Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1610
Peer-review started: June 23, 2016
First decision: August 10, 2016
Revised: September 13, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 28, 2016
Processing time: 188 Days and 12.8 Hours
To assess how serum gamma-glutamyltransferase (GGT) fractions vary in patients with alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD).
Serum samples were obtained from 14 patients with biopsy-proven alcoholic liver diseases and 9 patients with biopsy proven non-alcoholic fatty liver disease. In addition to these biopsy-proven cases, 16 obese (body mass index > 25) patients without any history of alcohol consumption but with a fatty liver on ultrasound examination and with elevated GGT were included for an additional analysis. Serum GGT fractionation was conducted by high-performance gel filtration liquid chromatography and was separated into the four fractions, big-GGT, medium-GGT, small-GGT (s-GGT), and free-GGT (f-GGT).
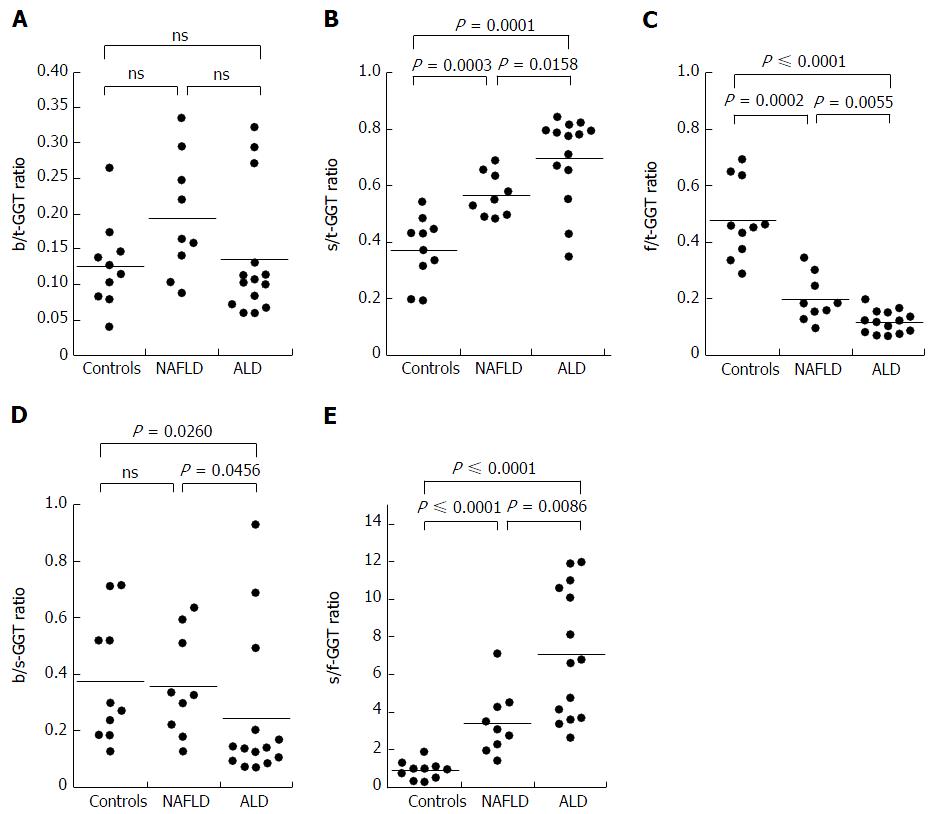
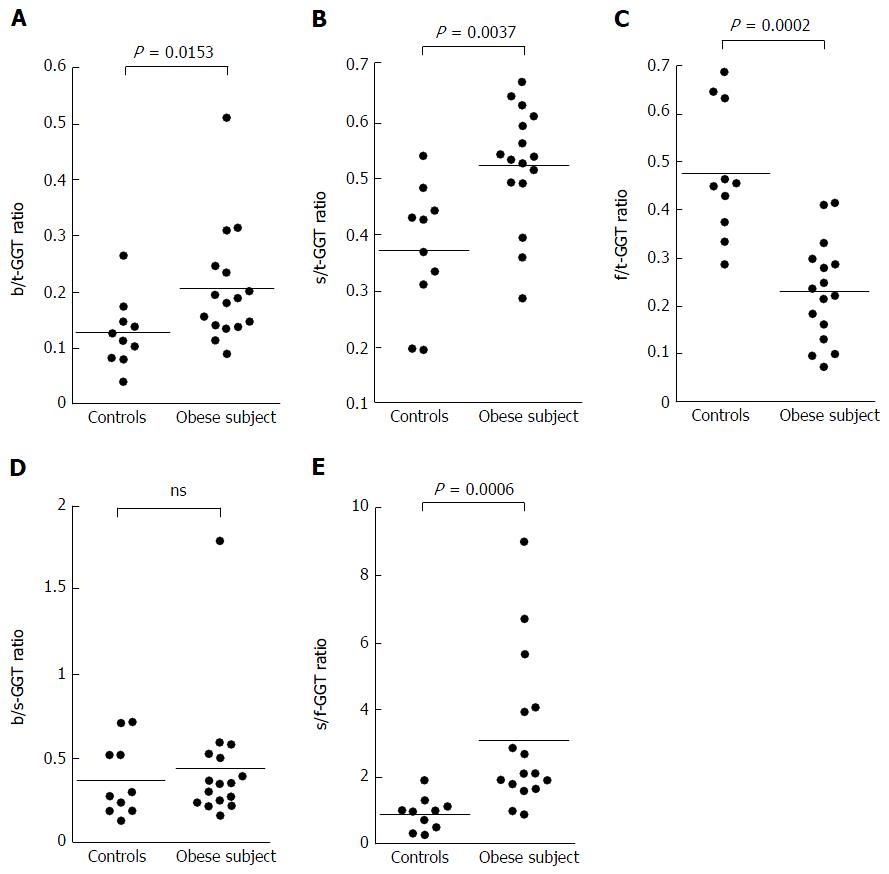
The results were expressed as a ratio of each fraction including the total GGT (t-GGT). The s-GGT/t-GGT ratios were lowest for the control group and highest for the ALD group. The differences between the control and NAFLD groups and also between the NAFLD and ALD groups were statistically significant. In contrast, the f-GGT/t-GGT ratios were highest in the control group and lowest in the ALD group, with the differences being statistically significant. As a result, the s-GGT/f-GGT ratios were markedly increased in the NAFLD group as compared with the control group. The increase of the s-GGT/t-GGT ratios, the decrease of the f-GGT/t-GGT ratios, and the increase of s-GGT/F-GGT ratios as compared with the control group subjects were also found in obese patients with clinically diagnosed fatty change of the liver.
Serum GGT fractionation by high-performance gel filtration liquid chromatography is potentially useful for the differential diagnosis of ALD and NAFLD.
Core tip: The aim of this study was to assess whether fractionation of serum gamma-glutamyltransferase (GGT) into four fractions by high-performance gel filtration chromatography is useful for the differential diagnosis of alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD). In patients with ALD, small-GGT (s-GGT)/total GGT (t-GGT) ratios were significantly higher and free-GGT (f-GGT)/t-GGT ratios were lower than in those in NAFLD. Consequently, there were marked differences in the s-GGT/f-GGT ratio between ALD and NAFLD. These preliminary results indicate that a large-scale study to clarify the diagnostic values of serum GGT fractionation in the differential diagnosis of ALD and NAFLD is warranted.
- Citation: Sueyoshi S, Sawai S, Satoh M, Seimiya M, Sogawa K, Fukumura A, Tsutsumi M, Nomura F. Fractionation of gamma-glutamyltransferase in patients with nonalcoholic fatty liver disease and alcoholic liver disease. World J Hepatol 2016; 8(36): 1610-1616
- URL: https://www.wjgnet.com/1948-5182/full/v8/i36/1610.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i36.1610
Gamma-glutamyltransferase (GGT) [(5-glutamyl)-peptide: Amino acid 5-glutamyltransferase, EC 2.3.2.2] is present in many tissues, including the kidneys, pancreas, and liver[1]. GGT in serum is mainly derived from the liver and this enzyme is often used as a marker of hepatobiliary diseases. Although sensitive, GGT elevation is not specific enough for the differential diagnosis of hepatobiliary disorders. GGT is present in serum in multiple forms in molecular complexes that vary in size, charge, and density[2]. These forms were evaluated in the past by electrophoretic methods to enhance the diagnostic value of GGT measurements[3]. These methods, however, were not sensitive enough to facilitate the differential diagnosis of liver diseases. To overcome this limitation, Franzini et al[4] developed a high-performance liquid chromatography method to quantify four plasma GGT fractions on the basis of molecular size exclusion chromatography, followed by a GGT-specific post-column reaction.
GGT is widely used as a marker of excessive alcohol intake in patients with alcoholic liver disease (ALD)[5]. Induction of hepatic microsomal GGT by chronic alcohol consumption may account, at least in part, for GGT elevation in alcoholics[6]. In addition, serum GGT levels are often increased in patients with non-alcoholic fatty liver disease (NAFLD)[7].
Distinguishing ALD from NAFLD is difficult because self-reported history of alcohol consumption is unreliable. Detection of patients with high alcohol intake by general practitioners is not necessarily easy[8,9]. Accurate diagnosis of NAFLD relies on a liver biopsy; hence, a less-invasive evaluation strategy is desirable[10].
The aim of this preliminary study was to assess how serum GGT fraction patterns, obtained by a high-performance liquid chromatography method, vary in patients with ALD and NAFLD.
Serum samples were obtained from 23 patients with biopsy-proven NAFLD or ALD at the Department of Hepatology, Kanazawa Medical University. Fourteen patients (11 males and 3 females, age 53.0 ± 10.6 years) with biopsy-proven ALD (3 patients with fatty liver, 2 alcoholic fibrosis, 5 alcoholic hepatitis, 3 alcoholic hepatitis, and 3 liver cirrhosis) and 9 patients (6 males and 3 females, age 57.2 ± 9.86 years) with biopsy-proven NAFLD (6 patients with non-alcoholic steatohepatitis and 3 with simple steatosis) were included in the study. In addition to these biopsy-proven cases, 16 obese (body mass index > 25) patients (16 males, age 48.3 ± 6.97 years) without any history of alcohol consumption but with a fatty liver on ultrasound examination and with elevated GGT were included for an additional analysis. Subjects suspected to have autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, Wilson’s disease and alpha 1 anti-trypsin deficiency were excluded from this study. Serum samples were also obtained from 10 apparently healthy and age-matched subjects for a control group. The clinical data for these 49 patients are presented in Table 1. All samples were frozen by liquid nitrogen and were stored at -80 °C until analysis. Written informed consent was obtained from all the patients. The ethics committees of each institute approved the protocol.
| Biopsy-proven ALD | Biopsy-proven NAFLD | Clinically diagnosed NAFLD | Controls | P value | |
| (n = 14) | (n = 9) | (n = 16) | (n = 10) | Biopsy-proven ALD vs NAFLD | |
| Age (yr) | 53 (45-60) | 54 (49-66) | 49 (43-54) | 51 (39-60) | NS |
| Gender (male:female) | 11:3 | 6:3 | 16:0 | 10:0 | NS |
| AST (U/L) | 74 (41-105) | 45 (38-95) | 32 (24-42) | 19 (15-21) | NS |
| ALT (U/L) | 19 (13-22) | 35 (27-67) | 48 (35-74.5) | 18 (14-20) | 0.0166 |
| Albumin (g/dL) | 4.1 (3.2-4.4) | 4.6 (4.1-4.7) | 4.4 (4.3-4.6) | 4.6 (4.3-4.8) | NS |
| Total bilirubin (mg/dL) | 1 (0.6-3.1) | 0.7 (0.6-0.8) | 0.8 (0.6-0.9) | 0.9 (0.6-1.2) | NS |
| Triglyceride (mg/dL) | 186 (99-284) | 184 (101-298) | 187 (102-212) | 130 (111-139) | NS |
| HDL-cholesterol (mg/dL) | 40 (30-56) | 43 (29-52) | 42 (39-54) | 50 (42-56) | NS |
| LDL -cholesterol (mg/dL) | 75 (43-105) | 116 (97-153) | 122 (98-157) | 113 (101-136) | 0.0181 |
| GGT (U/L) | 368 (296-421) | 94 (62-170) | 74 (57-112) | 24 (19-42) | 0.0018 |
| b/t-GGT ratio | 0.1 (0.07-0.13) | 0.16 (0.13-0.26) | 0.18 (0.14-0.24) | 0.12 (0.08-0.15) | NS |
| m/t-GGT ratio | 0.04 (0.02-0.05) | 0.04 (0.02-0.05) | 0.04 (0.03-0.05) | 0.02 (0.02-0.03) | NS |
| s/t-GGT ratio | 0.78 (0.65-0.80) | 0.55 (0.49-0.64) | 0.54 (0.49-0.60) | 0.4 (0.31-0.44) | 0.0158 |
| f/t-GGT ratio | 0.12 (0.08-0.15) | 0.18 (0.15-0.26) | 0.23 (0.15-0.29) | 0.45 (0.37-0.63) | 0.0055 |
| b/s-GGT ratio | 0.14 (0.09-0.20) | 0.33 (0.21-0.53) | 0.35 (0.24-0.51) | 0.29 (0.19-0.52) | 0.0456 |
| s/f-GGT ratio | 6.68 (3.67-10.58) | 3.1 (2.18-4.32) | 2.09 (1.69-3.98) | 0.96 (0.48-1.11) | 0.0086 |
Serum GGT fractionation by high-performance liquid chromatography was conducted on the basis of the methods described by Franzini et al[4]. A 100-μL aliquot of serum was injected into a Superose 6 HR 10/300 GL column (diameter 10 mm, length 300-310 mm; GE Healthcare, Parsippany, NJ, United States) attached to a LC-10AD high-performance liquid chromatography system (Shimadzu Co., Kyoto, Japan). Gel filtration chromatography was performed using the isocratic mode with a binary mobile phase composed of 0.1 mol/L sodium phosphate buffer (pH 7.4), containing 0.2 mol/L NaCl, 0.1 mmol/L EDTA, and 5.4 mmol/L Gly-Gly to support the GGT reaction[11,12]. The flow rate of the mobile phase was 0.5 mL/min. Total run time was 60 min, and fractions were collected every 30 s. Serum containing high GGT (> 150 U/L) levels was difficult to separate into small-GGT (s-GGT) and free-GGT (f-GGT) fractions. To make an appropriate comparison of elution profiles of GGT fractions, serum samples with high-GGT-level sera were diluted to approximate 30-50 U/L with the mobile phase solvent prior to analysis. All results were expressed as compared with total GGT activity subjected to the high-performance liquid chromatography analysis.
Serum total GGT (t-GGT) activities were determined using an enzymatic assay (Serotec Co. Ltd., Sapporo, Japan) with an autoanalyzer (JCA-2250; JEOL Ltd., Tokyo, Japan). This measurement conformed to the International Federation of Clinical Chemistry reference mode for GGT, implemented at a serum volume of 1.2 μL and a reagent volume of 75 μL[4]. Moreover, GGT activity in each fraction improved in the sensitometer mode, which was implemented at a serum volume of 25 μL and a reagent volume of 40 μL. The limit of quantitation at a 10% coefficient of variation was 0.102 U/L.
Total GGT activity and those in each high-performance liquid chromatography fraction in the NAFLD and ALD groups were analyzed using the non-parametric Wilcoxon-Mann-Whitney U test. Between-group comparisons of the laboratory data were made with Spearman’s rank correlation coefficient. P values of < 0.05 were considered significant.
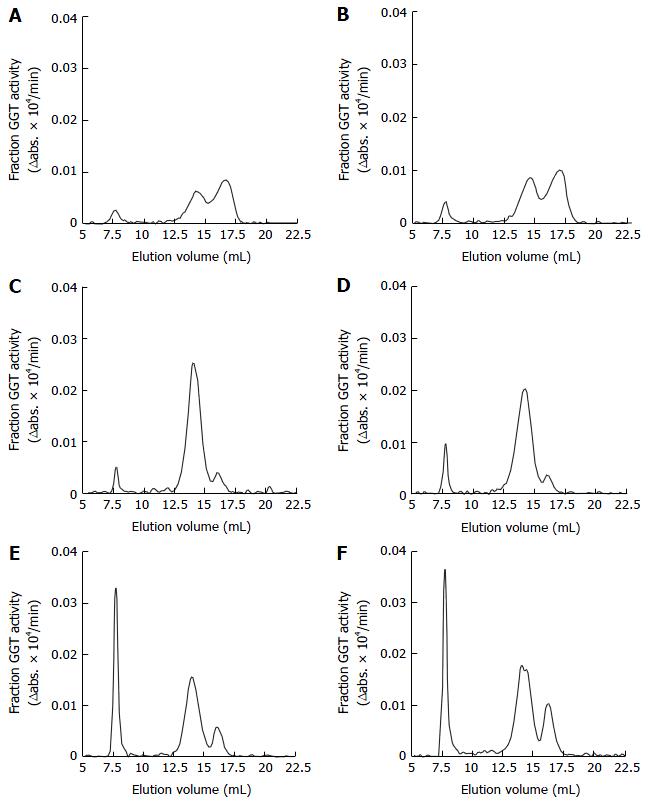
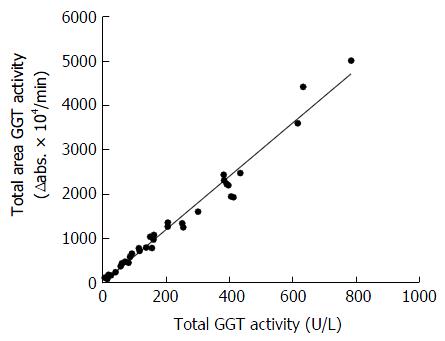
Figure 1A-E shows the GGT-specific elution profiles of representative serum samples obtained from the control group (Figure 1A and B), and patients with ALD (Figure 1C and D) and NAFLD (Figure 1E and F). Three distinct peaks and a low one were found by fractionation every 30 s. The area of the peaks was calculated using a blank for the average GGT eluted with elution volumes of 5.00-6.25 mL. Each GGT fraction was calculated by dividing the area of each single peak. As indicated in Figure 2, it was confirmed that the area under the chromatogram curve was proportional to the GGT enzyme activities.
On the basis of the molecular weight calibration curve (data not shown), these four peaks are equivalent to big-GGT (b-GGT) (MW > 2000 kDa, eluted between 6.25-9.50 mL), medium GGT (m-GGT) (MW 940 Da, eluted between 9.5-12.25 mL), s-GGT (MW 140 kDa, eluted between 12.25-15.5 mL) and f-GGT (MW 70 kDa, eluted between 15.5-20 mL), respectively.
The s-GGT/t-GGT ratios were lowest for the control group and highest for the ALD group. The differences between the control and NAFLD groups and also between the NAFLD and ALD groups were statistically significant, as indicated in Figure 3B. In contrast, the f-GGT/t-GGT ratios were highest in the control group and lowest in the ALD group, with the differences being statistically significant (Figure 3C). As a result, the s-GGT/f-GGT ratios were markedly increased in the NAFLD group as compared with the control group (Figure 3E). The increase of the s-GGT/t-GGT ratios, the decrease of the f-GGT/t-GGT ratios, and the increase of s-GGT/f-GGT ratios as compared with the control group subjects were also found in obese patients with clinically diagnosed fatty change of the liver (Figure 4C-E). There was also a positive correlation between b-GGT activity and levels of low-density lipoprotein and apolipoprotein B in the NAFLD group, but not in the ALD group, in the present study (data not shown).
Franzini et al[4] described a high-performance gel filtration chromatography method for plasma GGT fraction analysis. This method permitted the quantification of four GGT fractions; b-GGT, m-GGT, s-GGT (likely lipoprotein-bound, molecular masses > 2000, 940 and 140 kDa, respectively) and a f-GGT fraction.
It is common for serum GGT levels to be elevated in patients with ALD[5] or obesity-related NAFLD[7]. Patients with elevated serum GGT levels who are obese and are also excessive, habitual alcohol drinkers are frequently encountered in clinical practice. It is necessary to have non-invasive measures to assess the relative contribution of overweight and excessive alcohol consumption on GGT elevations. We wondered how serum GGT fraction patterns obtained by the high-performance liquid chromatography method vary in patients with ALD and NAFLD.
The results of this preliminary study indicate that in patients with ALD, s-GGT/t-GGT ratios were significantly increased and f-GGT/t-GGT ratios were lower, compared with those in NAFLD patients. As a result, there was a marked difference in the s-GGT/f-GGT ratios between patients with ALD and NAFLD. These results indicate that a large-scale study to clarify the diagnostic value of serum GGT fractionation in the differential diagnosis of ALD and NAFLD is warranted.
High-sensitivity GGT fraction patterns of various liver diseases were evaluated by Franzini et al[13-17], Elawdi et al[18], Fornaciari et al[19] and Corti et al[20,21]. They reported that the b-GGT/s-GGT ratio was significantly lower in both alcoholics and abstainers than in the control group, consistent with our study[13]. Patients with NAFLD and chronic hepatitis C have different GGT fraction patterns: b-GGT is increased in NAFLD, but not in chronic hepatitis C[14]. More recently, GGT fractions were measured in cirrhosis patients, revealing that, irrespective of etiology, s-GGT showed the greatest increase in cirrhotic patients and the b-GGT/s-GGT ratio was even lower than that in patients with chronic hepatitis C[18].
To the best of our knowledge, the present study is the first direct comparison of serum GGT fraction profiles between patients with ALD and NAFLD. However, there are several limitations to the present study. The numbers of the biopsy-proven cases was small. In addition, how serum GGT profiles change with disease progression from fatty liver to liver cirrhosis in patients with NAFLD and ALD remains unclear. Also, the diagnostic value of GGT profiles remains to be compared with other markers including cytochrome C[22].
In addition to the well-known alterations in hepatobiliary disorders, GGT is associated with cardiovascular disease (CVD)[23]. In a recent review article, the predictive value of GGT for assessing CVD and cancer mortality was described, including assessment at the physiological level of the enzyme activity[24]. Taking advantage of the high-performance gel filtration chromatography method for plasma GGT fraction analysis, Franzini et al[15] demonstrated that CVD risk factors were associated with b-GGT.
In conclusion, the serum GGT fraction patterns in patients with NAFLD are significantly different from those in patients with ALD. In patients with ALD, s-GGT/t-GGT ratios were significantly higher and f-GGT/t-GGT ratios were lower than in those in NAFLD. Consequently, there were marked differences in the s-GGT/f-GGT ratio between ALD and NAFLD. A large-scale study is needed to further evaluate the diagnostic value of serum GGT fractionation in the differential diagnosis of ALD and NAFLD.
Distinguishing alcoholic liver disease (ALD) from non-alcoholic fatty liver disease (NAFLD) is difficult because self-reported history of alcohol consumption is unreliable. Detection of patients with high alcohol intake by general practitioners is not necessarily easy. Accurate diagnosis of NAFLD relies on a liver biopsy; hence, a less-invasive evaluation strategy is desirable.
Gamma-glutamyltransferase (GGT) in serum is mainly derived from the liver and this enzyme is often used as a marker of hepatobiliary diseases. Although sensitive, GGT elevation is not specific enough for the differential diagnosis of hepatobiliary disorders. GGT is present in serum in multiple forms in molecular complexes that vary in size, charge, and density. These methods, however, were not sensitive enough to facilitate the differential diagnosis of liver diseases. To overcome this limitation, Franzini et al developed a high-performance liquid chromatography method to quantify four plasma GGT fractions on the basis of molecular size exclusion chromatography, followed by a GGT-specific post-column reaction.
To the best of our knowledge, the present study is the first direct comparison of serum GGT fraction profiles between patients with ALD and NAFLD.
Although preliminary, determination of serum GGT profiles may serve for differential diagnosis of ALD and NAFLD.
High-performance liquid chromatography (previously called high-pressure liquid chromatography), is a useful analytical tool which is able to separate various compounds based on their size, electrical charge and biochemical affinity.
The research presents a screening for future investigations about AFLD and NAFLD diagnosis differentiation. It is an interesting approach of ALD and AFLD diagnosis which was not executed in the best possible way.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dajani A, de Medeiros IC, Livero FA, Tarantino G S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Castellano I, Merlino A. γ-Glutamyltranspeptidases: sequence, structure, biochemical properties, and biotechnological applications. Cell Mol Life Sci. 2012;69:3381-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Nemesánszky E, Lott JA. Gamma-glutamyltransferase and its isoenzymes: progress and problems. Clin Chem. 1985;31:797-803. [PubMed] |
| 3. | Cohen DE, Carey MC. Rapid (1 hour) high performance gel filtration chromatography resolves coexisting simple micelles, mixed micelles, and vesicles in bile. J Lipid Res. 1990;31:2103-2112. [PubMed] |
| 4. | Franzini M, Bramanti E, Ottaviano V, Ghiri E, Scatena F, Barsacchi R, Pompella A, Donato L, Emdin M, Paolicchi A. A high performance gel filtration chromatography method for gamma-glutamyltransferase fraction analysis. Anal Biochem. 2008;374:1-6. [PubMed] |
| 5. | Rosalki SB, Rau D. Serum γ-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta. 1972;39:41-47. [PubMed] |
| 6. | Teschke R, Rauen J, Neuefeind M, Petrides AS, Strohmeyer G. Alcoholic liver disease associated with increased gamma-glutamyltransferase activities in serum and liver. Adv Exp Med Biol. 1980;132:647-654. [PubMed] |
| 7. | Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramírez JC, Castro MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Lívero FA, Acco A. Molecular basis of alcoholic fatty liver disease: From incidence to treatment. Hepatol Res. 2016;46:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Reid AL, Webb GR, Hennrikus D, Fahey PP, Sanson-Fisher RW. Detection of patients with high alcohol intake by general practitioners. Br Med J (Clin Res Ed). 1986;293:735-737. [PubMed] |
| 10. | Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Shaw LM, Strømme JH, London JL, Theodorsen L. International Federation of Clinical Chemistry, (IFCC), Scientific Committee, Analytical Section. IFCC methods for the measurement of catalytic concentration of enzymes. Part 4. IFCC method for gamma-glutamyltransferase [(gamma-glutamyl)-peptide: amino acid gamma-glutamyltransferase, EC 2.3.2.2]. J Clin Chem Clin Biochem. 1983;21:633-646. [PubMed] |
| 12. | Schiele F, Muller J, Colinet E, Siest G. Production and certification of an enzyme reference material for gamma-glutamyltransferase (CRM 319). Part 1: Preparation and characterization. Clin Chem. 1987;33:1971-1977. [PubMed] |
| 13. | Franzini M, Fornaciari I, Vico T, Moncini M, Cellesi V, Meini M, Emdin M, Paolicchi A. High-sensitivity γ-glutamyltransferase fraction pattern in alcohol addicts and abstainers. Drug Alcohol Depend. 2013;127:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Franzini M, Fornaciari I, Fierabracci V, Elawadi HA, Bolognesi V, Maltinti S, Ricchiuti A, De Bortoli N, Marchi S, Pompella A. Accuracy of b-GGT fraction for the diagnosis of non-alcoholic fatty liver disease. Liver Int. 2012;32:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Franzini M, Paolicchi A, Fornaciari I, Ottaviano V, Fierabracci V, Maltinti M, Ripoli A, Zyw L, Scatena F, Passino C. Cardiovascular risk factors and gamma-glutamyltransferase fractions in healthy individuals. Clin Chem Lab Med. 2010;48:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Franzini M, Fornaciari I, Rong J, Larson MG, Passino C, Emdin M, Paolicchi A, Vasan RS. Correlates and reference limits of plasma gamma-glutamyltransferase fractions from the Framingham Heart Study. Clin Chim Acta. 2013;417:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Franzini M, Fierabracci V, Bolognesi V, Maltinti S, Fornaciari I, Marchi S, Paolicchi A. Plasma gamma-glutamyltransferase (GGT) activity in inflammatory bowel disease: is the clinical laboratory plasma GGT assay sensitive enough for gastroenterology? Inflamm Bowel Dis. 2013;19:E21-E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Elawdi HA, Franzini M, Paolicchi A, Emdin M, Fornaciari I, Fierabracci V, De Simone P, Carrai P, Filipponi F. Circulating gamma-glutamyltransferase fractions in cirrhosis. Liver Int. 2014;34:e191-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Fornaciari I, Fierabracci V, Corti A, Aziz Elawadi H, Lorenzini E, Emdin M, Paolicchi A, Franzini M. Gamma-glutamyltransferase fractions in human plasma and bile: characteristic and biogenesis. PLoS One. 2014;9:e88532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169-1181. [PubMed] |
| 21. | Corti A, Fierabracci V, Caponi L, Paolicchi A, Lorenzini E, Campani D, Belcastro E, Franzini M. Effect of the three-dimensional organization of liver cells on the biogenesis of the γ-glutamyltransferase fraction pattern. Biomarkers. 2016;21:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47-56. [PubMed] |
| 23. | Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H; Vorarlberg Health Monitoring and Promotion Program Study Group. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 24. | Long Y, Zeng F, Shi J, Tian H, Chen T. Gamma-glutamyltransferase predicts increased risk of mortality: a systematic review and meta-analysis of prospective observational studies. Free Radic Res. 2014;48:716-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |