR-NASH (35.1 kg/m2, 95%CI: 34.7-35.5 vs 31.8 kg/m2, 95%CI: 31.5-32.0, P < 0.001) as was renal dysfunction (mean creatinine 1.98 g/dL, 95%CI: 1.85-2.11 vs 1.78 g/dL, 95%CI: 1.73-1.85, P = 0.003).
| High-risk NASH (n = 465) | Low-risk NASH (n = 2775) | P value | |
| Recipient characteristics | |||
| Age at listing, mean years (95%CI) | 64.0 (63.8-64.3) | 56.7 (56.2-56.9) | < 0.001 |
| Age at transplant, mean years (95%CI) | 64.5 (64.2-64.7) | 57.0 (56.8-57.4) | < 0.001 |
| Male gender | 247 (52.4) | 1500 (53.0) | NS |
| African American race | 7 (0.2) | 58 (1.6) | NS |
| Hispanic race | 43 (9.1) | 305 (10.8) | NS |
| BMI at transplant, kg/m2, mean (95%CI) | 35.1 (34.7-35.5) | 31.8 (31.5-32.0) | < 0.001 |
| Portal vein thrombosis | 66 (14.2) | 326 (11.8) | NS |
| Severity of liver disease | |||
| MELD score at listing, mean (95%CI) | 19.5 (18.7-20.3) | 20.1 (19.8-20.4) | NS |
| MELD score at transplantation, mean (95%CI) | 22.8 (21.9-23.6) | 23.7 (23.3-24.0) | NS |
| Laboratory values | |||
| Serum bilirubin, mg/dL, mean (95%CI) | 6.2 (5.4-7.0) | 7.7 (7.3-8.0) | 0.002 |
| INR, mean (95%CI) | 1.81 (1.75-1.86) | 1.94 (1.91-1.97) | 0.002 |
| Serum albumin, g/dL, mean (95%CI) | 3.1 (3.0-3.2) | 3.0 (2.9-3.1) | 0.006 |
| Creatinine, g/dL, mean (95%CI) | 1.98 (1.85-2.11) | 1.78 (1.73-1.83) | 0.003 |
| On dialysis at transplantation | 73 (15.5) | 415 (14.7) | NS |
| Portal hypertension manifestations | |||
| Moderate-severe ascites at transplant | 178 (37.8) | 1032 (36.5) | NS |
| Moderate-severe hepatic encephalopathy at transplant | 51 (10.8) | 324 (11.5) | NS |
Severity of liver disease based on MELD scores and portal hypertensive manifestations of ascites and encephalopathy were similar between the two groups. Interestingly, non-NASH recipients with HR features were not at increased odds of PVT (P = 0.11), albeit only 58 patients met criteria for this subgroup.
In adjusted multivariable analysis (Table 3), recipients with HR-NASH had the greatest risk of pre-transplant PVT with OR = 2.11 (95%CI: 1.60-2.76, P < 0.001) when referenced to the non-NASH cohort and 30% increased odds when compared to LR-NASH recipients (OR = 1.71, 95%CI: 1.49-1.96, P < 0.001). Other significant associations with pre-transplant PVT included male gender (OR = 1.18, 95%CI: 1.07-1.29, P < 0.001), Hispanic race (OR = 1.24, 95%CI: 1.10-1.39, P < 0.001) moderate-to-severe ascites (OR = 1.14, 95%CI: 1.04-1.25, P = 0.007) and autoimmune liver disease (OR = 1.43, 95%CI: 1.14-1.79, P = 0.002). African Americans were less likely to have pre-transplant PVT with OR = 0.75 (95%CI: 0.64-0.89, P < 0.001), similar to our previous findings[16].
| Odds ratio | 95%CI | P value | |
| African American race | 0.75 | 0.64-0.89 | < 0.001 |
| AIH | 1.43 | 1.14-1.79 | 0.002 |
| Hispanic race | 1.24 | 1.10-1.39 | < 0.001 |
| HR-NASH | 2.11 | 1.60-2.76 | < 0.001 |
| LR-NASH | 1.71 | 1.49-1.96 | < 0.001 |
| Male gender | 1.18 | 1.07-1.29 | < 0.001 |
| Moderate-severe ascites | 1.14 | 1.04-1.25 | 0.007 |
While MELD was significantly different on univariate analysis, the individual factors (bilirubin, INR, creatinine) involved in the MELD regression equation were not clinically important statistically significant predictors in adjusted multivariable analysis nor was pre-transplantation dialysis.
Based on a large national liver transplant database and building on our previous work in transplant recipients with NASH[16], we have shown an independent cross-sectional association documenting an increased risk of pre-transplant PVT in recipients undergoing transplantation for HR-NASH. This association was significant despite adjustment for multiple established risk factors for pre-transplant PVT. Given the technical difficulties associated with pre-transplant PVT, careful recipient selection is paramount in preventing post-transplant vascular complications.
Patients with NASH and in particular NASH cirrhosis, have in vivo abnormalities in primary, secondary and tertiary hemostasis[20,21]. The role of platelet dysfunction via increased activation, adherence and aggregation is the best described abnormality in primary hemostasis[22-24], however multiple investigators have shown elevations in vonWillebrand factor as a surrogate for endothelial dysfunction as well[21,25]. Secondary hemostasis is impaired in NASH due to elevations in fibrinogen, factor VIII, IX, XI, XII, increased clotting activity of factor VII, and low levels of antithrombin III[20,21,25-27]. Protein C levels may be increased or decreased in patients with NASH[20]. Tertiary hemostasis is disrupted due to elevations in plasminogen activator inhibitor-1 and low levels in both thrombin activatable fibrinolysis inhibitor and tissue plasminogen activator[20,21,27]. The aggregate of these impaired mechanisms of coagulation leads to the hypercoagulable milieu responsible in part for the development of PVT.
Whether or not the presence of pre-transplant PVT plays a role in the decreased survival of HR-NASH[6,7] remains unknown as our study did not investigate patient centered outcomes such as graft and overall patient survival. We did not attempt to do this due to the limitations of the UNOS/OPTN dataset, which does not contain information on the use of anticoagulants and data on post-transplant vascular complications is hindered by a large degree of missing data. The lack of this information introduces significant heterogeneity into the dataset and concrete post-transplant outcomes based conclusions for patients with PVT are problematic. However, what is clear is that the high-risk subgroup of NASH is the most at risk for PVT both in comparison to other NASH recipients not meeting the HR definition and also to all other etiologies of liver disease. We have previously shown that the independent factors of diabetes and obesity do not predispose to PVT on an individual basis[16]. It is only in combination with advanced age > 60 years and hypertension that these factors interact in a way to produce clinically meaningful thrombotic disease, perhaps due to increased physiologic endothelial dysfunction with advancing age[28].
In general, treatment outcomes for patients with PVT are impaired by a lack of large-scale, randomized, placebo-controlled trials that are generalizable. Villa et al[29] demonstrated in an un-blinded, single center randomized controlled trial that daily prophylactic dosing of low molecular weight heparin (40 mg daily) for twelve months prevented the development of PVT in patients with compensated cirrhosis at 48 wk, an effect that persisted through the 5-year follow-up period when compared to standard of care[29]. This study also demonstrated significantly less hepatic decompensation in the low molecular weight heparin arm and a survival benefit in the absence of a single bleeding event[29]. Building on this, Cui et al[30] recently published a controlled trial evaluating the efficacy and safety of anticoagulation therapy with different doses of enoxaparin (1 mg/kg twice a day vs 1.5 mg/kg daily) for PVT in patients with cirrhosis secondary to chronic hepatitis B in 65 patents, the majority of which had partial thrombosis. Importantly, 79% of patients achieved partial or complete response with anticoagulation based on follow-up imaging, however, non-variceal bleeding was significantly greater in the daily group (23.5% vs 6.4%) and the authors concluded that dosing at 1 mg/kg of enoxaparin subcutaneously twice a day was the preferred anticoagulation regimen. While the inclusion criteria were stringent limiting generalizability and the imaging guided definition of PVT open for criticism in these studies, the findings are nonetheless intriguing. A recent meta-analysis by Qi et al[31] of 16 studies (the authors did not include either of the aforementioned studies) and 960 patients found a pooled OR of 4.16 (95%CI: 1.88-9.20, P < 0.001) for complete portal vein recanalization with anticoagulation. Interestingly, the pooled rate of bleeding was only 3.3% (95%CI: 1.1%-6.7%). A recently published animal model found that 3 mo of dabigatran significantly reduced fibrin deposition, inflammation, hepatocellular injury, steatosis and weight gain[32] through mitigation of thrombin generation, the end-result of the coagulation abnormalities in NASH, suggesting a potential novel therapeutic approach. Newer data regarding the potential use of prothrombin complex concentrates in combination with antithrombin with or without concurrent fibrinogen administration to restore the delicate homeostasis of coagulation and normal thrombin and fibrinogen is emerging[33,34]. However, this combination of therapy has not been broadly studied in patients with chronic liver disease and concrete recommendations about the utility of this treatment cannot be made at this juncture.
In general, prospective, randomized, placebo-controlled studies are sorely needed in all patients with cirrhosis, however, targeting those most at risk including patients with HR-NASH, may provide the most substantial benefits including the potential to reduce disease burden from cerebrovascular accidents that this population is at risk for. If the reduction in inflammation and fibrosis with the direct thrombin inhibitors is validated in human subjects, these agents may provide an antifibrotic therapy which could alter the prognosis of liver disease.
Our study has several limitations. Despite containing a large number of transplant recipients in the MELD era, it is a retrospective study. Furthermore, missing data and correct diagnostic coding are potentially problematic with all large datasets. We attempted to control for missing data by excluding the small percentage of patients with unknown PVT status who were demographically similar to our included cohort to ensure bias towards or away from the null was not introduced. Although transplant centers are gaining increased experience transplanting recipients with PVT, it is possible that small volume centers may not have the same surgical technique or experience and pre-transplant PVT may in fact preclude transplantation in a subset of patients that would go on to be excluded from this study. The PVT variable in the dataset also has inherent heterogeneity as there is no differentiating between partial and complete thrombus or the chronicity of the clot. Our analysis also could not account for thrombophilia disorders and therapy in the pre-transplant phase. Additionally, our study excluded HCC patients in the event that PVT was associated with HCC as tumor thrombus, which may limit generalizability.
In conclusion, as the in vivo evidence of a thrombophilic state in patients with NASH continues to grow, epidemiologic evidence continues to lag behind. Building on our previous work, we have shown that liver transplant candidates with HR-NASH are at the highest risk for PVT development when compared to other NASH patients and also to all other etiologies of liver disease. Prospective study enrolling HR-NASH patients in anticoagulation trials seems warranted in order to determine a direct benefit in improving patient centered outcomes including the potential for overall and post-transplantation graft survival.
We would like to thank Dr. Timothy L McMurray for his statistical review of our manuscript. This work was presented at Digestive Diseases Week 2016.
Non-alcoholic steatohepatitis (NASH) is increasing in prevalence and will soon be the leading indication for liver transplantation in western nations. Patients with NASH are at increased risk for thrombosis. Patients with high-risk NASH have inferior liver transplantation outcomes. Whether or not this high-risk group has an increased risk of portal vein thrombosis (PVT) remains unknown.
The field of coagulation disorders in chronic liver disease continues to grow. Much of the research focuses on PVT and/or venothromboembolic disease. Identifying high-risk groups for possible preventative intervention through clinical trials remains a goal of the liver and hematology fields alike.
In the present study, the authors investigated the association between high-risk NASH and PVT in liver transplant recipients with cirrhosis. This is the first report of PVT risk in patients with high-risk NASH.
The present report furthers understanding regarding the thrombophilic state of NASH and highlights a potential high-risk group who may benefit from further prospective study.
This is an excellent very large retrospective review that clearly shows that high risk NASH patients are more thrombophilic than low risk NASH patients and much more thrombophilic than non-NASH cirrhotic patients.
| 1. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1734] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 2. | Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 3. | Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 628] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 4. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 5. | Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation. 2016;100:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Kennedy C, Redden D, Gray S, Eckhoff D, Massoud O, McGuire B, Alkurdi B, Bloomer J, DuBay DA. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford). 2012;14:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:394-402.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, Watt KD. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Søgaard KK, Horváth-Puhó E, Montomoli J, Vilstrup H, Sørensen HT. Cirrhosis is Associated with an Increased 30-Day Mortality After Venous Thromboembolism. Clin Transl Gastroenterol. 2015;6:e97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S, Santoliquido A, Flore R. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 361] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol. 2010;16:6119-6122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, Avolio AW. Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow-up. Transplant Rev (Orlando). 2014;28:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, Lynch RJ, Marrero JA, Pelletier SJ. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (2)] |
| 16. | Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1772] [Cited by in RCA: 2769] [Article Influence: 153.8] [Reference Citation Analysis (1)] |
| 19. | Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 718] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 20. | Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, Peyvandi F, Bertelli C, Valenti L, Fargion S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, Van Marck E, Staels B, Michielsen P, Van Gaal L. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Sobol AB, Watala C. The role of platelets in diabetes-related vascular complications. Diabetes Res Clin Pract. 2000;50:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, Onder E. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Celikbilek M, Gürsoy S, Deniz K, Karaman A, Zararsiz G, Yurci A. Mean platelet volume in biopsy-proven non-alcoholic fatty liver disease. Platelets. 2013;24:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 26. | Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, Lundbom N, Rissanen A, Lassila R, Yki-Järvinen H. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Cigolini M, Targher G, Agostino G, Tonoli M, Muggeo M, De Sandre G. Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men--role of the metabolic syndrome. Thromb Haemost. 1996;76:69-73. [PubMed] |
| 28. | Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 980] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 552] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 30. | Cui SB, Shu RH, Yan SP, Wu H, Chen Y, Wang L, Zhu Q. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. 2015;27:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Qi X, De Stefano V, Li H, Dai J, Guo X, Fan D. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Kopec AK, Joshi N, Towery KL, Kassel KM, Sullivan BP, Flick MJ, Luyendyk JP. Thrombin inhibition with dabigatran protects against high-fat diet-induced fatty liver disease in mice. J Pharmacol Exp Ther. 2014;351:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 33. | Mitrophanov AY, Rosendaal FR, Reifman J. Therapeutic correction of thrombin generation in dilution-induced coagulopathy: computational analysis based on a data set of healthy subjects. J Trauma Acute Care Surg. 2012;73:S95-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Mitrophanov AY, Wolberg AS, Reifman J. Kinetic model facilitates analysis of fibrin generation and its modulation by clotting factors: implications for hemostasis-enhancing therapies. Mol Biosyst. 2014;10:2347-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Mitrophanov AY, Ramsay MA S- Editor: Gong ZM L- Editor: A E- Editor: Li D