Published online Jun 28, 2017. doi: 10.4254/wjh.v9.i18.823
Peer-review started: February 9, 2017
First decision: March 7, 2017
Revised: March 28, 2017
Accepted: April 18, 2017
Article in press: April 19, 2017
Published online: June 28, 2017
Processing time: 142 Days and 16.4 Hours
To investigate the additional clinical impact of hepatic ischaemia reperfusion injury (HIRI) on patients sustaining acute kidney injury (AKI) following liver transplantation.
This was a single-centre retrospective study of consecutive adult patients undergoing orthotopic liver transplantation (OLT) between January 2013 and June 2014. Early AKI was identified by measuring serum creatinine at 24 h post OLT (> 1.5 × baseline) or by the use of continuous veno-venous haemofiltration (CVVHF) during the early post-operative period. Patients with and without AKI were compared to identify risk factors associated with this complication. Peak serum aspartate aminotransferase (AST) within 24 h post-OLT was used as a surrogate marker for HIRI and severity was classified as minor (< 1000 IU/L), moderate (1000-5000 IU/L) or severe (> 5000 IU/L). The impact on time to extubation, intensive care length of stay, incidence of chronic renal failure and 90-d mortality were examined firstly for each of the two complications (AKI and HIRI) alone and then as a combined outcome.
Out of the 116 patients included in the study, 50% developed AKI, 24% required CVVHF and 70% sustained moderate or severe HIRI. Median peak AST levels were 1248 IU/L and 2059 IU/L in the No AKI and AKI groups respectively (P = 0.0003). Furthermore, peak serum AST was the only consistent predictor of AKI on multivariate analysis P = 0.02. AKI and HIRI were individually associated with a longer time to extubation, increased length of intensive care unit stay and reduced survival. However, the patients who sustained both AKI and moderate or severe HIRI had a longer median time to extubation (P < 0.001) and intensive care length of stay (P = 0.001) than those with either complication alone. Ninety-day survival in the group sustaining both AKI and moderate or severe HIRI was 89%, compared to 100% in the groups with either or neither complication (P = 0.049).
HIRI has an important role in the development of AKI post-OLT and has a negative impact on patient outcomes, especially when occurring alongside AKI.
Core tip: Acute kidney injury (AKI) is common after liver transplantation (LT), and has a significant impact on patient outcomes. It is multifactorial in aetiology and has been shown to correlate with the use of higher risk grafts, due to an increased risk of hepatic ischaemia reperfusion injury. In context of the growing use of marginal grafts to meet demands, this study has demonstrated that hepatic ischaemia reperfusion injury was the only variable that predicted early AKI post-LT and that the presence of both HIRI and AKI led to worse clinical outcomes and higher mortality than either complication alone.
- Citation: Rahman S, Davidson BR, Mallett SV. Early acute kidney injury after liver transplantation: Predisposing factors and clinical implications. World J Hepatol 2017; 9(18): 823-832
- URL: https://www.wjgnet.com/1948-5182/full/v9/i18/823.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i18.823
Acute kidney injury (AKI) developing immediately after orthotopic liver transplantation (OLT) is common, and is associated with increased morbidity, mortality and resource utilisation[1]. It affects between 25% and 60% of recipients[2-4], the variation being largely related to the definition of AKI utilised[5,6]. The incidence of AKI following OLT is much higher than with other non-cardiac major surgery, in patients with previously normal renal function[7]. This reflects the additional risks facing the liver transplant recipient during the intra and post-operative course.
Multiple factors predisposing liver transplant patients to post operative AKI have been identified. Pre-operatively, existing renal impairment, increasing Model for End stage Liver Disease Score, diabetes mellitus, hypertension[4] and obesity have all been associated with post OLT renal dysfunction. Intraoperative mean arterial pressure, vasopressor requirements, blood loss and transfusion of blood products are additional risk factors[8,9]. Post-operatively, graft dysfunction and immunosuppression therapy have been regarded as the main renal insults.
More recently, the growing demand for organs in LT has led to the use of increasingly higher risk grafts, in order to reduce the waiting list mortality[10-12]. These include grafts from older donors, prolonged preservation period, graft steatosis, split or partial liver allografts and donation after cardiac death (DCD). In the United Kingdom, DCD organs in particular have been increasingly used over the last decade, accounting for 20% of all liver transplants in 2014/15 (compared to 17% in 2012 and 5% in 2005)[13-15]. This group have been shown to be at a higher risk of both hepatic and extra-hepatic complications, including AKI, which is the most common complication encountered following the transplantation of marginal grafts. The post-operative systemic inflammatory response that occurs as a result of the hepatic ischaemia reperfusion injury (HIRI) following warm ischaemia at retrieval is thought to play a critical role in the pathogenesis of renal injury in these patients[16]. In keeping with this, peak peri-operative serum aspartate aminotransferase (AST), which is a surrogate marker for HIRI[17,18], has been found to be a significant variable related to renal outcomes[19-21].
The additional impact of HIRI on early post-operative renal dysfunction of liver transplant recipients has not been widely investigated. Previous studies, especially those based on large national databases do not usually have sufficient clinical information to analyse the predisposing factors. Early identification of those at risk, followed by prevention and management of HIRI may have important implications on the outcomes of these patients.
The aims of this study were firstly to identify the incidence, risk factors and clinical outcomes of early AKI in our cohort of patients, and secondly to investigate the incidence of HIRI, its correlation with AKI and its additional impact on patient outcomes.
Single-centre retrospective observational study of consecutive adults (≥ 18 years of age) undergoing OLT between January 2013 and June 2014 at the Royal Free London NHS Foundation Trust, one of the 8 Liver Transplant centres in the United Kingdom and Ireland. Exclusion criteria were those requiring urgent transplantation for acute liver failure and those receiving a combined liver-kidney transplant.
To analyse possible factors associated with post OLT AKI we included recipient age, gender, weight, aetiology of liver disease, Model for End-Stage Liver Disease (MELD) score, presence of anaemia (haemoglobin) and the presence of diabetes mellitus, hypertension and pre-existing renal dysfunction [serum creatinine > 100 μmol/L on the day of admission prior to OLT (baseline)]. Donor data was taken from a prospectively compiled database and included donor age, donor status [DCD or donation after brain death (DBD)] and cold ischaemic time.
Intraoperative factors assessed included surgical technique (piggy back or caval replacement), blood products transfused during OLT (packed red cell units, fresh frozen plasma and platelets), transfused cell salvage blood (as a reflection of blood loss) and noradrenaline infusion rate on admission to intensive care (as a reflection of possible ischaemia reperfusion injury).
Post-operative AKI was determined from serum creatinine at midnight on Day 1 and the need for continuous veno-venous haemofiltration (CVVHF) during the post-operative stay on intensive care unit (ICU). Peak serum AST within the first 24 h post-OLT was used as a measure of ischaemia reperfusion injury.
Clinical outcomes measured aside from the presence of AKI included time to extubation, intensive care length of stay, the incidence of chronic renal failure (CRF), as demonstrated by an estimated glomerular filtration rate of < 60 mL/min per 1.73 m2 at 6 mo post transplantation and 90-d patient survival.
Extent of AKI was assessed using the AKIN criteria[22], with the multiple rise in creatinine at 24 h post OLT compared to baseline, categorising the post-operative renal status into: No AKI (< 1.5-fold rise in creatinine); Stage 1 (1.5-2 fold rise); Stage 2 (2-3 fold rise) and Stage 3 (> 3 fold rise or the commencement of renal replacement therapy). This time frame was chosen to ensure that the renal complication was not due to post-operative factors such as nephrotoxicity secondary to immunosuppression.
The incidence of AKI at 24 h post OLT was determined and patients with no AKI vs those with any grade of AKI were compared in terms of baseline recipient characteristics, donor graft characteristics and intraoperative variables. The two groups were then compared with respect to time taken until extubation, intensive care length of stay (ICU LOS), incidence of CRF and 90 d patient survival. Risk factors for early AKI were then determined including the variables outlined above.
Incidence and severity of HIRI were determined using peak serum AST within 24 h of OLT, putting recipients into the groups: Mild HIRI (AST < 1000 IU/L); moderate HIRI (AST 1000-5000 IU/L) and severe HIRI (AST > 5000 IU/L). These groups were compared in terms of time taken until extubation, ICU LOS, incidence of CRF and 90 d patient survival. Peak AST levels within 24 h post OLT were correlated with the presence of early AKI and organ status.
Clinical outcomes (time taken until extubation, ICU LOS, CRF and 90 d patient survival) were then compared between those with neither AKI nor HIRI, either complication or both AKI and HIRI. In this context, HIRI was identified as those with moderate or severe HIRI.
Continuous parametric variables were expressed as means with SDs, and compared using student’s t test and ANOVA analysis of variance. Continuous non-parametric variables were expressed as medians with interquartile ranges, and compared using the Mann Whitney U test and Kruskal Wallis analysis of variance. Normality of data was confirmed using both Shapiro Wilk test and histogram analysis. Categorical variables were analysed using χ2 test or Fisher’s exact test and correlations between variables were analysed using Spearman’s or Pearson’s rank correlation for non-parametric and parametric data respectively. Kaplan Meier plots were used to analyse survival with log rank tests for differences and logistic regression analysis was performed to identify variables associated with AKI. A P value of < 0.05 was considered statistically significant unless otherwise stated. Statistical analysis was carried out using Microsoft Excel and IBM SPSS Statistics Version 24.
One hundred and forty OLTs were performed in adult recipients over the study period, using either the caval replacement technique or the piggyback technique, with or without a temporary porto-caval shunt. Veno-venous bypass was not used in any of these cases. Twenty patients underwent urgent transplantation, 3 received a combined liver-kidney transplant and 1 patient died intra-operatively. These patients were excluded from further analysis. The remaining 116 patients were then grouped according to the absence or presence of post-operative AKI and compared with regards to demographics, aetiology, severity of liver disease and relevant co-morbidity (Table 1). These groups were further compared in context of the donor graft and intraoperative characteristics (Tables 2 and 3 respectively).
| Variables | No AKI (n = 58) | Any grade of AKI (n = 58) | P value |
| Patient characteristics | |||
| Age (yr) | 54 (18) | 56 (6) | 0.197 |
| Female | 17 (29%) | 16 (28%) | 0.837 |
| Weight (kg) | 75 (13) | 79 (15) | 0.163 |
| Aetiology | |||
| Alcohol | 13 | 14 | |
| Viral hepatitis | 20 | 25 | |
| NASH | 2 | 7 | |
| Autoimmune | 16 | 7 | |
| Hepatocellular carcinoma | 10 | 13 | |
| Other | 7 | 6 | |
| MELD score | 16 (7) | 16 (5.75) | 0.421 |
| Hypertension | 8 (14%) | 9 (16%) | 0.733 |
| Diabetes mellitus | 12 (21%) | 20 (34%) | 0.074 |
| Pre-operative Haemoglobin (g/L) | 114 (20) | 107 (26) | 0.072 |
| Baseline creatinine | 76 (39) | 75 (26) | 0.932 |
| Variables | No AKI (n = 58) | Any AKI (n = 58) | P value |
| Graft characteristics | |||
| Donor age (yr) | 50 (14) | 47 (15) | 0.219 |
| Organ status | |||
| DCD (%) | 7 (12%) | 10 (17%) | 0.454 |
| Cold Ischaemic time (min) | 493 (133) | 493 (106) | 0.502 |
| Variable | No AKI (n = 58) | Any AKI (n = 58) | P value |
| Intraoperative variables | |||
| Surgical technique | |||
| Piggyback | 33 (57%) | 22 (38%) | 0.041 |
| Intraoperative blood products | |||
| RCC transfusion (units) | 1 (3) | 4 (5) | 0.001 |
| FFP transfusion (units) | 0 (2) | 2 (6) | 0.001 |
| Platelet transfusion (units) | 0 (1) | 0 (2) | 0.024 |
| Cell salvage (mL) | 281 (550) | 764 (929) | 0.001 |
| Noradrenaline infusion rate on arrival to ICU (μg/kg per minute) | 0.16 (0.10) | 0.18 (0.13) | 0.343 |
Out of the 116 patients included in the study, 58 (50%) developed early AKI post OLT using the AKIN criteria[22]. In those sustaining this post-operative complication, 19 were classified as stage 1, 7 as stage 2 and 32 as stage 3 acute kidney injuries. Twenty-eight/116 (24%) patients required CVVHF during the post-operative admission on ICU. The indication for commencement of renal replacement therapy was AKI in all these cases.
Ninety/116 (77.6%) patients had a baseline serum creatinine < 100 μmol/L and 26/116 (22.4%) had a baseline serum creatinine > 100 μmol/L. The incidence of AKI post OLT was 48/90 (53%) in the former group, compared to 10/26 (38%) in the latter group, this difference not being statistically significant (χ2 = 1.78, P = 0.182). However, a greater proportion of those with pre-existing renal impairment (baseline creatinine > 100 μmol/L) developed stage 3 AKI (35% vs 26%) P = 0.081 and required CVVHF (35% vs 21%) P = 0.156 during the post-operative admission to ICU, compared to the group with a normal baseline creatinine although again this was not statistically significant.
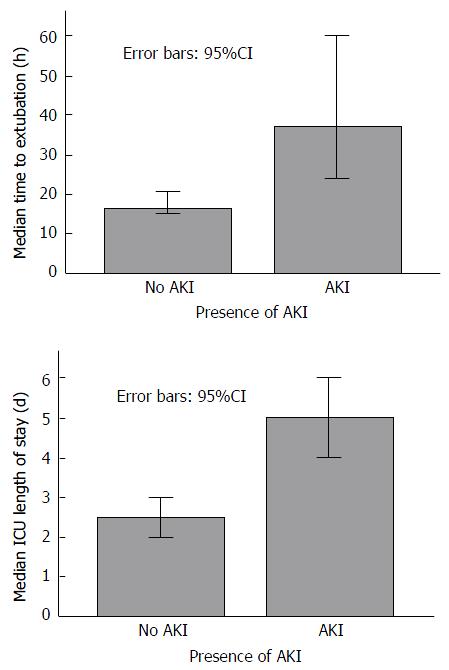
The median time to extubation was 37 h in the group sustaining AKI compared to 16 h in those without AKI (P < 0.0001) (Figure 1). Additionally, median intensive care length of stay was 5 d in the AKI group compared to 2.5 d in the no AKI group (P < 0.0001) (Figure 1). At the 6-mo follow-up period 39% of those with early post OLT AKI had developed CRF compared to 25% of those without AKI, although this did not reach statistical significance (P = 0.142).
Variables associated with AKI on regression analysis are described in Table 4. On univariate analysis surgical technique, transfusion of red cell concentrate, fresh frozen plasma, platelets and cell salvage blood and peak AST within 24 h after OLT were all associated with an increased risk of the development of AKI. In a multivariate model that included all clinically relevant variables, only peak AST within the first 24 h post OLT remained statistically significant in predicting early AKI (P = 0.020).
| Univariate analysis | Multivariate model | |||
| Variables | Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value |
| Male gender | 1.088 (0.49-2.44) | 0.837 | 0.430 (0.07-2.55) | 0.352 |
| Weight | 1.020 (0.99-1.05) | 0.164 | 1.026 (0.96-1.10) | 0.455 |
| Age | 1.034 (0.999-1.07) | 0.058 | 1.121 (1.02-1.23) | 0.019 |
| Pre-transplant | ||||
| Diabetes mellitus | 2.130 (0.92-4.92) | 0.077 | 2.429 (0.48-12.41) | 0.286 |
| Hypertension | 1.197 (0.43-3.36) | 0.733 | 0.593 (0.04-8.04) | 0.694 |
| Serum creatinine | 1.000 (0.99-1.01) | 0.937 | 0.979 (0.96-1.002) | 0.078 |
| MELD | 1.010 (0.97-1.05) | 0.639 | 0.992 (0.91-1.08) | 0.845 |
| Haemoglobin | 0.985 (0.97-1.002) | 0.076 | 0.977 (0.93-1.02) | 0.311 |
| Graft characteristics | ||||
| DCD organ | 1.488 (0.52-4.23) | 0.455 | 2.638 (0.30-23.12) | 0.381 |
| Donor age | 0.984 (0.96-1.01) | 0.218 | 0.964 (0.92-1.01) | 0.160 |
| Cold ischaemic time | 1.001 (0.99-1.004) | 0.498 | 1.000 (0.99-1.01) | 0.984 |
| Peak serum AST < 24 h post OLT | 1.001 (1.00-1.001) | 0.001 | 1.001 (1.00-1.001) | 0.020 |
| Perioperative course | ||||
| Caval replacement surgical technique | 2.160 (1.03-4.54) | 0.042 | 3.289 (0.71-15-25) | 0.128 |
| Noradrenaline infusion rate on ICU arrival | 4.830 (0.19-125.48) | 0.343 | 47.468 (0.12-1836.27) | 0.204 |
| RCC transfusion | 1.137 (1.03-1.26) | 0.014 | 0.917 (0.64-1.32) | 0.645 |
| FFP transfusion | 1.201 (1.07-1.35) | 0.003 | 1.118 (0.84-1.48) | 0.440 |
| Platelet transfusion | 1.499 (1.04-2.16) | 0.030 | 1.948 (0.871-4.36) | 0.104 |
| Cell salvage volume | 1.001 (1.00-1.001) | 0.025 | 1.00 (0.999-1.002) | 0.808 |
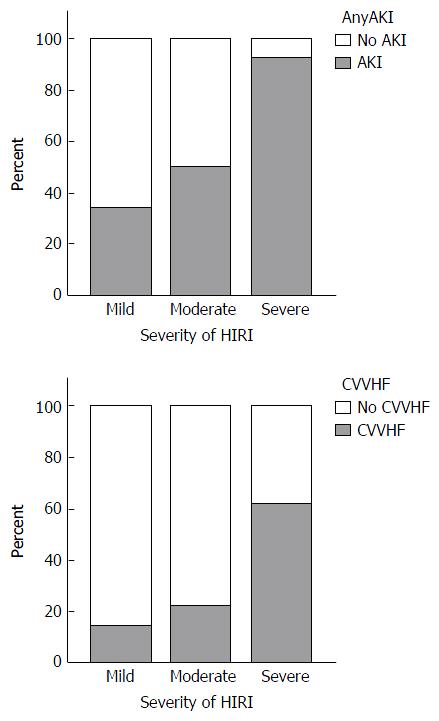
To assess the severity of HIRI, patients were divided into groups based on peak AST levels within the first 24 h after OLT: AST < 1000 IU/L (minor HIRI); AST 1000-5000 IU/L (moderate HIRI); AST > 5000 IU/L (severe HIRI)[23]. Thirty-five/116 (30%) of patients developed mild HIRI, 68/116 (59%) developed moderate HIRI and 13/116 (11%) developed severe HIRI. The effect of organ status and surgical technique on HIRI severity and the renal implications of increasing ischaemia reperfusion injury are summarised in Table 5.
| Severity of HIRI | ||||
| Mild (n = 35) | Moderate (n = 68) | Severe (n = 13) | P value | |
| DCD organs | 1/35 (2.9) | 11/68 (16.2) | 5/13 (38.5) | 0.007 |
| Caval replacement | 16/35 (45.7) | 36/68 (52.9) | 9/13 (69.2) | 0.348 |
| Incidence of AKI | 12/35 (34.3) | 34/68 (50.0) | 12/13 (92.3) | 0.002 |
| Need for CVVHF | 5/35 (14.3) | 15/68 (22.1) | 8/13 (61.5) | 0.003 |
| Development of CRF | 6/30 (20.0) | 19/53 (35.8) | 5/11 (45.5) | 0.195 |
Median peak AST levels within the first 24 h post OLT were 1248 IU/L and 2059 IU/L in the No AKI and AKI groups respectively (P = 0.0003). Furthermore, increasing levels of peak AST correlated well with increasing severity of AKI (Spearman’s r = 0.334, P = 0.0003). Finally, increasing severity of HIRI was associated with both a higher incidence of AKI (P = 0.002) and more frequent use of CVVHF (P = 0.003) (Figure 2).
Median peak AST levels within the first post-operative day were 1307 IU/L and 2060 IU/L in those who underwent DBD and DCD transplantation respectively (P = 0.001). A spearman’s rank-order correlation was run to examine the relationship between organ status and HIRI, which revealed a positive correlation between the two (spearman’s r = 0.322, P = 0.0005). Five thirteenths (38.5%) of those with severe HIRI had received a DCD graft, compared to only 1/35 (3%) of those with mild HIRI, P = 0.007 (Table 5).
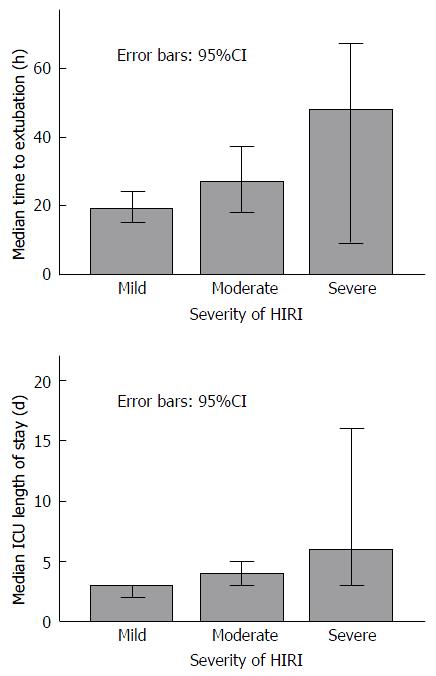
Increasing severity of HIRI was associated with a trend towards longer median time to extubation (Figure 3) P = 0.07, longer median ICU length of stay (Figure 3) P = 0.01, and a trend towards a higher incidence of CRF at 6 mo (Table 5) P = 0.195.
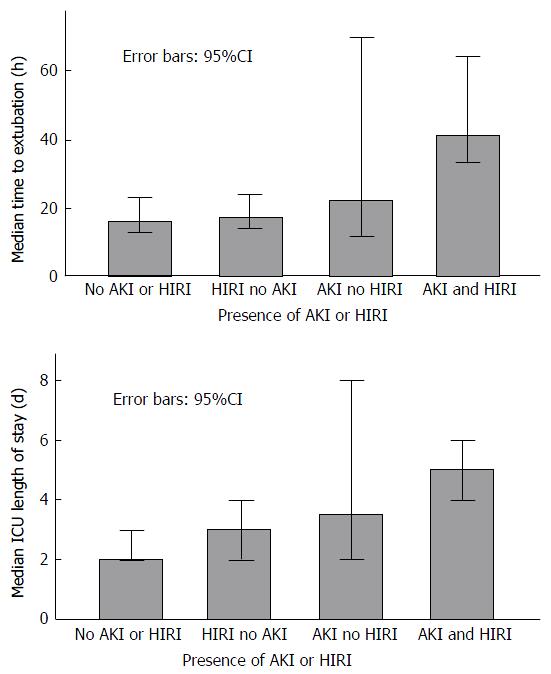
To examine the clinical impact of having both the complications of AKI and HIRI, the cohort was divided into 4 groups: Those with neither AKI nor HIRI (group 1); HIRI but no AKI (group 2); AKI but no HIRI (group 3) and those with both complications (group 4). The presence of HIRI included any patient that sustained either moderate or severe HIRI (peak AST within 24 h post OLT > 1000 IU/L). These groups were then compared for median time to extubation, median ICU length of stay and the incidence of CRF.
The median time to extubation (hours) differed between the groups (P < 0.001) with the lowest time observed in group 1 and the highest observed in group 4 (Figure 4). Pairwise comparisons revealed statistically significant differences between groups 1 and 4 (P = 0.001) and groups 2 and 4 (P = 0.003).
Similarly, the median ICU length of stay (days) increased between the groups (P = 0.001), with the highest value observed in the patients sustaining both AKI and HIRI (Figure 4). Pairwise comparisons revealed statistically significant differences between groups 1 and 3 (P = 0.04), groups 1 and 4 (P < 0.0001) and groups 2 and 4 (P = 0.005). Finally, there was a trend towards a higher incidence of CRF in those with any one of, or both AKI and HIRI compared to those with neither, with an incidence of only 15% in group 1 and 45% in group 4 (P = 0.238).
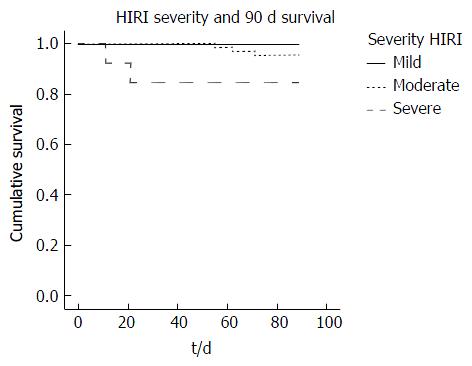
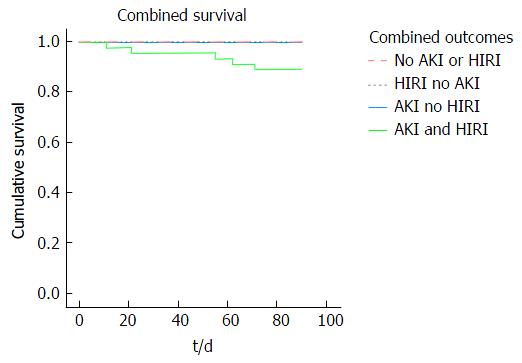
Kaplan Meier analysis revealed a reduction in 90-d patient survival associated with the presence of early AKI compared to no AKI (91.4% vs 100% respectively, P = 0.024) and increasing severity of HIRI (severe 84.6%; moderate 95.5%; mild 100.0%, P = 0.053) (Figure 5). Furthermore, early AKI and moderate/severe HIRI occurring in combination had a greater impact on 90-d patient survival than either complication when occurring in isolation (89% vs 100% respectively, P = 0.049) (Figure 6).
This single centre study has allowed detailed analysis of factors influencing post-operative AKI in United Kingdom patients undergoing LT, allowing strategies for intervention to be designed. In particular it has investigated the impact of HIRI, which is becoming more prevalent in an era that has seen a steady rise in the use of marginal grafts. The aetiology of AKI following OLT is complex and multifactorial, so our study has benefitted from the analysis of details that are not collected in national databases.
The importance of AKI and HIRI to the outcome of patients undergoing OLT is emphasised by major differences being demonstrated in this small single centre study in important patient centred outcomes. Patients who sustained both AKI and HIRI had a longer time to extubation, longer ICU length of stay and a lower 90-d patient survival. Furthermore, in a multivariate model of all clinically relevant variables, HIRI was shown to be the single most important factor predicting post-operative AKI, suggesting that it plays a critical role in the pathogenesis of renal dysfunction after LT.
The association between HIRI and AKI has previously been reported, with Leithead et al[16] demonstrating peak postoperative AST as the main predictor of renal dysfunction after DCD transplantation. Renal outcomes were examined for those undergoing DCD transplantation, but not specifically correlated with the degree of HIRI. Glanemann et al[23] examined the clinical implications of increasing severity of hepatic preservation injury, and found it to be associated with initial graft non-function and, as in the current study, to be correlated with an increased duration of post-operative ventilation and haemodialysis. However Glanemann et al[23] did not define the cohort that developed AKI. For the first time we have shown that the combination of early AKI and moderate to severe HIRI leads to worse post OLT outcomes than either complication alone.
The aetiology of AKI following LT is thought to be multifactorial, and contributory causes include exposure to high levels of toxic free radicals, renal ischaemia, use of nephrotoxic medications and the effects of end stage liver disease on the kidneys. Perioperative risk factors for the development of AKI post OLT have included pre-existing renal dysfunction, diabetes mellitus, hypertension, previous ascites, MELD score, surgical technique, intraoperative transfusion of blood products, ischaemia time, post-reperfusion syndrome and post OLT immunosuppression[24-26]. In our study, peak serum AST within 24 h of OLT, surgical technique and transfusion of blood products were all statistically significant in univariate analysis in being predictors of early AKI. However only peak serum AST within 24 h of OLT remained so in the multivariate model.
Previously it has been shown that DCD transplantation is associated with post-operative renal dysfunction[16]. This would be expected as the use of DCD grafts is associated with increased warm ischaemia, incidence of poor and non function of the graft and patient and graft mortality[27]. In our study we were not able to reproduce these results. This perhaps was secondary to the fact that only 15% of our cohort received DCD grafts, or that to compensate for the use of DCD organs the donors may have been younger, had lower cold ischaemia times or were transplanted into younger, fitter patients.
It has been reported that pre-operative renal dysfunction is an independent predictor of post-OLT AKI and the need for CVVHF[28,29]. However, in our study, pre-operative serum creatinine was not a predictor for early post-OLT AKI in logistic regression analysis. In fact, a greater proportion of those with a pre-operative creatinine < 100 μmol/L developed early AKI (53%) than those with a pre-operative creatinine > 100 μmol/L (38%). One possibility to explain this may have been that better quality grafts with a lower donor risk index were matched to the higher risk recipients.
Interestingly though, in those with pre transplant CRF who did develop AKI it was likely to be severe (stage 3 AKI) and require CVVHF suggesting a predisposition to an increased severity of the complication in the setting of pre-operative dysfunction. The difference in outcome of our logistic regression analysis may reflect the discrepancies in the definitions used to categorise AKI. For example, Cabezuelo et al[30] categorised post-op AKI as an increase in pre-operative serum creatinine > 50% (compared to our definition of > 150%). In addition, their team defined pre-operative acute renal impairment as an increase in creatinine > 50% from baseline, compared to our pre-operative renal function being defined by the serum creatinine on the day of transplantation alone.
The main limitation of this study lies in its retrospective nature and the inability to control for factors with inter-individual variability, such as the indications and timing in use of CVVHF, which remained reliant on the judgment of the clinician. Also, the variable definitions of AKI mean that interpretation of results needs to be considered in context of the methodologies used.
The frequency of AKI has increased in recent years, and this increase has occurred in parallel with a marked increase in the use of high-risk grafts. In the United Kingdom 29% of donors are over 60 years of age, over 20% are DCD, and clinically obese donors have doubled in the last 10 years. It is known that grafts from these extended criteria donors are more prone to HIRI and poor outcome[31]. HIRI is often more severe when implanting steatotic organs, and it has been reported that the incidence of AKI is significantly higher in patients receiving grafts from donors with a high BMI, although long term survival was not significantly different when corrected for other variables, such as diabetes[32]. The accelerated search in recent years for methods to expand the organ donor pool has lead to the increasing use of higher risk grafts. This trend in activity has important implications on the recipient population in terms of increased morbidity and mortality post OLT, however, as extended criteria donor grafts are usually allocated to patients with lower MELD scores, this may impact on increased hospital stay, complications and costs but not necessarily poorer graft or patient survival figures.
The role of graft injury and HIRI injury in the pathogenesis of AKI is being increasingly recognised[16,30]. It is one of the most important causes of organ dysfunction, and is a major determinant of successful LT. The deleterious effects are not limited to the liver, but are seen in other organs, including the lungs and kidney[33]. IRI can trigger a systemic inflammatory response and subsequent multi-organ failure, the injury being characterised by intravascular oxidative stress and functional impairment of the mitochondria[34,35]. Peak serum AST is a surrogate marker of the severity of HIRI, and is closely correlated with the development of AKI, as confirmed in this study. Low values of AST following transplantation are associated with superior outcomes[36], and a reduction in AST levels have been used as a primary end point for liver IRI studies in animal models. Preliminary results from a proof of concept study of normothermic machine perfusion compared to a standard cold preservation demonstrated a marked reduction in peak AST levels (417 IU/L vs 902 IU/L respectively), indicating that this method of preservation, by “reconditioning” the graft, may reduce HIRI and its attendant consequences, including AKI[37].
In summary, our study has shown that renal dysfunction and use of CVVHF after OLT is common, and rises in proportion to the level of hepatic-ischaemia-reperfusion-injury (as determined by AST levels) and its coexisting systemic inflammatory response. Further work should focus on novel therapies that prevent and treat this graft-related injury to improve recipient outcomes and broaden the donor pool with more extended criteria grafts.
The authors would like to thank Dr. Ciara I Donohue for her assistance in data acquisition.
Acute kidney injury (AKI) is a common complication following liver transplantation (LT) and has significant clinical implications on patient outcomes. In recent years, the growing demand for organs in transplantation has prompted a search for methods to expand the donor pool, which has included the consideration of use of higher risk grafts, including those from donation after cardiac death donors. This has conferred an increased risk of hepatic ischaemia reperfusion injury (HIRI) to the recipients, of which one of the consequences is AKI.
It is not fully clear what additional extra-hepatic clinical impact the use of these higher risk grafts have on the recipient in LT. A few reports have addressed the association between HIRI and renal dysfunction post LT but none have explored the clinical impact of having both HIRI and AKI as a combined outcome.
In this study, the authors have shown for the first time that not only do early AKI and moderate to severe HIRI as individual complications, lead to poorer patient outcomes, but combined have a worse impact on time to extubation, intensive care unit length of stay and 90-d survival when compared to each complication alone.
This study has highlighted the adverse extra-hepatic consequences of HIRI and the subsequent need to develop novel therapies that prevent and treat this graft-related injury to improve recipient outcomes and broaden the donor pool with more extended criteria grafts.
AKI: Acute kidney injury, as defined by the AKIN criteria (multiple rise in creatinine from baseline or the need for renal replacement therapy); HIRI: Hepatic ischaemia reperfusion injury; A graft related injury causing a systemic inflammatory response that has, in this study been categorised according to peak serum aspartate aminotransferase levels on day one post LT.
The study was conducted well in terms of identifying the predictors for and impact of HIRI on early AKIs.
| 1. | Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2401] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 2. | Charlton MR, Wall WJ, Ojo AO, Ginès P, Textor S, Shihab FS, Marotta P, Cantarovich M, Eason JD, Wiesner RH. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 3. | O’Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. 2007;7:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Lebrón Gallardo M, Herrera Gutierrez ME, Seller Pérez G, Curiel Balsera E, Fernández Ortega JF, Quesada García G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004;10:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003;14:2178-2187. [PubMed] |
| 6. | Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 402] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 7. | Abelha FJ, Botelho M, Fernandes V, Barros H. Determinants of postoperative acute kidney injury. Crit Care. 2009;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Chen J, Singhapricha T, Hu KQ, Hong JC, Steadman RH, Busuttil RW, Xia VW. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011;91:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Karapanagiotou A, Dimitriadis C, Papadopoulos S, Kydona C, Kefsenidis S, Papanikolaou V, Gritsi-Gerogianni N. Comparison of RIFLE and AKIN criteria in the evaluation of the frequency of acute kidney injury in post-liver transplantation patients. Transplant Proc. 2014;46:3222-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1526] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 12. | Dudek K, Kornasiewicz O, Remiszewski P, Zieniewicz K, Wróblewski T, Krawczyk M. Results of liver transplantation from old donors. Transplant Proc. 2014;46:2762-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Johnson RJ, Bradbury LL, Martin K, Neuberger J. Organ donation and transplantation in the UK-the last decade: a report from the UK national transplant registry. Transplantation. 2014;97 Suppl 1:S1-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Neuberger J. Liver transplantation in the United Kingdom. Liver Transpl. 2016;22:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | NHS. Interim report on liver transplantation (2015) - ODT Clinical. 2015; Available from: http://www.odt.nhs.uk/pdf/interim_liver_report.pdf. |
| 16. | Leithead JA, Tariciotti L, Gunson B, Holt A, Isaac J, Mirza DF, Bramhall S, Ferguson JW, Muiesan P. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am J Transplant. 2012;12:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Shaked A, Nunes FA, Olthoff KM, Lucey MR. Assessment of liver function: pre- and peritransplant evaluation. Clin Chem. 1997;43:1539-1545. [PubMed] |
| 18. | Gaffey MJ, Boyd JC, Traweek ST, Ali MA, Rezeig M, Caldwell SH, Iezzoni JC, McCullough C, Stevenson WC, Khuroo S. Predictive value of intraoperative biopsies and liver function tests for preservation injury in orthotopic liver transplantation. Hepatology. 1997;25:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 20. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 21. | Robertson FP, Bessell PR, Diaz-Nieto R, Thomas N, Rolando N, Fuller B, Davidson BR. High serum Aspartate transaminase levels on day 3 postliver transplantation correlates with graft and patient survival and would be a valid surrogate for outcome in liver transplantation clinical trials. Transpl Int. 2016;29:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 5067] [Article Influence: 266.7] [Reference Citation Analysis (0)] |
| 23. | Glanemann M, Langrehr JM, Stange BJ, Neumann U, Settmacher U, Steinmüller T, Neuhaus P. Clinical implications of hepatic preservation injury after adult liver transplantation. Am J Transplant. 2003;3:1003-1009. [PubMed] |
| 24. | Aksu Erdost H, Ozkardesler S, Ocmen E, Avkan-Oguz V, Akan M, Iyilikci L, Unek T, Ozbilgin M, Meseri Dalak R, Astarcioglu I. Acute Renal Injury Evaluation After Liver Transplantation: With RIFLE Criteria. Transplant Proc. 2015;47:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Hilmi IA, Damian D, Al-Khafaji A, Planinsic R, Boucek C, Sakai T, Chang CC, Kellum JA. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Yalavarthy R, Edelstein CL, Teitelbaum I. Acute renal failure and chronic kidney disease following liver transplantation. Hemodial Int. 2007;11 Suppl 3:S7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Laing RW, Scalera I, Isaac J, Mergental H, Mirza DF, Hodson J, Wilkin RJ, Perera MT, Muiesan P. Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant. 2016;16:1795-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Contreras G, Garces G, Quartin AA, Cely C, LaGatta MA, Barreto GA, Roth D, Gomez E. An epidemiologic study of early renal replacement therapy after orthotopic liver transplantation. J Am Soc Nephrol. 2002;13:228-233. [PubMed] |
| 29. | Lafayette RA, Paré G, Schmid CH, King AJ, Rohrer RJ, Nasraway SA. Pretransplant renal dysfunction predicts poorer outcome in liver transplantation. Clin Nephrol. 1997;48:159-164. [PubMed] |
| 30. | Cabezuelo JB, Ramírez P, Ríos A, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Bueno FS. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Andert A, Becker N, Ulmer F, Schöning W, Hein M, Rimek A, Neumann U, Schmeding M. Liver Transplantation and Donor Body Mass Index & gt; 30: Use or Refuse? Ann Transplant. 2016;21:185-193. [PubMed] |
| 33. | Fernández L, Heredia N, Peralta C, Xaus C, Roselló-Catafau J, Rimola A, Marco A, Serafín A, Deulofeu R, Gelpí E. Role of ischemic preconditioning and the portosystemic shunt in the prevention of liver and lung damage after rat liver transplantation. Transplantation. 2003;76:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Ramsay M. The reperfusion syndrome: have we made any progress? Liver Transpl. 2008;14:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Grattagliano I, Vendemiale G, Lauterburg BH. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J Surg Res. 1999;86:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Eisenbach C, Encke J, Merle U, Gotthardt D, Weiss KH, Schneider L, Latanowicz S, Spiegel M, Engelmann G, Stremmel W. An early increase in gamma glutamyltranspeptidase and low aspartate aminotransferase peak values are associated with superior outcomes after orthotopic liver transplantation. Transplant Proc. 2009;41:1727-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (1)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Akamatsu N, Yu JS, Zhu X S- Editor: Ji FF L- Editor: A E- Editor: Li D