Published online Mar 18, 2016. doi: 10.4254/wjh.v8.i8.395
Peer-review started: August 10, 2015
First decision: September 21, 2015
Revised: January 27, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: March 18, 2016
Processing time: 221 Days and 5.6 Hours
This review focuses on the management of iron metabolism and iron overload experienced in the hereditary condition, human factors engineering (HFE)-associated hemochromatosis. Hemochromatosis refers to a group of genetic diseases that result in iron overload; the major one globally is HFE-associated hemochromatosis. The evolution in understanding of the most common form of hereditary hemochromatosis, being the substation of cysteine to a tyrosine at position 282 in the HFE gene, has been extensively studied Novel mutations in both HFE and non-HFE genes have been indicated in this disease which hold significance in its application for the Asia-Pacific region. In conditions with iron overload, the storage of excess iron in various body tissues leads to complications and toxic damage. The most common presenting complaint for this disease is malaise, lethargy and other non-specific symptoms. In order to diagnose hereditary hemochromatosis, there are biochemical, imaging and genetic testing options. Currently, cascade screening of affected families is preferred over population-level screening. The mainstay of treatment is venesection and the appropriate approach to treatment has been consolidated over the years. Recently, the indications for venesection therapy of hemochromatosis have been challenged and are the subject of ongoing research.
Core tip: The concept of hemochromatosis as a single disease entity has changed to an iron storage disease resulting from several genetic disorders although the final common metabolic pathway is inappropriate iron absorption from the intestine and progressive tissue iron loading. The most common form of the disease is due to a mutation in the human factors engineering gene resulting in cysteine tyrosine substitution at position 282 in the molecule. This mutation is relatively common in populations of northern European extraction but is rare in other populations. In contrast other rarer forms of hemochromatosis resulting from other mutations in the hepcidin pathway are quite ubiquitous. The main stay of treatment remains venesection although new oral iron-chelating agents show promise.
- Citation: Sivakumar M, Powell LW. Management of human factors engineering-associated hemochromatosis: A 2015 update. World J Hepatol 2016; 8(8): 395-400
- URL: https://www.wjgnet.com/1948-5182/full/v8/i8/395.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i8.395
The clinical and molecular research surrounding the clinical syndrome of hemochromatosis has been substantial in the last two decades even though it has been recognized in its advanced state for more than 100 years[1]. A mutation in the human factors engineering (HFE) gene was identified as the cause for more than 90% of cases of classic hemochromatosis[2] in most countries except for the Mediterranean region where it is responsible for around 65% of the cases. The genetic cause for hemochromatosis is more common in individuals with a northern European ancestry; however, the clinical manifestation, or incidence of biochemical abnormalities and clinical disease, is not as common in these populations. Although mutations in the HFE gene are most common, there are other forms of iron overload caused by mutations in other iron regulatory molecules that present as distinct clinical diseases. Over time, population studies have served the purpose of outlining the risk to an individual with a genetic mutation and the clinical investigations available for assessment and monitoring have improved. The treatment of hemochromatosis is the one aspect of this condition that has evolved the least over the years with phlebotomy still being the main therapy available. However, the treatment has potential for change with increased research on new therapeutic agents under trial. Although the European Association of the Study of Liver (EASL) and the American Association for the Study of Liver Disease have outlined appropriate treatment regimens, recent research have challenged these guidelines suggesting there is a benefit in beginning treatment early for patients with even mildly elevated iron levels but with or without clinical manifestation. According to current guidelines, the threshold of serum ferritin at which to start treatment is currently taken as above the normal range where the normal range for serum ferritin in men is 24-336 μg/L and in women is 11-307 μg/L. The current clinical standard is to maintain the serum ferritin at 50-100 μg/L[3].
The role of iron in the body is a crucial one from oxygen transport in hemoglobin and oxidative phosphorylation to the production of red blood cells and other functions[4,5]. In situations with overload, there are consequences in disease and mortality to be discussed later in this paper however the extent of this risk is still debated[6-9]. Beginning with iron, when it is consumed, it can enter the body in two forms: Either heme or non-heme[10,11]. Heme is mostly commonly ingested as animal protein and non-heme is via vegetables. However, there is no mechanism for the excretion of iron which is toxic in overload. Uncontrolled loss (1-2 mg) in menses, bleeding and the sloughing of skin are the only methods for iron removal.
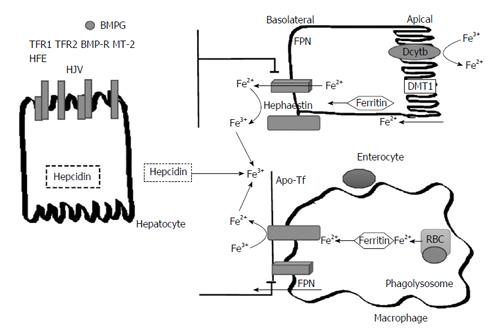
In order to understand iron homeostasis, a discussion regarding the pathway of iron is necessary. Iron is absorbed on the apical surface of enterocytes in the duodenum and proximal small bowel. Non-heme iron can be either ferrous (Fe2+) or ferric (Fe3+)[4]. It is important to note that since ferrous iron is more soluble, it is necessary for ferric iron to be reduced to ferrous iron prior to absorption[12]. In order to reduce ferric iron found in non-heme iron to the ferrous state both gastric acidity and duodenal cytochrome B (DCytB1) have been identified as well as other non-enzymatic pathways[13,14]. On the apical surface of enterocytes, the divalent metal transport 1 (DMT1) protein takes in ferrous iron[14]. The DMT1 protein also serves to transport manganese and copper (Figure 1).
From the enterocyte, iron uptake into tissue is mediated by transferrin receptors (TfR1 and TfR2). In transportation, iron is consistently bound to a molecule due to its ability to form free radicals. Transferrin, the carrier protein for iron binds to the TfR1 and is taken up by endosomes, where transferrin is cleaved and the receptor recycled back to the cell surface[15]. In the case of iron overload, excess iron is stored in complexes of hemosiderin or ferritin. Another form of iron storage is hemosiderin which is a by-product of ferritin degradation[13].
On the basal surface of enterocytes, ferroportin (FPN1) is the sole expressed exporter in cells. Iron is released into circulation when FPN1 interacts with ferroxidase and hephestin. Hephestin next acts to oxidize the iron and the iron is then immediately bound to the transport molecule transferrin (Tf)[4]. Another important regulator of iron homeostasis is ferroportin, a protein which acts to export stored iron from enterocytes and other intracellular stores. A small hepatic peptide, hepcidin, negatively regulates ferroportin[12] by causing the internalization and degradation of this protein thereby affecting the export of iron. In summary, hepcidin reduces iron uptake and serum iron[12,16]. There have been certain factors such as iron, inflammation and oxidative stress that have been demonstrated to have an inhibitory effect on the expression hepcidin. However, hepcidin regulation is not a topic that is completely understood.
Hereditary Hemochromatosis is caused by different mutations that alter the regulatory proteins involved in iron homeostasis and hepcidin pathways. The genetic causes for hemochromatosis can be categorized into HFE gene mutations and non-HFE gene mutations (FPN, TFR HJV)[2]. While non-HFE gene mutations are not as common as HFE gene mutations, there is an increased proportion of these mutations in non-Northern European populations[4]. Therefore, this information is of significance in Asia-Pacific populations[4].
The knowledge and classification of hemochromatosis and other iron overload diseases has become more detailed in the last 2 decades (Table 1). Mutations in the genes encoding HFE, TfR2, hemojuvelin and hepcidin all lead to decreased hepcidin activity and increased iron absorption, resulting in the syndrome of hemochromatosis[5]. Mutations in HFE, HJV, HAMP, TFR2 and SLC40A1 have been linked to the various types of hemochromatosis[2,5].
| Genetic iron overload (primary) |
| Type 1 HFE-associated hemochromatosis |
| C282Y homoyzygosity |
| C282Y/H63D compound heterozygosity |
| Type 2 juvenile hemochromatosis |
| 2A hemojuvelin mutations |
| 2B hepcidin mutations |
| Type 3 TfR2-related hemochromatosis |
| Transferrin receptor 2 |
| Type 4 ferroportin disease |
| Loss of function mutations, also called type 4A or "M" |
| Hepcidin resistance mutations, also called type 4B or "H" |
| Aceruloplasminemia |
| Ceruloplasmin mutations |
| A(hypo)transferrinemia |
| Acquired iron overload (secondary) |
| Ineffective erythropoiesis |
| Thalassemia major |
| Sideroblastic anemia |
| Chronic hemolytic anemia |
| Dietary iron overload (African) |
| Parenteral iron overload (including transfusional overload) |
In Northern European ancestry, an amino acid substitution specifically at position 282 of the HFE protein is the mutation most responsible for iron overload in this population[5]. The C282Y substitution is rare outside those of white ethnicity[17-19]. HFE is tightly linked to the HLA-A locus on chromosome 6p. Persons who are homozygous for the mutation are at increased risk of iron overload and account for 80% to 90% of clinical hereditary hemochromatosis in persons of northern European descent[6-9]. Pietrangelo suggest that between 10% and 33% of homozygous patients develop hereditary hemochromatosis[4,20]. This suggests that there are other genetic and non-genetic factors in the disease[21].
There have been alternative mutations of HFE identified, primarily H63D and S65C; however, these mutations have not been proven to cause substantial iron overload[4]. In order to produce symptomatic disease, a heterozygous mutation is necessary. Since there is an increased prevalence of C282Y, and H63D is more relevant clinically, compound heterozygotes with symptomatic disease are usually C282Y/H63D[2,22-24].
Discussion regarding non-HFE associated hemochromatosis is beyond the scope of this paper.
Hereditary hemochromatosis is most commonly associated with liver disease including cirrhosis, but the clinical manifestations of iron overload are diverse and involve many other organs. Hemochromatosis is an overall underdiagnosed disease due to the idea that it is a rare condition and also associating diagnosis with clinical features seen in advanced disease such as cirrhosis, diabetes and skin pigmentation[3]. Genetic susceptibility for hemochromatosis is seen in approximately one in 250 Caucasians; however, fully expressed disease with end-organ manifestations is seen in fewer than 10% of these individuals[3]. Hemochromatosis patients mostly present with non-specific symptoms such as lethargy, arthralgia and weakness[25,26]. The other more commonly affected organ systems include liver, heart, pancreas, pituitary, skin and joints. Iron deposition in the conducting bundles and parenchyma of the heart result in cardiac arrhythmias and cardiomyopathy in 2%-19% of symptomatic patients[27,28]. Diabetes mellitus (DM) can be seen in up to 60% of symptomatic homozygotes but the rates of DM in asymptomatic patients are comparable to controls[7,29]. Endocrine dysfunction can occur as a result of iron deposition in pituitary and parathyroid glands[27,30]. Arthropathy is also observed in symptomatic and asymptomatic patients due to calcium pyrophosphate deposition in the articular cartilage, not iron sequestration and primarily involves the 2nd and 3rd metacarpophalangeal joints[25,31].
Treatment for hemochromatosis with venesection (phlebotomy) has remained unchanged over the years[5]. Venesection as a treatment has two purposes: Directly reduce serum iron by depleting hemoglobin levels and to replace the depleted circulating serum iron by mobilizing iron stores from tissues. Early intervention, prior to the onset of symptoms, improves patient prognosis[32]. Furthermore, venesection in symptomatic individuals improves certain symptoms, such as skin pigmentation, while not having an effect on others such as cirrhosis and arthropathy[32].
According to EASL clinical practice guidelines, the threshold of serum ferritin at which to start treatment is currently taken as above the normal range. In regards to maintenance, the advocated standard practice is to maintain the serum ferritin at 50-100 μg/L and this is usually achieved with 3-6 mo of venesection[32]. It has been identified that the morbidity and mortality related to hereditary hemochromatosis can be greatly reduced by beginning treatment (phlebotomy) before the development of cirrhosis and/or diabetes. As a result of these findings, it is generally recommended that individuals at risk have prompt identification and pre-emptive treatment[32]. The pre-emptive treatment should be extended to involve those with homozygous HH that are asymptomatic and have markers of iron overload. Also, individuals with indications or evidence of increased level of hepatic iron should be treated. In summary, the American Association for the Study of Liver Disease recommends that in the absence of indicators suggestive of significant liver disease (alanine amiotransferase, aspartate transaminase elevation), C282Y homozygotes who have an elevated ferritin (but < 1000 μg/L) should proceed to prophylactic phlebotomy without a liver biopsy where target levels of phlebotomy should be a ferritin level of 50-100 μg/L[3,23,32].
Traditionally, it was suggested that serum ferritin be maintained below 50 μg/L, but this has been updated to the range stated. Treatment guidelines also suggest yearly follow-up for the patients whose ferritin levels are at the normal range. This treatment strategy works for types 1-3 hereditary hemochromatosis but patients with type 4a may not tolerate venesection due to the irregular iron export from cells therefore treatment must be intermittent and is more complicated[32].
Generally, 1 unit of blood is understood to contain approximately 200-250 mg of iron but the amount of iron that is removed each venesection can be variable[3]. It has been reported that on average, phlebotomy removes around 200-250 mg of iron per session[33]. Therefore, treatment must be provided on a personalized and case by case basis for each patient for appropriate venesection intervals and treatment regiments.
Although the treatment has remained the same for many years, there is still debate regarding the appropriate serum ferritin levels for maintenance of hemochromatosis. A recent study conducted by Bardou-Jacquet et al[6] found that early and sustained iron removal is beneficial as patients with serum ferritin levels between normal and 1000 μg/L, when treated, have reduced cardiovascular and extra-hepatic related mortality rates despite normal liver-related mortality rates. This study suggests that patients with even mild iron overload should be treated which builds on current management guidelines. However, this subject remains controversial.
There is a continuing need to study the factors contributing to hemochromatosis due to the variable clinical penetrance of HFE mutations and the worldwide prevalence of hemochromatosis in the absence of HFE mutations. Hemochromatosis has been divided into HFE-associated hemochromatosis related to mutations affection iron transport and absorption and also HFE negative hemochromatosis or disease without HFE mutations. There is an incomplete understanding of the reasons for incomplete penetrance of disease phenotype in those with HFE mutations but recent research has revealed the presence of at least one other significant modifying genetic mutation[34]. Individuals at risk for hemochromatosis with genetic mutations and with or without symptomatic disease are recommended to pursue treatment at the earliest time possible and prior to any disease as this can help prevent further morbidity and mortality associated with hemochromatosis. Research advancement is opening doors for the management and treatment of iron overload as recent research has begun to develop the importance of treating mild iron overload due to its identified relation with reduced cardiovascular and extrahepatic related mortality rates.
P- Reviewer: Bardou-Jacquet E, Castro JA, Musci G S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Von-Recklinghausen F. Uber haemochromatose. Tageblatt Versammlung Dtsche Naturforscher Arzte Heidelberg. 1889;62:324-325. |
| 2. | Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2676] [Cited by in RCA: 2555] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 3. | Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 4. | Ekanayake D, Roddick C, Powell LW. Recent advances in hemochromatosis: a 2015 update : a summary of proceedings of the 2014 conference held under the auspices of Hemochromatosis Australia. Hepatol Int. 2015;9:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int. 2009;3:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Bardou-Jacquet E, Morcet J, Manet G, Lainé F, Perrin M, Jouanolle AM, Guyader D, Moirand R, Viel JF, Deugnier Y. Decreased cardiovascular and extrahepatic cancer-related mortality in treated patients with mild HFE hemochromatosis. J Hepatol. 2015;62:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 507] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 8. | Powell LW, Dixon JL, Ramm GA, Purdie DM, Lincoln DJ, Anderson GJ, Subramaniam VN, Hewett DG, Searle JW, Fletcher LM. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med. 2006;166:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 9. | Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 547] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Han O. Molecular mechanism of intestinal iron absorption. Metallomics. 2011;3:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Bourdon E, Kang DK, Ghosh MC, Drake SK, Wey J, Levine RL, Rouault TA. The role of endogenous heme synthesis and degradation domain cysteines in cellular iron-dependent degradation of IRP2. Blood Cells Mol Dis. 2003;31:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 804] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 13. | Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal. 2013;18:2473-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2329] [Article Influence: 80.3] [Reference Citation Analysis (7)] |
| 15. | Bartnikas TB. Known and potential roles of transferrin in iron biology. Biometals. 2012;25:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 909] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 17. | Khusainova RI, Khusnutdinova NN, Khusnutdinova EK. [Analysis of the hemochromatosis gene (HFE) mutations, C282Y and H63D, in the populations of Central Asia]. Genetika. 2006;42:421-426. [PubMed] |
| 18. | Hayashi H, Wakusawa S, Motonishi S, Miyamoto K, Okada H, Inagaki Y, Ikeda T. Genetic background of primary iron overload syndromes in Japan. Intern Med. 2006;45:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Lee JY, Yoo KH, Hahn SH. HFE gene mutation, C282Y causing hereditary hemochromatosis in Caucasian is extremely rare in Korean population. J Korean Med Sci. 2000;15:179-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393-408, 408.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 21. | Fracanzani AL, Piperno A, Valenti L, Fraquelli M, Coletti S, Maraschi A, Consonni D, Coviello E, Conte D, Fargion S. Hemochromatosis in Italy in the last 30 years: role of genetic and acquired factors. Hepatology. 2010;51:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bassett ML, Hickman PE, Dahlstrom JE. The changing role of liver biopsy in diagnosis and management of haemochromatosis. Pathology. 2011;43:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Kanwar P, Kowdley KV. Diagnosis and treatment of hereditary hemochromatosis: an update. Expert Rev Gastroenterol Hepatol. 2013;7:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 594] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Cadet E, Capron D, Perez AS, Crépin SN, Arlot S, Ducroix JP, Dautréaux M, Fardellone P, Leflon P, Merryweather-Clarke AT. A targeted approach significantly increases the identification rate of patients with undiagnosed haemochromatosis. J Intern Med. 2003;253:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Swinkels DW, Aalbers N, Elving LD, Bleijenberg G, Swanink CM, van der Meer JW. Primary haemochromatosis: a missed cause of chronic fatigue syndrome? Neth J Med. 2002;60:429-433. [PubMed] |
| 27. | Adams PC, Deugnier Y, Moirand R, Brissot P. The relationship between iron overload, clinical symptoms, and age in 410 patients with genetic hemochromatosis. Hepatology. 1997;25:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 146] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 557] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 29. | Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 541] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Bacon BR, Olynyk JK, Brunt EM, Britton RS, Wolff RK. HFE genotype in patients with hemochromatosis and other liver diseases. Ann Intern Med. 1999;130:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Timms AE, Sathananthan R, Bradbury L, Athanasou NA, Wordsworth BP, Brown MA. Genetic testing for haemochromatosis in patients with chondrocalcinosis. Ann Rheum Dis. 2002;61:745-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | European Association For The Study Of The Liver. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol. 2010;53:3-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 33. | Harrison SA, Bacon BR. Hereditary hemochromatosis: update for 2003. J Hepatol. 2003;38 Suppl 1:S14-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | McLaren CE, Emond MJ, Subramaniam VN, Phatak PD, Barton JC, Adams PC, Goh JB, McDonald CJ, Powell LW, Gurrin LC. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology. 2015;62:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |