Published online Jul 8, 2017. doi: 10.4254/wjh.v9.i19.833
Peer-review started: February 7, 2017
First decision: March 28, 2017
Revised: April 13, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: July 8, 2017
Processing time: 153 Days and 23.3 Hours
The prevalence of hepatitis C virus (HCV) infection amongst patients with chronic kidney disease (CKD) and end-stage renal disease exceeds that of the general population. In addition to predisposing to the development of cirrhosis and hepatocellular carcinoma, infection with HCV has been associated with extra-hepatic complications including CKD, proteinuria, glomerulonephritis, cryoglobulinemia, increased cardiovascular risk, insulin resistance, and lymphoma. With these associated morbidities, infection with HCV is not unexpectedly accompanied by an increase in mortality in the general population as well as in patients with kidney disease. Advances in the understanding of the HCV genome have resulted in the development of direct-acting antiviral agents that can achieve much higher sustained virologic response rates than previous interferon-based protocols. The direct acting antivirals have either primarily hepatic or renal metabolism and excretion pathways. This information is particularly relevant when considering treatment in patients with reduced kidney function. In this context, some of these agents are not recommended for use in patients with a glomerular filtration rate < 30 mL/min per 1.73 m2. There are now Food and Drug Administration approved direct acting antiviral agents for the treatment of patients with kidney disease and reduced function. These agents have been demonstrated to be effective with sustained viral response rates comparable to the general population with good safety profiles. A disease that was only recently considered to be very challenging to treat in patients with kidney dysfunction is now curable with these medications.
Core tip: Advances in the understanding of the molecular biology of hepatitis C virus (HCV) have ushered in a new era in treatment. Recent studies have shifted the focus to the more difficult-to-treat cohorts of patients. The presence of chronic kidney disease and end stage renal disease were exclusion criteria for the pivotal clinical direct-acting antiviral agents trials, creating a group of patients with a large unmet medical need. This review will update the reader on the use of the direct acting antiviral agents in the HCV-infected patient with kidney disease. Recommendations for the timing of therapy, choice of agents and management of the kidney transplant candidate will be presented.
- Citation: Ladino M, Pedraza F, Roth D. Opportunities for treatment of the hepatitis C virus-infected patient with chronic kidney disease. World J Hepatol 2017; 9(19): 833-839
- URL: https://www.wjgnet.com/1948-5182/full/v9/i19/833.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i19.833
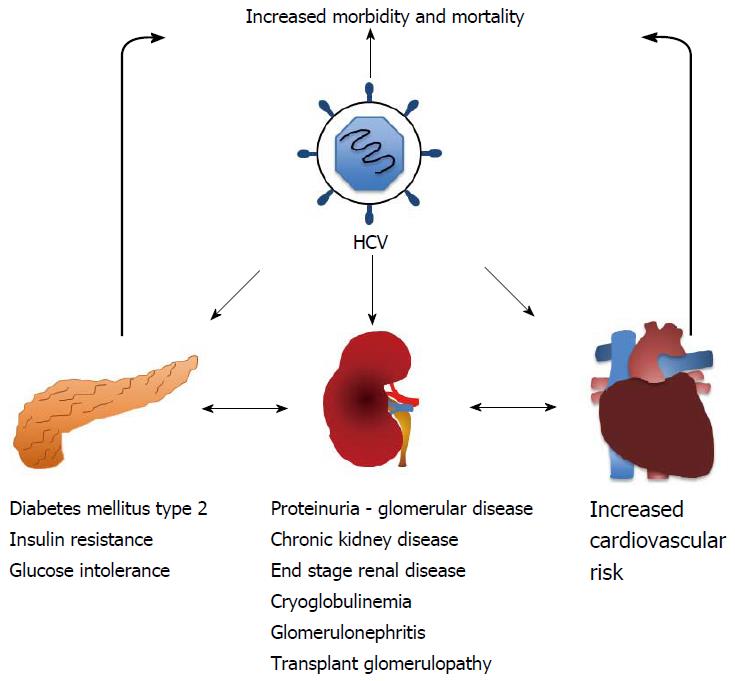
Hepatitis C virus (HCV) infection is a recognized public health concern with global implications that affects approximately 170 million individuals worldwide[1-4]. Infection with HCV is associated with an increased morbidity and mortality secondary to hepatic injury and associated complications[4]. The infection, however, can also affect other organs with significant extrahepatic manifestations (Figure 1). Most noteworthy of these include insulin resistance, cryoglobulinemic vasculitis, sicca syndrome, neurocognitive dysfunction, B-cell non-Hodgkin lymphoma and an increase in cardiovascular adverse events[5-11]. On note, patients with HCV infection also have an increased incidence of proteinuria and chronic kidney disease (CKD)[5], often in the setting of essential mixed cryoglobulinemia or “idiopathic” membranoproliferative glomerulonephritis[5,9,12]. Furthermore, it has also been well established that patients with end stage renal disease (ESRD) have an even higher prevalence of HCV infection that is likely a consequence of greater blood product exposure and patient-to-patient transmission of disease within the dialysis clinics due to breakdowns in universal precautions[12,13].
This review will summarize the most recent data and treatment options recommended for HCV-infected patients with kidney disease. A population of patients that for years had extremely limited options for therapy can now be successfully and safely treated for eradication of HCV.
The HCV has an unusual tropism for B lymphocytes through linkage of envelope protein 2 and the CD81 molecule on the B cell. B cell activation can result in expansion of malignant cell lines or the production of unique antibodies that are of the IgM isotype and possess rheumatoid factor like activity[14-16]. As a consequence of these events, clinical syndromes including mixed cryoglobulinemia, lymphoproliferative disorders and glomerulonephritis with distinct histological patterns including membranous or membranoproliferative glomerulonephritis can be seen[5,6,17,18]. Of note, co-infected HIV/HCV patients have an increased mortality and an overall worse prognosis[19,20].
The glomerular diseases commonly associated with HCV infection are a consequence of the formation of circulating immune complexes that become trapped in the glomerular basement membrane. The clinical expression of this process can occur through type 2 mixed cryoglobulinemia with resulting type 1 membranoproliferative glomerulonephritis (GN), mesangial proliferative and focal proliferative GN, IgA nephropathy, membranous GN and polyarteritis nodosa[6,14,18]. Typically, the patient that develops cryoglobulinemia has been infected with HCV for many years. These patients may present with a skin rash (palpable purpura), polyneuropathy, multi-organ vasculitis, hypertension and the nephritic syndrome[14].
Suppression of viral replication is necessary to interrupt immune-complex production and subsequent injury to the kidney. The VASCUVALDIC study described the use of sofosbuvir and ribavirin in 24 patients with HCV-vasculitis syndrome and cryoglobulinemia. Patients were treated with direct-acting antiviral agents (DAAs) for 24 wk and achieved a sustained viral response at week 12 (SVR12) of 74% with minimal side effects[21]. The less common presentation of an active vasculitic syndrome as part of the cryoglobulinemic syndrome requires a more aggressive treatment strategy targeted at the ongoing endothelial inflammatory process. Options include high dose corticosteroids, rituximab and therapeutic plasma exchange in addition to appropriate DAA therapy to eradicate viral replication[21-24].
HCV infection is highly prevalent in CKD patients[5] and HCV-infected patients have an increased risk for the development of CKD and proteinuria[5,25,26]. Furthermore, emerging data suggests that the rate of CKD progression to ESRD is greater when compared to non-infected patients[26-31]. In this context, HCV-infected patients with CKD stages I (GFR > 90 mL/min per 1.73 m2), II (GFR 60-89 mL/min per 1.73 m2) and IIIa (GFR 45-59 mL/min per 1.73 m2) should be considered for DAA therapy with the goal to slow the progression of CKD. HCV-infected patients with CKD stages IIIb (GFR 30-44 mL/min per 1.73 m2), IV (GFR 15-29 mL/min per 1.73 m2) and V (GFR < 15 mL/min per 1.73 m2) will require a more individualized approach depending on the renal replacement therapy options being considered. The major decision point in this context is whether treatment should be recommended before or after kidney transplantation. Patients with a living kidney donor should be treated to achieve a SVR prior to transplantation. For the patient that is going to receive a deceased donor kidney the options may include delaying antiviral treatment in order to receive a kidney from an anti-HCV positive donor with the initiation of DAA treatment post transplantation. Alternatively, the patient could be treated pre-transplant and then transplanted with a kidney from an anti-HCV negative donor. Since not all centers currently accept kidneys from anti-HCV positive donors, this option is not available for all patients. Initial reports have demonstrated that accepting a kidney from a positive donor is associated with substantially shortened waiting time on the deceased donor waiting list in the United States[32-34]. Recent studies have demonstrated the safety and efficacy of DAAs in the kidney transplant recipient, with sustained viral response rates equal to that obtained in the general population with minimal side effects[35-37].
It is estimated that 5%-10% of the United States dialysis population is infected with HCV[38]. Many studies have demonstrated that HCV infection is associated with an increased risk of mortality and worse clinical outcomes in ESRD patients[39-43]. In a meta-analysis of ESRD patients, Fabrizi et al[41] found that HCV infection was associated with a relative risk of mortality of 1.35 (95%CI: 1.25-1.47). The increased morbidity and mortality associated with HCV infection emphasizes the systemic impact of this disease which can manifest with multiple extrahepatic manifestations and complications[5,40]. In this context, an increased cardiovascular risk attributable to HCV infection has been demonstrated in the ESRD patient[40]. In a recent update from the Dialysis Options and Practice Patterns Study data, it was concluded that HCV infection in ESRD patients was associated with an increased risk of death and hospitalization, anemia and worse quality of life scores for physical function, pain, vitality and mental health[44]. Relevant to any discussion on the associated risks accompanying HCV infection is whether successful treatment delivers a positive impact on outcomes. In this context, Hsu et al[45] reported that IFN-based therapy increased survival in HCV-infected ESRD patients. In another report, ESRD patients receiving IFN plus ribavirin obtained improved renal and cardiovascular outcomes compared to those who were untreated[46]. Prospective studies in ESRD patients will be necessary to determine if viral eradication alters the long-term outcome of this challenging population of patients with multiple co-morbidities.
Kidney transplantation is associated with an increase in long-term survival for ESRD patients with HCV infection[47,48]. This was clearly demonstrated in a longitudinal cohort study in which there was a decreased risk of death post-transplantation for the HCV-infected kidney transplant recipients when compared to those remaining on the waiting list[49]. This survival benefit was largely the result of a decrease in cardiovascular events within the first-year post-transplant[50].
HCV infection has been linked to several extra-hepatic manifestations that combine to increase morbidity and mortality after kidney transplantation[51]. It has been well established that HCV is the primary cause of liver disease in kidney allograft recipients[52] and these patients express an increased risk of insulin resistance and diabetes mellitus[53-58]. Furthermore, HCV-infected kidney recipients have a higher probability of developing transplant glomerulopathy[59] and recurrent membranoproliferative glomerulonephritis secondary to immune-complex injury to the renal allograft[60,61].
The availability of DAAs with high SVR rates and favorable adverse event profiles allowed for the study of these drugs in patients with kidney disease, a group that had been excluded from of all the large pivotal trials. Emerging data are now demonstrating an excellent safety and efficacy profile in this patient population (Tables 1 and 2). The HCV-TARGET is a real-world study that collects data on the use of sofosbuvir-based regimens in HCV-infected patients. A total of 73 patients with a GFR ≤ 45 mL/min per 1.73 m2 (n = 18 with GFR ≤ 30 mL/min per 1.73 m2 and n = 5 on hemodialysis) were included in the analysis[62]. The SVR rate was 83% in patients with GFR ≤ 45 mL/min per 1.73 m2 which was similar to patients with GFR > mL/min per 1.73 m2, however patients with a GFR ≤ 45 mL/min per 1.73 m2 had higher rates of anemia, worsening kidney function and increased adverse events irrespective of the use of ribavirin[62]. Two open label treatment studies with simeprevir and dose-adjusted sofosbuvir exhibited high rates of SVR with a low incidence of adverse events in patients with advanced CKD and ESRD[63,64]. The RUBY-I trial evaluated the 3D regimen [ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) plus dasabuvir (DSV)] in patients with advanced CKD (stages 4/5) and on dialysis. SVR rates were 90% for patients with HCV genotype (GT) 1 with minimal side effects except for the patients with genotype 1a who received ribavirin as part of the protocol[65]. This group had more anemia events and required erythropoietin dose adjustments. Grazoprevir and elbasvir were studied in HCV-infected GT 1 patients with advanced CKD and ESRD in the C-SURFER trial. Sustained viral response rates of 99% were reported with a minimal adverse events profile[66]. The RUBY-I Cohort 2 study included patients with stage F4 fibrosis and GT 1a who were treated for 24 wk with the 3D regimen plus ribavirin. SVR24 rates of 89% were reported for this cohort with minimal side effects[67]. The RUBY-II study evaluated the use of the 3D regimen in CKD 4 and 5 patients with HCV GT 1a (n = 13) infection without the addition of ribavirin. Genotype 4 patients received OBV/PTV/r without DSV (n = 5). Modified intention to treat (mITT) SVR12 rates of 100% were obtained in both groups[68]. Finally, a recent report described the use of glecaprevir (NS3/4A inhibitor) and pibrentasvir (NS5A inhibitor) in patients with advance kidney disease and HCV genotype 1-6 infection (n = 104). In this trial, patients with a GFR < 30 mL/min per 1.73 m2 (n = 13 with GFR 15-29 mL/min per 1.73 m2, n = 6 with stage 5 CKD and n = 85 on hemodialysis) obtained a 98% ITT SVR12 with no serious adverse events[69] and no viral relapses.
| Medication dose | Use in CKD stage IV, V and ESRD | Use in kidney transplant patients - interactions with Immunosuppressant |
| Sofosbuvir/Simeprivir | CKD IV - GFR 15-29 mL/min: Not recommended | Decrease in TAC levels with Simeprivir |
| 400 mg daily/150 mg daily | CKD V - GFR < 15 mL/min: Not recommended | Increase levels of both CyA and Simeprivir |
| ESRD (dialysis): Not recommended | Increase or decrease levels of SRL with Simeprivir | |
| No changes in TAC, CyA and SRL with Sofosbuvir | ||
| Sofosbuvir/Velpatasvir | CKD IV - GFR 15-29 mL/min: Not recommended | Increase in TAC levels with Velpatasvir |
| 400 mg/100 mg daily | CKD V - GFR < 15 mL/min: Not recommended | No changes in CyA levels with Velpatasvir |
| ESRD (dialysis): Not recommended | Increase in SRL levels with Velpatasvir | |
| No changes in TAC, CyA and SRL with Sofosbuvir | ||
| Sofosbuvir/Daclastavir | CKD IV - GFR 15-29 mL/min: Not recommended | No changes in TAC levels with Daclastavir |
| 400 mg daily/60 mg daily | CKD V - GFR < 15 mL/min: Not recommended | No changes in CyA levels with Daclastavir |
| ESRD (dialysis): Not recommended | Increase in SRL levels with Daclastavir | |
| No changes in TAC, CyA and SRL with Sofosbuvir | ||
| Sofosbuvir/Ledipasvir | CKD IV - GFR 15-29 mL/min: Not recommended | No changes in TAC levels with Ledipasvir |
| 400 mg/90 mg daily | CKD V - GFR < 15 mL/min: Not recommended | No changes in CyA levels with Ledipasvir |
| ESRD (dialysis): Not recommended | No changes in SRL levels with Ledipasvir | |
| No changes in TAC, CyA and SRL with Sofosbuvir | ||
| Ombitasvir/Paritaprevir/ritonavir/Dasabuvir | CKD IV - GFR 15-29 mL/min: Dose adjustment not required | Increase in TAC levels (ritonavir) |
| 12.5 mg/75 mg/50 mg × 2 tabs/250 mg × 2 tabs | CKD V - GFR < 15 mL/min: Dose adjustment not required | Increase in CyA levels (ritonavir) |
| ESRD (dialysis): Dose adjustment not required. Dialysis population studied. Minimal adverse events in patients with advanced CKD and ESRD on hemodialysis | Increase in SRL levels (ritonavir) | |
| No changes in TAC, CyA and SRL with Ombitasvir/Paritaprevir/Dasabuvir | ||
| Grazoprevir/Elbasvir | CKD IV - GFR 15-29 mL/min: Dose adjustment not required | Increase in TAC levels with Grazoprevir |
| 100 mg/50 mg daily | CKD V - GFR < 15 mL/min: Dose adjustment not required | Use of both CyA and Grazoprevir increase levels of |
| ESRD (dialysis): Dose adjustment not required. Dialysis population studied. Minimal adverse events in patients with advanced CKD and ESRD on hemodialysis | Grazoprevir, contraindicated to use together | |
| Increase in SRL levels with Grazoprevir |
| HCV/kidney disease consideration | Complications and observations from HCV infection | DAA options | Other DAA options/notes |
| HCV related acute glomerulonephritis with or without cryoglobulinemia | HCV has tropism for B-cells with subsequent: Mixed cryoglobulinemia | Sofosbuvir 400 mg/d combined with | Can use: Grazoprevir 100 mg/elbasvir 50 mg/d |
| Simeprivir 150 mg/d | |||
| Daclastavir 60 mg/d | Ombitasvir 12.5 mg/paritaprevir 75 mg/ritonavir 50 mg × 2 tabs/dasabuvir 250 mg × 2 tabs | ||
| Glomerulonephritis with distinct histological patterns: Membranous nephropathy | Velpatasvir 100 mg/d | ||
| Ledipasvir 90 mg/d | |||
| Membranoproliferative GN | |||
| The HCV-infected patient with stage 1-3a chronic kidney disease (GFR > 45 mL/min) | Increased risk for CKD development | Sofosbuvir 400 mg/d combined with | Can use |
| Increased rate of CKD progression to ESRD | Simeprivir 150 mg/d | Grazoprevir 100 mg/elbasvir 50 mg/d | |
| Daclastavir 60 mg/d | |||
| Higher mortality rate | Velpatasvir 100 mg/d | Ombitasvir 12.5 mg/paritaprevir 75 mg/ritonavir 50 mg × 2 tabs/dasabuvir 250 mg × 2 tabs | |
| Ledipasvir 90 mg/d | |||
| The patient with advanced stage 3 and stage 4/5 chronic kidney disease (GFR < 45 mL/min) | Receiving an anti-HCV positive allograft decreases waiting times for a deceased donor kidney | Sofosbuvir 400 mg/d combined with | Sofosbuvir not recommended with GFR < 30 mL/min |
| Simeprivir 150 mg/d | |||
| Daclastavir 60 mg/d | Can use | ||
| Velpatasvir 100 mg/d | Grazoprevir 100 mg/Elbasvir 50 mg/d | ||
| Ledipasvir 90 mg/d | |||
| Ombitasvir 12.5 mg/Paritaprevir 75 mg/ritonavir 50 mg × 2 tabs/dasabuvir 250 mg × 2 tabs | |||
| The ESRD patient on dialysis | Increased risk of mortality and poor clinical outcomes in ESRD patients Increased cardiovascular risk | Grazoprevir 100 mg/Elbasvir 50 mg/d | Grazoprevir/elbasvir, ombitasvir/paritaprevir/ritonavir/dasabuvir, Dialysis population studied |
| Ombitasvir 12.5 mg/Paritaprevir 75 mg/ritonavir 50 mg × 2 tabs/dasabuvir 250 mg × 2 tabs | |||
| Minimal adverse events in patients with advanced CKD and ESRD on hemodialysis | |||
| The kidney transplant recipient with eGFR > 30 mL/min | DAA use after kidney transplant is safe and well tolerated with SVR > 97% | Sofosbuvir 400 mg/d combined with | Can use |
| Simeprivir 150 mg/d | Grazoprevir 100 mg/elbasvir 50 mg/d (caution with cyclosporin) | ||
| Daclastavir 60 mg/d | |||
| Velpatasvir 100 mg/d | Ombitasvir 12.5 mg/paritaprevir 75 mg/ritonavir 50 mg × 2 tabs/dasabuvir 250 mg × 2 tabs | ||
| Ledipasvir 90 mg/d |
IFN-based protocols have not been recommended after kidney transplantation due to an unacceptably high incidence of rejection events. In contrast, DAA use in kidney transplant recipients has been shown to be safe and effective with minimal side effects[34-37]. Caution to avoid drug-drug interactions related to different drug metabolism/interactions (Table 1) is necessary in addition to high vigilance to maintain therapeutic calcineurin inhibitor levels as HCV viremia is suppressed[34,37].
The availability of DAA agents has dramatically changed the way HCV-infected patients with CKD and ESRD can be managed. While providing outstanding results, these excellent outcomes raise new questions as to which patients should be treated and when is the best time to initiate therapy. Further studies will be necessary to answer these important questions.
| 1. | Bunchorntavakul C, Maneerattanaporn M, Chavalitdhamrong D. Management of patients with hepatitis C infection and renal disease. World J Hepatol. 2015;7:213-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Kwo PY, Agrawal S. HCV/HIV Coinfection: A New Treatment Paradigm. Gastroenterology. 2015;148:1470-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK. Hepatitis C in hemodialysis patients. World J Hepatol. 2015;7:548-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 5. | Cacoub P, Desbois AC, Isnard-Bagnis C, Rocatello D, Ferri C. Hepatitis C virus infection and chronic kidney disease: Time for reappraisal. J Hepatol. 2016;65:S82-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Jang JY, Chung RT. Chronic hepatitis C. Gut Liver. 2011;5:117-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46 Suppl 5:S165-S173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 9. | Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 577] [Article Influence: 17.5] [Reference Citation Analysis (6)] |
| 10. | Morales JM, Kamar N, Rostaing L. Hepatitis C and renal disease: epidemiology, diagnosis, pathogenesis and therapy. Contrib Nephrol. 2012;176:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Ferri C, Sebastiani M, Giuggioli D, Colaci M, Fallahi P, Piluso A, Antonelli A, Zignego AL. Hepatitis C virus syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World J Hepatol. 2015;7:327-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (5)] |
| 12. | Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008;49:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Pereira BJ, Milford EL, Kirkman RL, Levey AS. Transmission of hepatitis C virus by organ transplantation. N Engl J Med. 1991;325:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 268] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Kupin WL. Viral-Associated GN: Hepatitis C and HIV. Clin J Am Soc Nephrol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Mihăilă RG. Hepatitis C virus - associated B cell non-Hodgkin’s lymphoma. World J Gastroenterol. 2016;22:6214-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Rostaing L, Izopet J, Kamar N. Hepatitis C virus infection in nephrology patients. J Nephropathol. 2013;2:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. 1997;25:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Scherzer R, Shlipak MG. Risk factors: Individual assessment of CKD risk in HIV-positive patients. Nat Rev Nephrol. 2015;11:392-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Klein MB, Rollet-Kurhajec KC, Moodie EE, Yaphe S, Tyndall M, Walmsley S, Gill J, Martel-Laferriere V, Cooper C. Mortality in HIV-hepatitis C co-infected patients in Canada compared to the general Canadian population (2003-2013). AIDS. 2014;28:1957-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Saadoun D, Thibault V, Si Ahmed SN, Alric L, Mallet M, Guillaud C, Izzedine H, Plaisier A, Fontaine H, Costopoulos M. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann Rheum Dis. 2016;75:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Quartuccio L, Soardo G, Romano G, Zaja F, Scott CA, De Marchi G, Fabris M, Ferraccioli G, De Vita S. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford). 2006;45:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Pietrogrande M, De Vita S, Zignego AL, Pioltelli P, Sansonno D, Sollima S, Atzeni F, Saccardo F, Quartuccio L, Bruno S. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev. 2011;10:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Cacoub P, Delluc A, Saadoun D, Landau DA, Sene D. Anti-CD20 monoclonal antibody (rituximab) treatment for cryoglobulinemic vasculitis: where do we stand? Ann Rheum Dis. 2008;67:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Fabrizi F, Dixit V, Martin P, Messa P. The evidence-based epidemiology of HCV-associated kidney disease. Int J Artif Organs. 2012;35:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat. 2015;22:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, Hwang SJ. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol. 2013;14:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Fabrizi F, Messa P, Martin P. Recent advances on hepatitis C virus in dialysis population. Kidney Blood Press Res. 2014;39:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Ladino M, Pedraza F, Roth D. Hepatitis C Virus Infection in Chronic Kidney Disease. J Am Soc Nephrol. 2016;27:2238-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. N Engl J Med. 2015;373:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Sawinski D, Kaur N, Ajeti A, Trofe-Clark J, Lim M, Bleicher M, Goral S, Forde KA, Bloom RD. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant. 2016;16:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 36. | Lubetzky M, Chun S, Joelson A, Coco M, Kamal L, Ajaimy M, Gaglio P, Akalin E, Deboccardo G. Safety and Efficacy of Treatment of Hepatitis C in Kidney Transplant Recipients with Directly Acting Antiviral Agents. Transplantation. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, Del Bello A, Métivier S, Barange K. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Am J Transplant. 2016;16:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Ingsathit A, Kamanamool N, Thakkinstian A, Sumethkul V. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis. Transplantation. 2013;95:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P. Antiviral therapy of hepatitis C in chronic kidney diseases: meta-analysis of controlled clinical trials. J Viral Hepat. 2008;15:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Kopple JD, Greenland S. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, Hospitalization, and Quality of Life among Patients with Hepatitis C Infection on Hemodialysis. Clin J Am Soc Nephrol. 2017;12:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 45. | Hsu YH, Hung PH, Muo CH, Tsai WC, Hsu CC, Kao CH. Interferon-Based Treatment of Hepatitis C Virus Infection Reduces All-Cause Mortality in Patients With End-Stage Renal Disease: An 8-Year Nationwide Cohort Study in Taiwan. Medicine (Baltimore). 2015;94:e2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant. 2004;18:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Ruhı Ç, Süleymanlar İ, Koçak H, Yilmaz VT, Çolak D, Dınçkan A, Gürkan A, Ersoy F, Yakupoğlu G, Süleymanlar G. The impact of hepatitis C virus infection on long-term outcome in renal transplant patients. Turk J Gastroenterol. 2011;22:165-170. [PubMed] |
| 49. | Roth D, Gaynor JJ, Reddy KR, Ciancio G, Sageshima J, Kupin W, Guerra G, Chen L, Burke GW. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16:1545-1549. [PubMed] |
| 51. | Baid-Agrawal S, Pascual M, Moradpour D, Somasundaram R, Muche M. Hepatitis C virus infection and kidney transplantation in 2014: what’s new? Am J Transplant. 2014;14:2206-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18:97-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138:932-941.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] |
| 55. | Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, Baid S, Cosimi AB, Pascual M. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703-710. [PubMed] |
| 57. | Baid-Agrawal S, Frei U, Reinke P, Schindler R, Kopp MA, Martus P, Berg T, Juergensen JS, Anker SD, Doehner W. Impaired insulin sensitivity as an underlying mechanism linking hepatitis C and posttransplant diabetes mellitus in kidney recipients. Am J Transplant. 2009;9:2777-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Naing C, Mak JW, Wai N, Maung M. Diabetes and infections-hepatitis C: is there type 2 diabetes excess in hepatitis C infection? Curr Diab Rep. 2013;13:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Baid-Agrawal S, Farris AB, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, Collins AB, Frei U, Colvin RB. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int. 2011;80:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1:171-178. [PubMed] |
| 61. | Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, Carreno M, Esquenazi V, Miller J. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation. 1995;59:1676-1682. [PubMed] |
| 62. | Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, Nelson DR, Fried MW, Terrault NA. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 63. | Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, Roth D, Schiff E, O’Brien C, Martin P. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 64. | Sabucedo A, Antoine M, Jorge D, Andreu A, Pedraza F, Hernandez M, Jeffers L, Ladino M. Sofosbuvir use in patients with Hepatitis C virus infection and severe chronic kidney disease [Abstract]. J Am Soc Nephrol. 2015;26:663A. |
| 65. | Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, Bernstein DE, Cohen DE, Shulman NS, Wang D. Efficacy of Direct-Acting Antiviral Combination for Patients With Hepatitis C Virus Genotype 1 Infection and Severe Renal Impairment or End-Stage Renal Disease. Gastroenterology. 2016;150:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 66. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Martin P, Pol S, Londoño MC, Hassanein T. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 534] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 67. | Vierling JM. RUBY-I Study, cohort 2: ombitasvir/paritaprevir/ritonavir dasabuvir ± RBV for HCV genotype 1 with renal impairment. AASLD. 2016;Abs. 886 Available from: http://www.hcv-trials.com/showStudy.asp?Study=144. |
| 68. | Gane E. RUBY-II Study: ombitasvir/paritaprevir/ritonavir ± dasabuvir for HCV genotype 1a or 4 with severe renal impairment. AASLD. 2016;Abs. 935 Available from: http://www.hcv-trials.com/showStudy.asp?Study=139. |
| 69. | Gane E. EXPEDITION-IV Study: glecaprevir/pibrentasvir in patients with renal impairment. AASLD. 2016;Abs Available from: http://www.hcv-trials.com/showStudy.asp?Study=132. |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Grassi A, Irshad M, Komatsu H, Tsuchiya A, Zhu X S- Editor: Ji FF L- Editor: A E- Editor: Li D