Published online Aug 28, 2017. doi: 10.4254/wjh.v9.i24.1022
Peer-review started: March 2, 2017
First decision: May 3, 2017
Revised: May 19, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: August 28, 2017
Processing time: 177 Days and 3 Hours
The place of liver transplantation in the treatment of severe iatrogenic liver injuries has not yet been widely discussed in the literature. Bile duct injuries during cholecystectomy represent the leading cause of liver transplantation in this setting, while other indications after abdominal surgery are less common. Urgent liver transplantation for the treatment of severe iatrogenic liver injury may-represent a surgical challenge requiring technically difficult and time consuming procedures. A debate is ongoing on the need for centralization of complex surgery in tertiary referral centers. The early referral of patients with severe iatrogenic liver injuries to a tertiary center with experienced hepato-pancreato-biliary and transplant surgery has emerged as the best treatment of care. Despite widespread interest in the use of liver transplantation as a treatment option for severe iatrogenic injuries, reported experiences indicate few liver transplants are performed. This review analyzes the literature on liver transplantation after hepatic injury and discusses our own experience along with surgical advances and future prospects in this uncommon transplant setting.
Core tip: Liver transplantation may represent the only option to manage severe iatrogenic liver injuries. Despite widespread interest, reported experiences indicate only a minority of liver transplants are performed, and the place of liver transplantation in this setting has not yet been widely discussed. Causes other than severe bile duct injuries during cholecystectomy are less common indications for liver transplantation. Urgent liver transplantation for the treatment of severe iatrogenic liver injury may require technically difficult and time-consuming surgical procedures. The centralization of complex surgery in tertiary centers and the early referral of patients with severe iatrogenic liver injuries are crucial.
- Citation: Lauterio A, De Carlis R, Di Sandro S, Ferla F, Buscemi V, De Carlis L. Liver transplantation in the treatment of severe iatrogenic liver injuries. World J Hepatol 2017; 9(24): 1022-1029
- URL: https://www.wjgnet.com/1948-5182/full/v9/i24/1022.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i24.1022
At the end of the line, liver transplantation (LT) may represent the only curative and life-saving option to manage severe iatrogenic liver injuries. Whereas many recent articles have focused on different strategies in the multidisciplinary management of iatrogenic bile duct injuries (BDI) after cholecystectomy[1-4], the place of LT in the treatment of other severe iatrogenic liver injuries after hepatobiliary (HPB) surgery has not yet been widely discussed in the literature. This review analyzes the cases reported to date and discusses our own experience along with surgical advances and future prospects in this uncommon transplant setting.
There are basically two main types of severe iatrogenic liver injury requiring urgent LT: Biliary or vascular injuries, or a combination of the two. Some patients were indicated for LT due to acute liver failure (ALF) resulting from vascular injury secondary to a first biliary injury or other less common severe iatrogenic liver injuries.
The incidence of BDI during cholecystectomy varies from 0.1% to 0.3%, rising to 0.6% when considering the laparoscopic approach[5,6]. The type and extent of BDI play an important role in surgical planning for appropriate timing and treatment.
Different systems have been proposed to classify and grade the severity of BDI. In 1982, Professor Bismuth[7] first classified postoperative bile duct strictures in a chapter of the “Blumgart book”. He subsequently proposed a useful classification of biliary strictures based on the principles of surgical treatments[8]. Like the Bismuth classification, Strasberg’s scale[9] incorporates other biliary injuries commonly encountered after laparoscopic cholecystectomy. To prevent bile duct injury, the Stewart-Way classification incorporates the mechanism of injury as well as its anatomy, separating resectional damage from stricture and providing a guide to pre-operative evaluation and biliary reconstruction[10]. Although other classifications of BDI after laparoscopic cholecystectomy have been reported and recently reviewed by Chun[11], the Strasberg scale remains the classification of choice for defining the types of BDI.
Some recently reported series on LT for cholecystectomy-induced BDI provide important insights. In 2011, Ardiles et al[2] analyzed their experience using LT as a definitive treatment for BDI, reporting data from a retrospective national survey performed in 18 LT centers over 20 years in Argentina. Among 2766 LT performed from 1990 to 2009, 19 (0.7%) were secondary to BDI arising during 16 cholecystectomies (open in 10, and laparoscopic in 6), two hydatid cyst resections, and one right hepatectomy. Seven patients had associated vascular injuries. The indication for LT was liver cirrhosis in 18 cases and ALF in the remaining one. No intraoperative mortality was reported but four patients died during the first month after LT, and another four died in the late postoperative period. The remaining 11 patients showed a good quality of life in the long-term follow-up and recipient survival rates at one, three, five and ten years were 73%, 68%, 68% and 45% respectively. The authors reported a higher rate of major post-operative complications (52%), according to the Clavien classification[12], compared with other etiologies and secondary biliary cirrhosis[13]. Interestingly, the significant decrease over time in the incidence of LT for this indication in their cohorts (3.1% of all LT in the period 1990-1994; and 0.2% in the period 2005-2009 - P < 0.001) reflects improvements in the prevention and management of BDI related to a multidisciplinary and specialized approach to injury-related complications.
In 2013, Parilla et al[4], on behalf of the Spanish Liver Transplantation Study Group, reviewed the indications and outcome of 27 patients with BDI after cholecystectomy and listed for LT in Spain over a 24-year period. Emergency LT for ALF was indicated in seven patients all after laparoscopic cholecystectomy. Two of them died while on the waiting list, one from multiorgan failure (MOF) secondary to BDI-related sepsis, and the other was anhepatic after a total hepatectomy required for massive liver necrosis. Another 20 patients underwent elective LT for secondary biliary cirrhosis after BDI (13 after open and 7 after laparoscopic cholecystectomy). Four of the five recipients who underwent emergency LT for ALF died within 30 d after LT, and the estimated overall five-year survival rate was 68%. The Spanish study confirms that BDI after laparoscopic cholecystectomy tends to be more severe than that after the open approach.
Very recently, an Italian group from Genoa reviewed the literature and reported another two cases of LT for iatrogenic injuries among 12 patients referred to their tertiary center for the management of complicated cholecystectomy[14]. The timing for LT differed in this series. The first patient was transplanted after several endoscopic and radiological attempts to solve recurrent cholangitis that led to secondary biliary cirrhosis five years after BDI. He initially underwent open cholecystectomy with a biliary lesion described as type E2 (according to the Strasberg-Bismuth classification), and referred to the tertiary center five years after the first injury. Conversely, the second patient was listed for an emergency LT after a laparoscopic cholecystectomy converted to the open approach because of bleeding from the liver parenchyma. Eight days after surgery the patient had bile leaks and underwent endoscopic biliary stent placement complicated by a large intrahepatic hematoma and bleeding initially treated by right hepatic embolization. The patient required emergency surgical exploration and a total hepatectomy with temporary portocaval shunt (TPCS) was required to overcome the bleeding after a right hepatectomy. The intraoperative field showed a massive liver hematoma involving the right lobe, deep parenchyma lacerations, and a type D injury. After a two-day anhepatic bridging period the patient was successfully transplanted and underwent long-term follow-up. The same authors also described another patient with chronic cirrhosis who underwent LT after acute liver decompensation caused by open cholecystectomy for common bile duct lithiasis.
In addition to biliary damage, severe vascular iatrogenic injuries during HPB surgery can result in devastating complications. While the BDI rate after cholecystectomy is estimated up to 0.6% (6), and concomitant hepatic artery damage has been reported in 12%-47% of patients[15], isolated portal vein (PV) injury is uncommon. In 2011, Strasberg et al[16] published an analytical review of vasculobiliary injury in cholecystectomy, evaluating frequencies, causes clinical implications, and their management. A year later, the same team addressed the pathogenesis of “extreme” vasculobiliary injury and reported on outcomes after cholecystectomy for severely inflamed gallbladders in eight patients[17]. Unfortunately, one patient developed infarction of the bile ducts after injury to the proper hepatic artery and died of sepsis in the postoperative period after urgent LT. In author’s opinion, in presence of inflammation a fundus-down cholecistectomy should be avoided for the prevention of extreme vasculobiliary injuries.
In 2013, Wang et al[15] analyzed the therapeutic strategies for iatrogenic PV injury after cholecystectomy, reporting their experience of 11 patients with vascular injuries in the absence of biliary damage. One of these patients, a 50-year-old woman, underwent LT due to chronic liver failure four months after the initial injury to the right branch of PV after an open cholecystectomy. In the authors’ opinion, delayed diagnosis and treatment may have led to difficult vein repair and liver revascularization resulting in PV thrombosis and hepatic necrosis. They highlighted the major role of thrombolytic and anticoagulation therapy in the treatment of acute massive thrombus. We agree with them that an immediate attempt to repair severe PV injury should be preferred in a hemodynamically stable patient.
Indications for LT to treat severe iatrogenic liver injuries after abdominal surgery or causes other than injuries during cholecystectomy are certainly less common, and very few cases have been reported.
In 2006, Huerta et al[18] described three lethal complications resulting from severe iatrogenic injuries during bariatric surgery performed in a high-volume bariatric center. They also described details of three cases of PV thrombosis that led to LT after two Roux-en-Y gastric bypass (RYGBP) procedures and one vertical banded gastroplasty. In the two cases of RYGBP, the porta hepatis was inadvertently stapled, while in the patient who underwent vertical banded gastroplasty the PV was divided and promptly reconstructed, but caused irreversible ischemic liver damage. Although the iatrogenic injuries were immediately recognized, a transplant surgeon consulted, and patients referred for emergency LT, the postoperative course was complicated by sepsis, MOF, and other severe medical complications resulting in the deaths of the patients. The authors claimed that PV ligation with immediate patient referral to a LT center for emergency transplant may improve the outcome in case of severe PV injury.
In 2009, the group from the University Medical Center, Nashville, Tennessee (United States) reported two cases of iatrogenic porta hepatis transection requiring an urgent two-stage liver LT[19]. In the first case, severe porta hepatis transection occurred during an open adrenalectomy in a 39-year-old woman with a history of cholecystectomy. Before transferring the patient to the authors’ tertiary LT center, primary PV repair was attempted, and a Roux-en-Y hepaticojejunostomy performed, while the hepatic artery was left divided. Due to progression of the hepatic dysfunction and worsening hemodynamics, the patient underwent urgent total hepatectomy and portocaval shunt, and was listed for an emergency LT. In the other case, severe iatrogenic injury occurred during a laparoscopic cholecystectomy converted to an open operation to control a massive bleed and complete cholecystectomy before emergency transfer of the patient to the authors’ tertiary center. A computed tomography (CT) scan showed infarction of the right hepatic lobe, transection of the right hepatic artery and right PV. Arterial perfusion of the left lobe was provided through a replaced left hepatic artery. A right hepatic lobectomy was planned and an urgent surgical re-exploration performed. Unfortunately, the extent of the left PV injury precluded successful reconstruction of the PV flow and a total hepatectomy with a portocaval shunt was performed. The patient underwent LT 20 h later. We agree with the author that patients presenting with severe portal transection cannot be treated expectantly, and prompt radiological evaluation and surgical intervention are mandatory to attempt to restore hepatic flow. Hepatic resections should not be the only options entertained and LT should be promptly evaluated on a case-by-case basis.
Another case of severe hepatic injury resulting from an open right adrenalectomy was reported in the same year by Tessier et al[20] in a review of high-grade complications after adrenalectomy. The surgical procedure was complicated by an unrecognized injury to and ligation of the proper hepatic artery. Three months after adrenalectomy, the patient underwent a Roux-en-Y hepaticojejunostomy for the treatment of multiple liver abscesses, recurrent episodes of cholangitis and later a bleeding cholecysto-enteric fistula. The patient was ultimately referred to a tertiary center where LT was performed because of recurrent cholangitis and bile duct sclerosis.
Interestingly, in 2010 Di Benedetto et al[21], reported details of their experience in the treatment of severe injuries after transjugular intrahepatic portosystemic shunt placements in two cirrhotic patients where surgical and radiological attempts had failed to stop the bleeding after parenchymal and vascular rupture. Although the indications for LT were liver failure after artery embolization, and uncontrollable hemobilia, this experience highlights the ability of a tertiary referral center to offer LT as the only curative option.
Our tertiary referral center offers both a specialist HPB referral service and an abdominal organ transplantation service with more than 1800 LTs performed by the end of 2016. Out of 64 patients referred to our center with BDI after cholecystectomy only four underwent LT for secondary biliary cirrhosis, while the injuries were repaired by surgical operations or radiological and endoscopic approaches in the other cases. Another three patients were listed for LT to manage severe iatrogenic liver injuries occurring during HPB surgery.
The first case of life-saving LT performed by our institution has been described in detail elsewhere together with a full description of the surgical technique adopted[22]. A 46-year-old man was initially considered for a liver resection due to a giant symptomatic hepatic hemangioma arising from the caudate lobe with compression of the retrohepatic inferior vena cava (IVC), and thrombosis of the left and middle hepatic veins. An uncontrollable bleeding from the confluence of the suprahepatic veins occurred during the liver resection and a total hepatectomy with retrohepatic IVC resection after a venous-venous by-pass was carried out to overcome the hemodynamic instability. The extensive liver congestion excluded any attempt to proceed to an ex-vivo major hepatectomy, and a request for urgent LT was launched. A Dacron interposition prosthesis replaced the retrohepatic vena cava, and an end-to-side TPCS was performed between the recipient PV and the Dacron prosthesis. The LT was carried out with a side-to-side cavocaval anastomosis between the graft retrohepatic vena cava and the Dacron interposition graft. There were no postoperative complications, and the patient was discharged 26 d after LT.
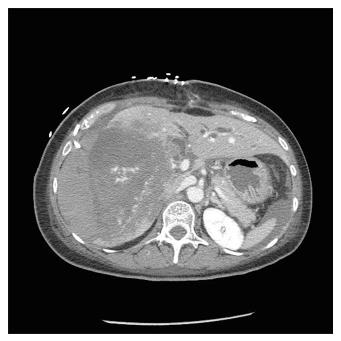
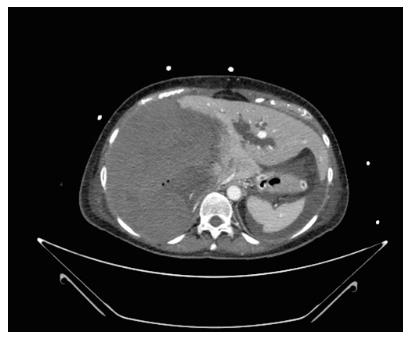
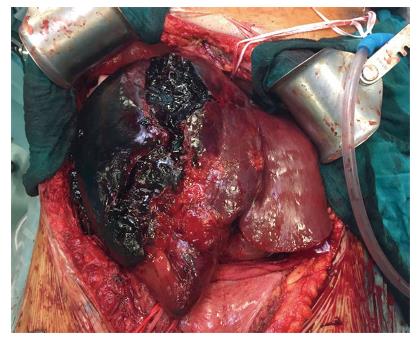
The second patient was a 52-year-old woman referred to our center from another HPB tertiary center without a LT program. She had ALF resulting from a radiologically assisted hepatic artery embolization in a patient initially affected by bilobar intrahepatic calculosis treated by bile duct exploration and a Roux-en-Y hepaticojejunal anastomosis. Before referral, after surgical bile duct exploration an intrahepatic bleed occurred with a rapid deterioration of the patient’s clinical status due to hemorrhagic shock. The CT scan showed a massive intrahepatic hematoma involving the right hepatic lobe and segment IV (Figure 1). After right hepatic artery embolization the bleeding stopped, but the patient developed severe ALF due to acute ischemic liver necrosis (Figure 2). After the patient was referred to our center, a conservative liver resection such as right extended hepatectomy was excluded because of the liver failure and the massive hepatic infarction extending to the left lobe. In our opinion, a liver resection could be a surgical option only when the hepatic infarction and necrosis is limited and liver function preserved, because any surgical or infectious complication after a major hepatectomy could represent a contraindication to proceed to LT. An urgent LT was planned and a liver graft from a deceased donor was immediately requested on a top priority basis from the Italian national organ sharing network. An AB0-compatible graft became available 16 h later, and the patient underwent LT. The intraoperative findings are summarized in Figure 3. Despite the huge right lobe hematoma extending to segment IV with signs of extrahepatic rupture, the hepatectomy was carried out with hemodynamic stability and a TPCS and a venovenous by-pass. The liver implant was performed in a piggy-back fashion, and a Roux-en-Y reconstruction carried out using the same intestinal loop created during the first surgery. The patient was transferred to the floor after two days spent in the ICU, discharged after 12 d, and alive three years after LT.
Another patient, a 42-year-old woman, was referred to our center the day after a complicated Whipple procedure for an ampullary adenoma with subsequent total pancreasectomy due to pancreatic fistula and hemoperitoneum. After surgical re-exploration patient was transferred to the ICU. Liver function tests, lactate, and her hemodynamic conditions continued to worsen and a CT scan showed massive liver necrosis with multiple abscesses excluding any attempt to proceed to a liver resection. A request for an urgent LT was launched, and a compatible donor was available eight hours later. Recipient laparotomy revealed massive intestinal necrosis, and complete hepatic artery and PV thrombosis. These findings, associated with severe MOF and hemodynamic instability, made the indication for LT impracticable and futile. Unfortunately, the patient failed to overcome MOF and the available liver graft was connected to oxygenated hypotermic machine perfusion after 12:15 h of static cold storage before the transplant in a back-up recipient[23].
Urgent LT to solve severe iatrogenic liver injuries may represent a surgical challenge requiring technically difficult and time-consuming procedures. Although a TPCS improves hemodynamic stability during LT, its role is still controversial and its use has remained limited since the technique was recommended in the early 1990s for recipients with portal hypertension caused by acute or subacute liver failure expected not to have adequate portosystemic collaterals[24]. A total hepatectomy and subsequent LT could be a useful strategy for patients presenting massive ischemic liver or exsanguinating hepatic injuries with uncontrollable vascular or parenchymal bleeding. In addition, urgent total hepatectomy and a TPCS may be performed awaiting a compatible deceased liver donor, or in the event of “toxic hepatic syndrome” secondary to massive hepatic necrosis. It is well known that total hepatectomy might improve the metabolic, coagulation and hemodynamic profiles of these patients while waiting for a suitable liver donor[21,25].
From a surgical point of view, portal blood could be shunted to the systemic circulation performing an end-to-side anastomosis between the main PV and the anterior wall of the anterior surface of the suprarenal IVC or performing a portosuprahepatic anastomosis[26].
Alternatively, an extracorporeal portocaval shunt-catheter connecting the PV to the femoral vein can be applied as described by the Munich transplant group[27] who reported the feasibility of this shunt technique, which does not require anticoagulation or an additional pump supply.
A venovenous by-pass may represent another possible option especially when a patient becomes hemodynamically unstable after a massive bleed and resection of the IVC required as previously reported by our Institution[22].
Vascular reconstruction in patients with severe iatrogenic injuries of hepatic hilum elements could be challenging, and extra-anatomical reconstruction with the use of arterial conduits remains an important tool in the transplant surgeon’s armamentarium. Banked or freshly procured vascular grafts from deceased donors should be considered for supraceliac or infrarenal aortohepatic conduits.
The use of aortohepatic conduits using deceased donor iliac artery as an interpositional graft in LT have already been investigated and recently reviewed[22-30].
In addition to deceased arterial grafts, the use of cryopreserved arterial grafts as conduits has been recently proposed in living donor LT[31].
A recently published paper by Hibi et al[32] advised proceeding with caution in primary adult LT, where the placement of an aortohepatic conduit should be strictly limited because of the greater risk of late hepatic artery thrombosis and impaired graft survival. Nevertheless, the use of arterial conduits could provide the only alternative option for graft vascularization during LT after severe iatrogenic injury of the hepatic artery. Baylor’s group recently published their center experience after twenty years’ follow-up of PV conduits in LT[33]. More than two thousand adult LTs were evaluated. All PV conduits were the donor’s iliac vein procured during liver retrieval. PV conduits were required during the first LT in 35/2370 patients (1.5%). Long-term graft survival after LT using PV conduits was excellent and comparable to that of the control group (65% with the conduit vs 66% without the conduit at five-year follow-up, 58% vs 51% at ten years, and 48% vs 35% at 15 years). The authors reported excellent long-term results proving the longevity of the PV conduits using the donor’s iliac vein. The reported results may also be applicable to other complex surgical settings such as severe iatrogenic vascular injuries requiring LT.
Resection and replacement of the IVC could occasionally be required during LT for severe iatrogenic injury of the liver or the vena cava. A variety of reconstruction strategies and materials including biological (autologous and heterologous) and synthetic grafts such as polytetrafluoroethylene (PTFE) and polypropylene (Dacron) have been reported to replace the vena cava[22]. Pulitanò et al[34] recently highlighted some important technical aspects in the use of biological tissues for IVC replacement. They reported advances in the use of glutaraldehyde-treated bovine pericardium and an autogenous peritoneo-fascial graft from a flap of parietal peritoneum backed by the posterior rectus sheath as alternatives to prosthetic IVC reconstruction. After 32 IVC reconstructions, the authors claimed that biological grafts allow greater flexibility and biocompatibility and long-term patency without permanent anticoagulation.
As previously mentioned for arterial and PV reconstructions, especially in LT centers, the use of cryopreserved banked or freshly procured venous allografts from deceased donors offers an option in IVC replacement. The use of allografts was first described long ago by Starzl et al[29] and is still common practice in the field of LT[28,29,35].
HPB surgery has had an extraordinary evolution and diffusion in recent years thanks to the success in reducing mortality and morbidity rates[36], especially in high-volume centers. A debate is ongoing on the need for centralization of complex surgery in tertiary referral centers. Clinicians are constantly reminded about the importance of early referral for patients with severe iatrogenic liver injuries to a tertiary center with experienced HPB and transplant surgery. Patients initially and repeatedly treated in non-specialist hospitals and referred for LT in the ALF setting have been reported to have worse outcomes[4].
The role of surgical experience in the repair process has been widely explored and demonstrated in the past[37]. In 2008, Silva et al[38] from the Queen Elisabeth Hospital, United Kingdom reported their experience as a specialist outreach service for on-table repair for iatrogenic BDI after laparoscopic cholecystectomy. They highlighted the role of this new kind of “travelling surgeon” reporting repeatable outcomes with no post-operative mortalities in 22 procedures avoiding transfer of the patient to a tertiary center, prolonged bile drainage, and a reoperation with a shorter hospital stay and a reduced risk of sepsis and liver failure. They also claimed that the proposed immediate approach has potential medicolegal advantages reducing the risk of litigation and costs.
Our experience highlighted the crucial role of a liver transplant program when referring a patient with complex and severe injuries after HPB surgery because LT may represent the patient’s only curative option in a small number of cases.
The literature lacks reports on severe iatrogenic liver injuries, likely because negative outcomes tend to be under-reported, and we have no information on those patients with severe iatrogenic liver injuries who died before referral to a tertiary center. This is detrimental to surgical education, and the topic was recently voiced by Cheah et al[39] who discussed improvement in care by close examination of “near-miss” cases.
Reported experiences on the place of LT in the treatment of severe iatrogenic injuries indicate few LTs are performed in this uncommon setting. Without an official comprehensive registry, it is exceedingly difficult to determine appropriate indications and long-term outcomes as detailed data are confined to individual case reports in the literature.
All the clinicians involved in the care of patients with severe iatrogenic liver injuries should clearly spell out information on their outcomes honestly and swiftly so that others can learn a lesson and not repeat the same errors.
| 1. | Thomson BN, Parks RW, Madhavan KK, Garden OJ. Liver resection and transplantation in the management of iatrogenic biliary injury. World J Surg. 2007;31:2363-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Ardiles V, McCormack L, Quiñonez E, Goldaracena N, Mattera J, Pekolj J, Ciardullo M, de Santibañes E. Experience using liver transplantation for the treatment of severe bile duct injuries over 20 years in Argentina: results from a National Survey. HPB (Oxford). 2011;13:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Lubikowski J, Chmurowicz T, Post M, Jarosz K, Białek A, Milkiewicz P, Wójcicki M. Liver transplantation as an ultimate step in the management of iatrogenic bile duct injury complicated by secondary biliary cirrhosis. Ann Transplant. 2012;17:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Parrilla P, Robles R, Varo E, Jiménez C, Sánchez-Cabús S, Pareja E; Spanish Liver Transplantation Study Group. Liver transplantation for bile duct injury after open and laparoscopic cholecystectomy. Br J Surg. 2014;101:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Roslyn JJ, Binns GS, Hughes EF, Saunders-Kirkwood K, Zinner MJ, Cates JA. Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 246] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 175] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Bismuth H. Postoperative strictures of the bile ducts. The biliary tract. 5th edition. Edinburgh: Churchill-Livingstone; 1982; 209-218. |
| 8. | Bismuth H, Majno PE. Biliary strictures: classification based on the principles of surgical treatment. World J Surg. 2001;25:1241-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125. [PubMed] |
| 10. | Stewart L, Domingez CO, Way LW. Bile duct injuries during laparoscopic cholecystectomy: a sensemaking analysis of operative reports. In: Mosier K, Fischer U, editors. Proceedings of the 8th International NDM Conference; Pacific Grove, CA, 2007 . . |
| 11. | Chun K. Recent classifications of the common bile duct injury. Korean J Hepatobiliary Pancreat Surg. 2014;18:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 12. | Clavien PA, Camargo CA Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 281] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Loinaz C, González EM, Jiménez C, García I, Gómez R, González-Pinto I, Colina F, Gimeno A. Long-term biliary complications after liver surgery leading to liver transplantation. World J Surg. 2001;25:1260-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Leale I, Moraglia E, Bottino G, Rachef M, Dova L, Cariati A, De Negri A, Diviacco P, Andorno E. Role of Liver Transplantation in Bilio-Vascular Liver Injury After Cholecystectomy. Transplant Proc. 2016;48:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Wang Z, Yu L, Wang W, Xia J, Li D, Lu Y, Wang B. Therapeutic strategies of iatrogenic portal vein injury after cholecystectomy. J Surg Res. 2013;185:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford). 2011;13:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 17. | Strasberg SM, Gouma DJ. ‘Extreme’ vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford). 2012;14:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Huerta S, Li Z, Livingston EH. Outcome of portal injuries following bariatric operations. Obes Surg. 2006;16:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zaydfudim V, Wright JK, Pinson CW. Liver transplantation for iatrogenic porta hepatis transection. Am Surg. 2009;75:313-316. [PubMed] |
| 20. | Tessier DJ, Iglesias R, Chapman WC, Kercher K, Matthews BD, Gorden DL, Brunt LM. Previously unreported high-grade complications of adrenalectomy. Surg Endosc. 2009;23:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Di Benedetto F, Mimmo A, D’Amico G, De Ruvo N, Cautero N, Montalti R, Guerrini GP, Ballarin R, Spaggiari M, Tarantino G. Liver transplantation due to iatrogenic injuries: two case reports. Transplant Proc. 2010;42:1375-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Aseni P, Lauterio A, Slim AO, Giacomoni A, Lamperti L, De Carlis L. Life-saving super-urgent liver transplantation with replacement of retrohepatic vena cava by dacron graft. HPB Surg. 2010;2010:pii: 828326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | De Carlis R, Lauterio A, Ferla F, Di Sandro S, Sguinzi R, De Carlis L. Hypothermic Machine Perfusion of Liver Grafts Can Safely Extend Cold Ischemia for Up to 20 Hours in Cases of Necessity. Transplantation. 2017;101:e223-e224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Tzakis AG, Reyes J, Nour B, Marino IR, Todo S, Starzl TE. Temporary end to side portacaval shunt in orthotopic hepatic transplantation in humans. Surg Gynecol Obstet. 1993;176:180-182. [PubMed] |
| 25. | Ringe B, Lübbe N, Kuse E, Frei U, Pichlmayr R. Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg. 1993;218:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Robles R, Parrilla P, Acosta F, Bueno FS, Ramirez P, Lujan JA, Rodriguez JM, López J, Fernandez JA. Portosuprahepatic shunt as an alternative to portocaval shunt in an hepatic patients waiting for an orthotopic liver transplant. Transplant Proc. 1999;31:2400-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Pratschke S, Meimarakis G, Bruns CJ, Kaspar M, Prix N, Zachoval R, Guba M, Jauch KW, Loehe F, Angele MK. Temporary intraoperative porto-caval shunt: useless or beneficial in piggy back liver transplantation? Transpl Int. 2013;26:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Cooke FN, Kurzweg FT, Starzl TE. Blood Vessel Bank: Organization and Function. Bull Univ Miami Sch Med Jackson Meml Hosp. 1957;2:26-31. [PubMed] |
| 29. | Starzl TE, Halgrimson CG, Koep LJ, Weil R 3rd, Taylor PD. Vascular homografts from cadaveric organ donors. Surg Gynecol Obstet. 1979;149:737. [PubMed] |
| 30. | Chatzizacharias NA, Aly M, Praseedom RK. The role of arterial conduits for revascularisation in adult orthotopic liver transplantation. Transplant Rev (Orlando). 2017;31:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Ali MA, Yong CC, Eng HL, Wang CC, Lin TL, Li WF, Wang SH, Lin CC, Yap A, Chen CL. Cryopreserved arterial grafts as a conduit in outflow reconstruction in living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2015;22:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Hibi T, Nishida S, Levi DM, Sugiyama D, Fukazawa K, Tekin A, Fan J, Selvaggi G, Ruiz P, Tzakis AG. Long-term deleterious effects of aortohepatic conduits in primary liver transplantation: proceed with caution. Liver Transpl. 2013;19:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Nikitin D, Jennings LW, Khan T, Vasani S, Ruiz R, Sanchez EQ, Chinnakotla S, Levy MF, Goldstein RM, Klintmalm GB. Twenty years’ follow-up of portal vein conduits in liver transplantation. Liver Transpl. 2009;15:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Pulitanó C, Crawford M, Ho P, Gallagher J, Joseph D, Stephen M, Sandroussi C. The use of biological grafts for reconstruction of the inferior vena cava is a safe and valid alternative: results in 32 patients in a single institution. HPB (Oxford). 2013;15:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Palma AF, Oberkofler CE, Raptis DA, Eshmuminov D, de Rougemont O, Schnyder A, Dimitroulis D, Lesurtel M, Dutkowski P, Clavien PA. Novel rescue procedure for inferior vena cava reconstruction in living-donor liver transplantation using a vascular graft recovered 25 h after donors’ circulatory death and systematic review. Transpl Int. 2014;27:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 427] [Article Influence: 32.8] [Reference Citation Analysis (4)] |
| 37. | Bismuth H, Franco D, Corlette MB, Hepp J. Long term results of Roux-en-Y hepaticojejunostomy. Surg Gynecol Obstet. 1978;146:161-167. [PubMed] |
| 38. | Silva MA, Coldham C, Mayer AD, Bramhall SR, Buckels JA, Mirza DF. Specialist outreach service for on-table repair of iatrogenic bile duct injuries--a new kind of ‘travelling surgeon’. Ann R Coll Surg Engl. 2008;90:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl. 2013;19:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Nishi H S- Editor: Gong ZM L- Editor: A E- Editor: Li D