Published online Jun 8, 2017. doi: 10.4254/wjh.v9.i16.746
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 19, 2017
Accepted: May 12, 2017
Article in press: May 15, 2017
Published online: June 8, 2017
Processing time: 126 Days and 13.7 Hours
To investigate the prevalence of osteopenia and osteoporosis in postoperative biliary atresia (BA) children and the association of bone mineral density (BMD) and biochemical parameters in postKasai BA subjects.
A total of 70 patients with postKasai BA were enrolled in this prospective study. The patients were classified into two groups according to their jaundice status. BMD of the lumbar spine was analyzed using dual energy X-ray absorptiometry.
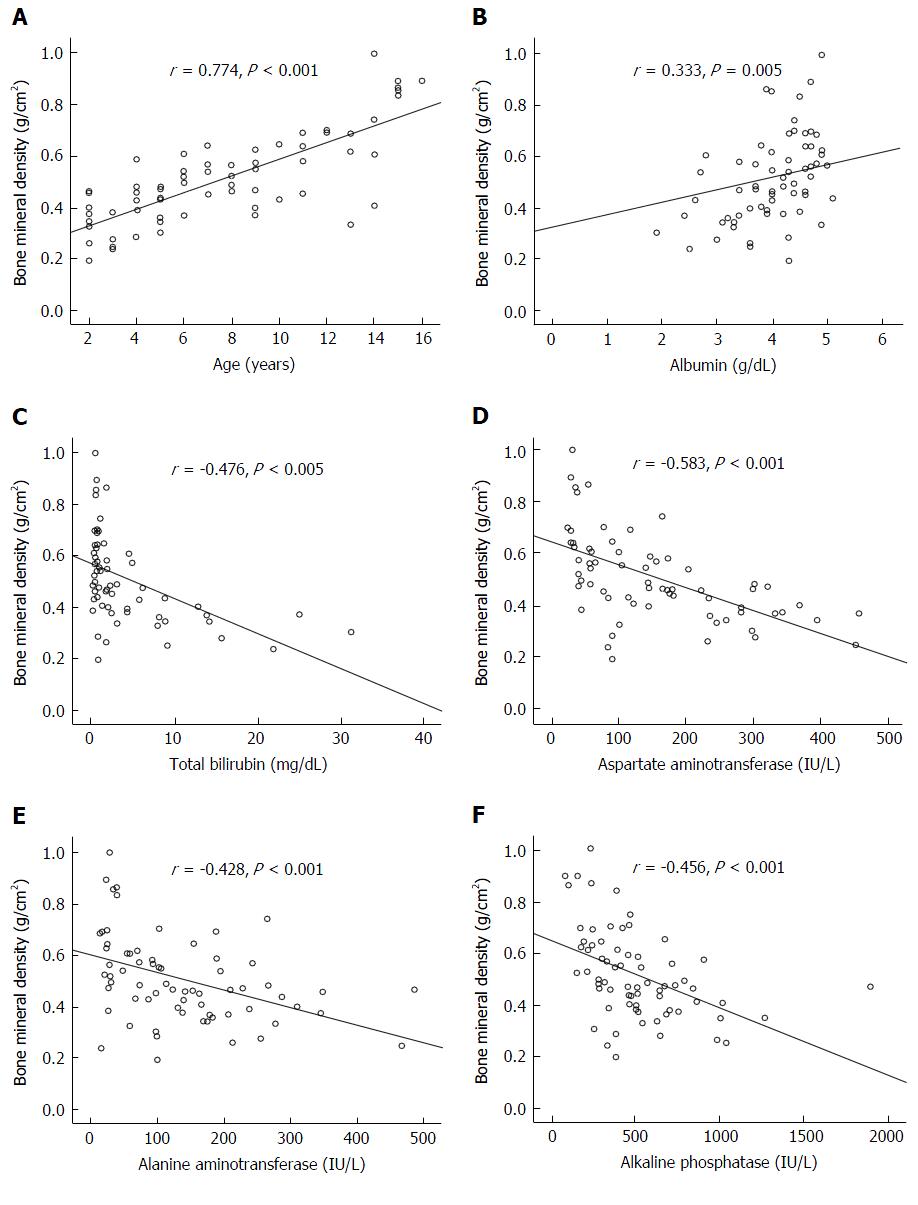
The prevalence of low bone mass (osteopenia and osteoporosis) in BA patients were 51.4% (36 out of 70). Ten patients (35.7%) in the jaundice group and 8 patients (19.0%) in the non-jaundice group had osteopenia. Sixteen patients (57.1%) in the jaundice group and 2 patients (4.8%) in the no jaundice group had osteoporosis. In addition, lumbar spine BMD Z-score was substantially lower in the jaundice BA patients compared with non-jaundice patients. BA subjects with persistent jaundice had significantly lower serum 25-hydroxyvitamin D than those without jaundice. Further analysis revealed that lumbar spine BMD was correlated with age (r = 0.774, P < 0.001), serum albumin (r = 0.333, P = 0.005), total bilirubin (r = -0.476, P < 0.001), aspartate aminotransferase (r = -0.583, P < 0.001), alanine aminotransferase (r = -0.428, P < 0.001), and alkaline phosphatase(r = -0.456, P < 0.001).
Low BMD was associated with biochemical parameters reflecting the severity of cholestasis in postKasai BA patients.
Core tip: Recent evidences have highlighted the importance of bone mineral density (BMD) in chronic liver disease including biliary atresia (BA). This study revealed that BA patients with persistent jaundice had significantly lower BMD and 25-hydroxyvitamin D than those without jaundice. Furthermore, lumbar spine BMD was correlated with hepatic dysfunction suggesting that low BMD was associated with outcome parameters reflecting the severity of cholestasis in postoperative BA patients.
- Citation: Homchan K, Chaiwatanarat T, Udomsinprasert W, Chongsrisawat V, Poovorawan Y, Honsawek S. Low bone mineral density and the severity of cholestasis in biliary atresia. World J Hepatol 2017; 9(16): 746-751
- URL: https://www.wjgnet.com/1948-5182/full/v9/i16/746.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i16.746
Biliary atresia (BA) is a progressive, idiopathic, necroinflammatory process resulting in obliteration of the extrahepatic biliary tree resulting in intrahepatic cholestasis, hepatic fibrosis, biliary cirrhosis, and advanced chronic liver failure[1]. It is a rare disease, with the reported prevalence ranging from 1 in 5000 to 1 in 19000 live births[2]. It is the most common cause of neonatal jaundice for which surgery is indicated and also the most common indication for liver transplantation in children. The pathogenesis of BA has remained a mystery. Most of the causal theories include defects resulting from a viral infection or toxin exposure, defects in morphogenesis, genetic predisposition, defects in prenatal circulation and immune dysregulation[3-5].
Low bone mass is frequent in patients with chronic liver disorder including BA. Metabolic bone disease is a common disorder that can be found in patients with hepatic osteodystrophy, particularly those affected by chronic cholestasis[6,7]. Its etiology is complex and multifactorial and presents as osteopenia and osteoporosis which should be investigated and diagnosed early in patients with chronic liver disease in order to minimize the risk of fractures and improve their quality of life[8,9]. The purpose of this study was to determine bone mineral density (BMD) from postKasai BA children and to investigate the association of BMD and outcome parameters in postoperative BA patients.
This investigation was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University and was conducted in compliance with the Declaration of Helsinki. All parents of BA children were informed of the study’s objectives, and written informed consent was derived from the parents prior to the participants entering the study.
A total of 70 postKasai BA subjects (30 males and 40 females; mean age 7.6 ± 0.5 years) who attended the follow-up visit in Pediatric Liver Clinic at King Chulalongkorn Memorial Hospital were recruited in the present study. Among the 70 BA children in this study, none of them had any evidence of residual infection or ascending cholangitis or clotting abnormalities during venipuncture. None had experienced liver transplantation. To compare the clinical outcomes among BA subjects, they were allocated into two groups corresponding to their levels of serum total bilirubin (TB): Non-jaundiced group (TB < 2.0 mg/dL, n = 42) and persistently jaundiced group (TB ≥ 2.0 mg/dL, n = 28).
Venous blood specimens were procured from each subject, centrifuged, and then kept at -80 °C until measurement. Liver function tests including TB, direct bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were assessed using Hitachi 912 automated chemical analyzer at the central laboratory of our hospital. Serum 25-hydroxyvitamin D [25(OH)D] levels were analyzed using automated chemiluminescent immunoassay (Diasorin, Saluggia, Italy).
Dual-energy X-ray absorptiometry scans (Hologic QDR 2000, Hologic Inc., Waltham, MA, United States) were performed on the lumbar spine (anteroposterior lumbar vertebrae L1-L4) of every subject for BMD assessments. BMD was reported as grams of mineral per square centimeter (g/cm2) and Z-scores. Z-scores of BMD were expressed as numbers of standard deviations from the mean BMD of age matched norms. Children were categorized into normal, osteopenia, and osteoporosis based on World Health Organization (WHO) criteria. Osteoporosis was designated as a lumbar spine BMD equal to or exceeding 2.5 standard deviations (SD) below the average values (Z score ≤ -2.5). Osteopenia was designated as a lumbar spine BMD below 2.5 SD but above 1 SD under the average values (-2.5 < Z score < -1.0). Normal BMD was designated as a lumbar spine BMD equal to or below 1 SD under the average values (Z score ≥ -1.0).
Statistical analysis was performed using the statistical package for social sciences software, version 22.0 for Windows. All values are expressed as a mean ± standard error. Demographic and clinical data between groups were compared by χ2 tests and unpaired Student’s t tests, where appropriate. Comparisons of clinical data and biochemical markers among patients with normal, osteopenia, and osteoporosis were analyzed using one-way analysis of variance (ANOVA) with Tukey post hoc test if ANOVA showed significance. Correlations between numerical data were acquired using the Pearson correlation coefficient (r). A P-value < 0.05 indicated statistically significant.
Seventy postKasai BA patients were enrolled in this prospective study. The characteristics and laboratory parameters of BA children with persistent jaundice compared to BA children without jaundice are described in Table 1. Jaundice BA subjects had markedly lower serum albumin levels than non-jaundice BA children. On the other hand, serum bilirubin, AST, ALT, ALP were considerably higher in BA cases with jaundice than those without jaundice. Subsequent analysis demonstrated that lumbar spine BMD and serum 25-hydroxyvitamin D values of jaundice BA subjects were significantly lower than those of non-jaundice BA subjects (P < 0.001).
| BA patients | Total | Jaundice | No jaundice | P-value |
| n | 70 | 28 | 42 | |
| Gender (male/female) | 30:40 | 12:16 | 18:24 | 0.5 |
| Age (yr) | 7.6 ± 0.5 | 6.3 ± 0.8 | 8.6 ± 0.6 | 0.01 |
| Albumin (g/dL) | 3.9 ± 0.1 | 3.2 ± 0.3 | 4.3 ± 0.1 | < 0.001 |
| Total bilirubin (mg/dL) | 3.8 ± 0.7 | 8.2 ± 1.5 | 0.9 ± 0.1 | < 0.001 |
| Direct bilirubin (mg/dL) | 2.5 ± 0.6 | 5.8 ± 1.1 | 0.2 ± 0.1 | < 0.001 |
| AST (IU/L) | 148.8 ± 13.7 | 235.9 ± 20.9 | 90.8 ± 11.3 | < 0.001 |
| ALT (IU/L) | 133.3 ± 12.8 | 183.4 ± 18.4 | 99.8 ± 15.7 | 0.001 |
| ALP (IU/L) | 501.7 ± 36.3 | 681.6 ± 46.3 | 381.8 ± 43.3 | < 0.001 |
| 25(OH)D (ng/mL) | 25.3 ± 1.1 | 16.0 ± 1.8 | 30.1 ± 0.7 | < 0.001 |
| Lumbar BMD (g/cm2) | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | < 0.001 |
| Lumbar BMD Z-score | -1.2 ± 0.2 | -2.3 ± 0.2 | -0.4 ± 0.1 | < 0.001 |
The prevalence of low bone mass (osteopenia and osteoporosis) in BA subjects were 51.4% (36 out of 70). Ten patients (35.7%) in the jaundice group and 8 patients (19.0%) in the non-jaundice group had osteopenia. Sixteen patients (57.1%) in the jaundice group and 2 patients (4.8%) in the no jaundice group had osteoporosis. Subsequently, BA patients were divided into tertiles based on the WHO criteria. The first tertile included 34 patients with BMD Z-scores from 0 to -1 (considered as normal), the second tertile included 18 patients with Z-scores from -1.0 to -2.5 (considered as osteopenia), and the third tertile included 18 patients with Z-score lower than -2.5 (considered as osteoporosis). There was no statistically significant difference in gender and age distribution among the three tertiles (Table 2). However, serum albumin, serum bilirubin, AST, ALT, serum 25(OH)D and lumbar spine BMD were significantly different between the three tertiles. Further analysis revealed that lumbar spine BMD was correlated with age (r = 0.774, P < 0.001), serum albumin (r = 0.333, P = 0.005), TB (r = -0.476, P < 0.001), AST (r = -0.583, P < 0.001), ALT (r = -0.428, P < 0.001), and ALP (r = -0.456, P < 0.001). The correlations between lumbar spine BMD, age, serum albumin, serum TB, AST, ALT, ALP are illustrated in Figure 1.
| Characteristics | Normal | Osteopenia | Osteoporosis | P-value |
| n | 34 | 18 | 18 | |
| Gender (male/female) | 15/19 | 7/11 | 8/10 | 0.3 |
| Age (yr) | 8.2 ± 0.7 | 7.7 ± 1.1 | 6.5 ± 1.0 | 0.4 |
| Albumin (g/dL) | 4.1 ± 0.2 | 4.0 ± 0.1 | 3.3 ± 0.2 | < 0.05 |
| Total bilirubin (mg/dL) | 1.0 ± 0.2 | 2.8 ± 0.7 | 10.0 ± 2.1 | < 0.001 |
| Direct bilirubin (mg/dL) | 0.4 ± 0.1 | 1.6 ± 0.5 | 7.3 ± 1.7 | < 0.001 |
| AST (IU/L) | 95.6 ± 13.7 | 177.1 ± 24.8 | 221.2 ± 31.2 | < 0.001 |
| ALT (IU/L) | 104.2 ± 18.2 | 164.6 ± 23.7 | 156.8 ± 25.1 | < 0.001 |
| ALP (IU/L) | 429.1 ± 55.7 | 538.4 ± 55.2 | 602.3 ± 71.3 | 0.08 |
| 25(OH)D (ng/mL) | 33.2 ± 0.7 | 26.3 ± 0.5 | 14.3 ± 1.5 | < 0.01 |
| Lumbar BMD (g/cm2) | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | < 0.001 |
BA is a serious cholestatic liver disease in neonates. The obstruction of bile flow in BA results in worsening cholestasis, liver fibrosis and cirrhosis, which lead to portal hypertension and eventually end-stage liver failure in children. Early diagnosis and timely Kasai portoenterostomy to restore bile flow can help avoid the need of liver transplantation during childhood in a number of patients[10]. Despite a number of extensive clinical research studies on BA, the etiology and pathogenesis of BA are largely unknown.
In the recent years, serum 25-hydroxyvitamin D level was decreased in BA patients with low BMD[11]. Additionally, circulating leptin and osteoprotegerin levels has been shown to be correlated with BMD and the presence of jaundice in BA, suggesting that leptin and osteoprotegerin could play a pontential role in maintaining bone mass of BA patients[12,13].
The current study showed that postoperative BA patients with jaundice had significantly lower lumbar spine BMD than those without jaundice. Moreover, we have illustrated that the prevalence rates of osteopenia and osteoporosis in jaundiced BA subjects were higher in comparison with those in non-jaundiced children. Further analysis revealed an inverse association between lumbar spine BMD and serum TB and liver synthetic function. The explanation for these findings may be attributable to decreased osteoblastic function or increased osteoclastic resorption in BA patients. It has been documented that osteoblast proliferation was inhibited by unconjugated bilirubin in vitro and by the serum of jaundiced patients, indicating that bilirubin might have a direct effect on bone metabolism[14,15]. A number of BA cases eventually become advanced stage of liver disease and pediatric liver transplantation is the treatment strategy of choice for improving quality of life in BA children. Recent study has reported that successful liver transplantation could improve biochemical markers of bone formation and resorption suggesting acceleration of growth process in BA children[16]. However, the connection between cholestasis and low bone mass in BA patients merits further investigations.
Some caveats need to be acknowledged regarding the current study. First, the number of patients and controls enrolled in the present study was relative small. This could reduce the statistical power of these results. Accordingly, prospective longitudinal study with a larger population is warranted to elucidate the exact relationship between BMD, outcome parameters, and the severity in BA subjects. Secondly, inadequate measurement of plausible confounding factors including comorbidities needed to be taken under advisement. Moreover, another limitation of our study is the lack of Child-Pugh and Model for End-Stage Liver Disease (MELD) scores. Future study is also required to evaluate the Child-Pugh and MELD values for predicting of chronic liver disease severity. Ultimately, the paucity of quantitative bone histomorphometry analysis which may render evidence as to whether bone was correlated with BMD data. Therefore, more research will be needed in order to better comprehend the precise role of bone mass in the severity of postKasai BA.
To summarize, the current study demonstrated that BA subjects with persistent jaundice had significantly lower BMD than those without jaundice. Additionally, lumbar spine BMD was correlated with hepatic dysfunction suggesting that low BMD was associated with outcome parameters reflecting the severity of cholestasis in postKasai BA patients.
Biliary atresia (BA) is a severe congenital cholestatic liver disease with an unknown etiology. Metabolic bone disorder (osteopenia and osteoporosis) can be complicated by existing chronic liver diseases including BA. There is evidence that serum markers of bone metabolism correlated with the degree of jaundice in BA.
In recent years, much research has revealed that vitamin D deficiency is associated with the severity of hepatic fibrosis or reduced bone mineral density (BMD) in patients with chronic liver disease. This study showed that lumbar spine BMD and 25-hydroxyvitamin D level in BA patients with jaundice were lower than those without jaundice. Moreover, low BMD was associated with serum bilirubin and liver function.
Jaundiced BA patients showed significantly lower lumbar spine BMD and 25-hydroxyvitamin D than in non-jaundiced BA patients. Additionally, lumbar spine BMD correlated with hepatic function markers, which reflect the severity of cholestasis in postKasai BA patients.
BMD could be used to assist clinicians in assessing the progression of cholestasis. This study highlights the need of vitamin D supplementation and its potential in maintaining bone mass in persistently jaundiced BA children.
BMD is the amount of bone mineral per unit volume of the bone tissue and is used as an indirect parameter of bone health. BMD measurements of the patients are generally compared to those from age-matched population and are expressed as Z-score. Osteopenia is defined as Z-score between -1 and -2.5, and osteoporosis as Z-score < -2.5.
A very interesting study to explore the prevalence of osteopenia and osteoporosis in post-Kasai BA children and the association of bone mineral density and biochemical parameters in postoperative BA patients.
| 1. | Kobayashi H, Stringer MD. Biliary atresia. Semin Neonatol. 2003;8:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 211] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Erlichman J, Hohlweg K, Haber BA. Biliary atresia: how medical complications and therapies impact outcome. Expert Rev Gastroenterol Hepatol. 2009;3:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | A-Kader HH, Abdel-Hameed A, Al-Shabrawi M, Mohsen N, El-Karaksy H, Hassanein B, Elsayed B, Abdel-Khalik MK, Karjoo M. Is biliary atresia an autoimmune disease? Eur J Gastroenterol Hepatol. 2003;15:447. [PubMed] |
| 6. | Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Pusl T, Beuers U. Extrahepatic manifestations of cholestatic liver diseases: pathogenesis and therapy. Clin Rev Allergy Immunol. 2005;28:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Sanchez AJ, Aranda-Michel J. Liver disease and osteoporosis. Nutr Clin Pract. 2006;21:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Okada T, Honda S, Miyagi H, Minato M, Taketomi A. Hepatic osteodystrophy complicated with bone fracture in early infants with biliary atresia. World J Hepatol. 2012;4:284-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 669] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 11. | Chongsrisawat V, Ruttanamongkol P, Chaiwatanarat T, Chandrakamol B, Poovorawan Y. Bone density and 25-hydroxyvitamin D level in extrahepatic biliary atresia. Pediatr Surg Int. 2001;17:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Honsawek S, Chaiwatanarat T, Chongsrisawat V, Thawornsuk N, Vejchapipat P, Poovorawan Y. Circulating leptin levels and bone mineral density in children with biliary atresia. Acta Paediatr. 2008;97:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Honsawek S, Chaiwatanarat T, Vejchapipat P, Chongsrisawat V, Thawornsuk N, Poovorawan Y. Relationships between OPG, RANKL, bone metabolism, and bone mineral density in biliary atresia. Pediatr Surg Int. 2009;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ruiz-Gaspà S, Dubreuil M, Guañabens N, Combalia A, Peris P, Monegal A, Parés A. Ursodeoxycholic acid decreases bilirubin-induced osteoblast apoptosis. Eur J Clin Invest. 2014;44:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Teisseyre M, Pawłowska J, Kryśkiewicz E, Karczmarewicz E, Czubkowski P, Dadalski M, Jankowska I, Teisseyre J, Ismail H, Lorenc R. Bone mineral metabolism in children with biliary atresia after living related liver transplantation. Evaluation of selected parameters. Ann Transplant. 2007;12:19-25. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Balaban YH, Chiu KW, Kaya M S- Editor: Ji FF L- Editor: A E- Editor: Li D