Published online Dec 18, 2016. doi: 10.4254/wjh.v8.i35.1584
Peer-review started: June 28, 2016
First decision: August 10, 2016
Revised: September 9, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 18, 2016
Processing time: 170 Days and 8.2 Hours
To examine the association of PNPLA3 polymorphisms in chronic hepatitis C patients and development of liver disease spectrum.
Literature was searched systematically from PubMed/MEDLINE, EMBASE, and Cochrane search engines for full-length articles written in English that examined PNPLA3 polymorphism in chronic hepatitis C (CHC) patients. Studies evaluating the association of PNPLA3 polymorphism spectrum (fatty liver, steatohepatitis, cirrhosis, and hepatocellular carcinoma) of CHC were included. Pooled data are reported as OR with 95%CI. Our study endpoint was the risk of the entire liver disease spectrum including: Steatosis/fatty liver, cirrhosis, and hepatocellular carcinoma in CHC patients with PNPLA3 polymorphisms.
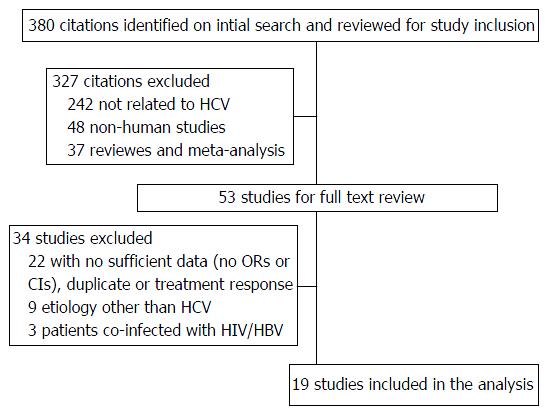
Of 380 studies identified, a total of 53 studies were included for full-text review. Nineteen on chronic hepatitis C were eligible for analysis. Pooled ORs for rs738409 GG compared to CC and CG among patients with fatty liver was 2.214 (95%CI: 1.719-2.853). ORs among advanced fibrosis/cirrhosis were 1.762 (95%CI: 1.258-2.468). Similar odds ratios among hepatocellular carcinoma patients were 2.002 (95%CI: 1.519-2.639). Pooled ORs for rs738409 GG and CG compared to CC among patients with fatty liver were 1.750 (95%CI: 1.542-1.986). Pooled ORs for advanced fibrosis/cirrhosis patients were 1.613 (95%CI: 1.211-2.147). All analyses were homogenous and without publication bias except one. The associations were maintained after adjusting for publication bias and heterogeneity.
PNPLA3 polymorphisms have strong association with increased risk and severity of the liver disease spectrum in CHC patients.
Core tip:PNPLA3 polymorphisms (rs738409 CG and GG) are associated with increased risk of steatosis, advanced fibrosis, cirrhosis, and hepatocellular carcinoma in chronic hepatitis C patients.
- Citation: Salameh H, Masadeh M, Al Hanayneh M, Petros V, Maslonka M, Nanda A, Singal AK. PNPLA3 polymorphism increases risk for and severity of chronic hepatitis C liver disease. World J Hepatol 2016; 8(35): 1584-1592
- URL: https://www.wjgnet.com/1948-5182/full/v8/i35/1584.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i35.1584
Hepatitis C virus (HCV) infection is one of the most important causes of chronic liver disease in the United States[1]. About 27% of cases of cirrhosis and 25% of hepatocellular carcinoma (HCC) worldwide are secondary to HCV infection[2]. Multiple genetic factors identified within the past few years have been shown to be associated with the predisposition to chronic liver disease and the progression to cirrhosis and HCC[3,4]. The single nucleotide polymorphism (SNP) rs738409 C>G (isoleucine to methionine substitution at position 148, I148M) in the PNPLA3 gene has been strongly linked to progression of liver disease in multiple studies, and this association was confirmed in meta-analyses of the spectrum of alcoholic liver disease (ALD)[5] as well as non-alcoholic fatty liver disease (NAFLD)[6-9].
The frequency of hepatic steatosis varies with ethnicity where it was reported as 45%, 33% and 24% in Hispanics, Whites and Blacks respectively[10]. At the same time the frequencies of the PNPLA3 rs738409[G] allele were 0.49, 0.23, and 0.17 in Hispanics, European Americans and African Americans[11]. In addition, the prevalence of the GG genotype in different races in fact correlates with the rate of NAFLD in each respective population, with nearly half of all Hispanics possessing the allele, who in turn are also most likely to have NAFLD. The same is true of the inverse, with less than one quarter of African Americans having the PNPLA3 rs738409[G] allele, and they are least likely to develop NAFLD compared to Hispanics and Caucasians[10,11].
Given that the association between PNPLA3 polymorphism and liver disease spectrum in chronic hepatitis C (CHC) patients has not been consistent, especially for HCC[12,13] and cirrhosis[14,15], we performed this meta-analysis to further examine the association of PNPLA3 polymorphisms with the predisposition to the entire spectrum of liver disease among patients with CHC.
Utilizing the Meta-analysis of Observational Studies in Epidemiology guidelines, literature was searched from PubMed/Medline, Embase, and Cochrane search engines for full-length articles written in English that examined PNPLA3 polymorphism in CHC patients[16]. The initial medical subject headings search terms were: “Hepatitis C, Chronic” and “adiponutrin, human”. The search was then expanded using the terms: “rs738409” and “patatin-like phospholipase domain-containing 3 protein”. All databases were searched from their inception date through March 2015. Meeting abstracts from major gastroenterology conferences over the past 3 years were also searched to identify studies that were potentially overlooked in our database search. Articles were selected for full text review based on title and abstract.
Three independent investigators (Masadeh M, Al Hanayneh M and Maslonka M) manually search the retrieved publications to ensure all appropriate articles were discovered and included. Two authors (HS and AKS) reviewed articles in question for possible inclusion. The following inclusion criteria were set for inclusion in this meta-analysis: (1) studies published as full-length articles which reported association of the PNPLA3 variant (rs738409 C>G) among CHC patients; and (2) studies which analyzed patients with other liver diseases and reported separate data on PNPLA3 polymorphisms for CHC.
The following exclusion criteria were set: (1) studies without available gene frequency data for analysis; and (2) studies including subjects with other liver diseases without separate data on CHC patients.
HCV infection was diagnosed with both positive serum anti-HCV antibodies and serum HCV ribonucleic acid (RNA). The disease spectrum was defined as the following: steatosis = fatty liver (FL) on imaging without evidence of cirrhosis or HCC; advanced fibrosis and cirrhosis = biopsy-proven bridging fibrosis, or clinical evaluation supported by hematological, biochemical, and radiologic imaging findings; and HCC = diagnostic imaging findings on triple phase magnetic resonance imaging or computed tomography, or using histological confirmation from liver tissue. Healthy controls were defined as subjects without liver disease and without HCV infection.
After determining eligibility for inclusion, two reviewers (Masadeh M and Al Hanayneh M) independently extracted data for (1) study characteristics: Author and year of publication, and study design (population based or not, using controls or not); (2) study population: Liver disease spectrum and sample size; (3) frequencies of PNPLA3 polymorphism genotypes (rs738409 CC, CG, and GG); and (4) OR: For association of PNPLA3 polymorphism and the spectrum of liver disease and for severity of liver disease. Any discrepancies amongst the reviewers were resolved by jointly reviewing the study in question. Among studies comparing diseased population with healthy controls, similar data were also extracted on healthy controls.
Our study endpoint was the risk of the entire liver disease spectrum including: Steatosis/fatty liver, cirrhosis, and HCC in CHC patients with PNPLA 3 polymorphisms.
The quality of included studies was assessed independently by three authors (Masadeh M, Al Hanayneh M and Maslonka M) using the Newcastle-Ottawa Quality Assessment Scale for case-control studies[17]. This scale has one instrument for assessing case-control studies and another one for cohort studies. Each of these instruments includes measures of quality in selection, comparability, and exposure domains. While one point is granted for each of the areas measured within the selection and exposure domains, a maximum of two points can be assigned within the comparability domain with highest possible total score of nine. Previous studies have reported that a score of seven or greater denotes a high-quality study[18]. Any discrepancies between the three coauthors were addressed by a joint reevaluation of the original article.
The strength of the association between rs738409 and CHC liver disease spectrum prevalence was expressed by OR and their corresponding 95%CI. The Random effects model was used for analyzing pooled data for all the analyses[19]. Heterogeneity was measured using I2 statistics for inter-study variance, with the χ2 test used for statistical analysis. Heterogeneity was defined with I2≥ 50% or χ2P < 0.10[20]. At least two studies are needed to examine and report heterogeneity. To examine the heterogeneous data and source of heterogeneity, sensitivity analyses were performed in a stepwise fashion for (1) study quality; and (2) excluding studies with the highest and lowest OR. Publication bias was assessed using Egger regression and the Begg-Mazumdar rank correlation tests[21-23]. Egger test is a regression method checking for association between effect sizes and standard error and uses actual effect size for each study[23]. Begg-Mazumdar is a rank correlation test examining the potential association between effect estimates (taken as a rank and not exact effect size) and sampling variance (or standard error)[22]. At least three studies are needed for examining and reporting publication bias. For analyses with publication bias, the analyses were repeated either by performing sensitivity analysis or using the Duval and Tweedie Trim and Fill method, a nonparametric (rank-based) data augmentation technique[24]. The method can be used to estimate the number of studies missing from a meta-analysis resulting in a skew of the data due to the suppression of the most extreme results on one side of the funnel plot. The method then amplifies the observed data so that the funnel plot is more symmetric and re-computes the summary estimate based on the comprehensive data. The method should not be regarded as a way of yielding a more “valid” estimate of the overall effect or outcome, but as a means of examining the sensitivity of the results to one particular selection mechanism[25]. All statistical analyses were performed using R (Foundation for Statistical Computing) utilizing the metaphor package, or Comprehensive Meta-analysis (Biostat, Englewood, NJ). Singal AK from University of Alabama, Birmingham, reviewed the statistical methods of this study.
A total of 380 citations were retrieved on initial search. After reviewing article titles and abstracts, a total of 53 studies were included for full-text review (Figure 1). Of these, twenty articles were excluded because they did not have sufficient data for our analysis. Nine studies were excluded for including subjects with liver disease not caused by HCV[11,26-33], and three studies were excluded for including subjects co-infected with human immunodeficiency virus and/or hepatitis B virus infection[34-36]. One duplicate study[37] and one study that looked at treatment response[38] were excluded. Nineteen studies evaluating 9093 patients (57.6% males, mean body mass index 25.1 kg/m2) on association of PNPLA3 polymorphisms in CHC[12-15,39-53] were included for the analysis. Data on study design, ethnicity and genotype frequency are summarized in Table 1.
| Ref. | Study design | Controls (n) | Cases | ||||||||
| n | Ethnicity | M% | Mean age | Mean BMI | rs 738409 genotype count (CC:CG:GG)4 | ||||||
| FL | Hepatitis | Cirrhosis | HCC | ||||||||
| Cai et al[39] (2011) | R | - | 626 | C | 61.8 | 44.7 | 23.7 | 62:281 | - | - | - |
| Valenti et al[40] (2011) | R | 179 | 819 | C + NA | 56.4 | 57.4 | 24.8 | 269:219:73 | - | 119:172:229 | 17:21:12 |
| Trépo et al[41] (2011) | R | - | 537 | C | 63 | 49.4 | 25.5 | 136:106:31 | - | 108:85:23 | - |
| Corradini et al[42] (2011) | P6 | - | 221 | C | 63 | 58 | - | - | - | - | - |
| Nischalke et al[13] (2011) | P | 190 | 162 | C | 57 | 56 | 28.4 | - | - | 45:31:05 | 40:33:08 |
| Valenti et al[43] (2012) | P | - | 567 | NS | - | - | - | - | - | - | - |
| Valenti et al[15] (2012) | P6 | - | 602 | NS | 51 | 51 | 25.1 | 364:422 | - | 158:212 | - |
| Guyot et al[12] (2013) | P | - | 253 | NS | 54.2 | 56.7 | 27.3 | - | - | 140:75:38 | 54:26:13 |
| Ezzikouri et al[44] (2013) | P | 132 | 230 | NA | 45.2 | 63.63 | - | - | 47:71:11 | - | 43:35:23 |
| Stättermayer et al[45] (2014) | R | - | 478 | NS | 65.7 | 44.9 | 25.6 | 190:232 | - | 101:572 | - |
| Ampuero et al[46] (2014) | P6 | - | 474 | M | 64.8 | 43.4 | 25.7 | 94:1263 | - | - | - |
| Sato et al[47] (2014) | R | - | 358 | A | 55.9 | 69.76 | - | 41:202 | - | 112:372 | 100:176:82 |
| Yasui et al[48] (2014) | P6 | - | 276 | A | 40.6 | 58.2 | 23 | 23:75:39 | 45:66:38 | 20:31:21 | - |
| Petta et al[14] (2015) | P | - | 434 | C | 53.9 | 51.7 | - | - | 40:35:12 | 71:36:13 | - |
| Nakaoka et al[49] (2015 ) | P | - | 231 | A | 44.6 | 62.9 | 22.5 | - | - | 90:272 | 12:22:14 |
| Tamaki et al[50] (2015) | R | - | 176 | A | 39.8 | 56.5 | 22.9 | - | - | 52:87:37 | - |
| Huang et al[51] (2015) | R | - | 1018 | A | 56.6 | 51.8 | 24.9 | 175:205:75 | - | - | - |
| Petta et al[52] (2016) | P6 | - | 694 | C | 53 | 54 | 26.5 | 151:151:455 | - | - | - |
| Ali et al[53] (2016) | P | - | 937 | M | 70.1 | 49.5 | - | - | - | 172:2123 | - |
| Summary | 501 | 9093 | 57.6 | 52.7 | 25.1 | ||||||
Based on the Newcastle-Ottawa Scale, nine studies were of “high quality” with a score of seven or more, and the remaining ten studies had a score of six or below (Table 2).
| Ref. | Selection | Comparability | Exposure | Total | |||||
| Independent validation | Case representation | Controls selection | Controls definition | Case and control design/analysis | Ascertainment of exposure | Same method of ascertainment | Same response rate | ||
| Cai et al[39] (2011) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
| Valenti et al[40] (2011) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Trépo et al[41] (2011) | 1 | 1 | 2 | 1 | 5 | ||||
| Corradini et al[42] (2011) | 1 | 1 | 2 | 1 | 5 | ||||
| Nischalke et al[13] (2011) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Valenti et al[43] (2012) | 1 | 1 | 2 | 1 | 1 | 6 | |||
| Valenti et al[15] (2012) | 1 | 1 | 2 | 1 | 1 | 6 | |||
| Guyot et al[12] (2013) | 1 | 1 | 2 | 1 | 5 | ||||
| Ezzikouri et al[44] (2013) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Stättermayer et al[45] (2014) | 1 | 1 | 2 | 1 | 5 | ||||
| Ampuero et al[46] (2014) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
| Sato et al[47] (2014) | 1 | 1 | 2 | 1 | 5 | ||||
| Yasui et al[48] (2014) | 1 | 1 | 2 | 1 | 5 | ||||
| Petta et al[14] (2015) | 1 | 1 | 2 | 1 | 5 | ||||
| Nakaoka et al[49] (2015 ) | 1 | 1 | 2 | 1 | 5 | ||||
| Tamaki et al[50] (2015) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
| Huang et al[51] (2015) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
| Petta et al[52] (2016) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
| Ali et al[53] (2016) | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||
Association between PNPLA3 polymorphism and liver disease spectrum (GG vs CG and CC analysis)
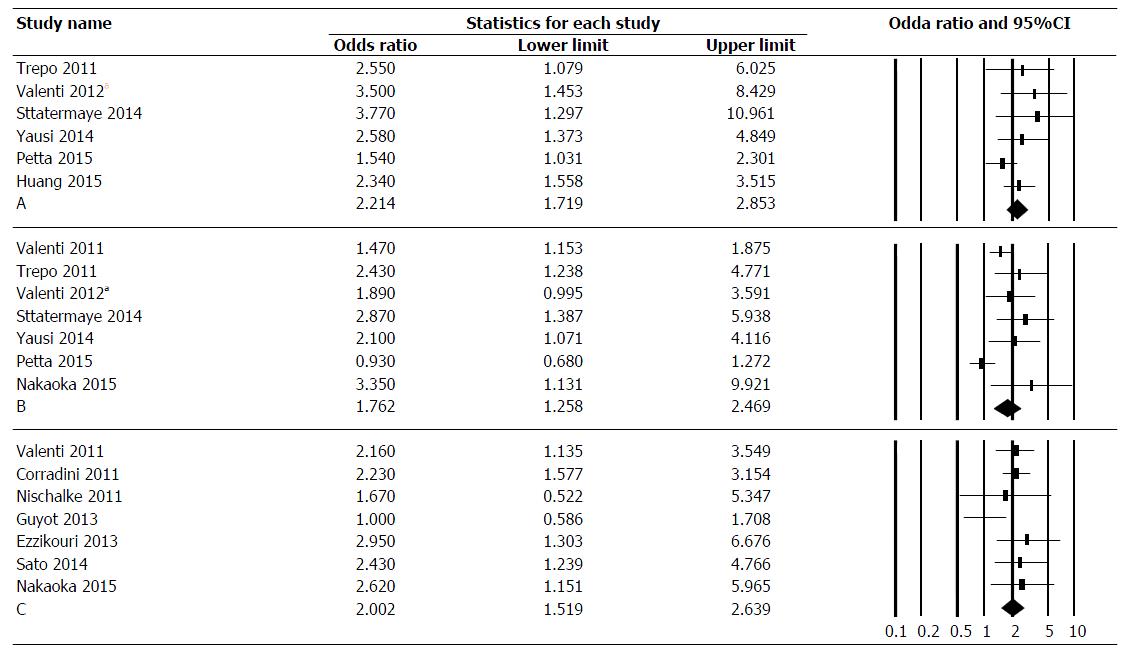
Association of PNPLA3 polymorphisms with FL in CHC patients: Among six studies on 3310 patients, the pooled OR for rs738409 GG genotype compared to CC and CG genotypes in CHC was 2.214 (95%CI: 1.719-2.853) (Figure 2A). The data was homogeneous (I2 = 9.4%, P = 0.36) and without publication bias as assessed by Egger test (P = 0.08) and Begg-Mazumdar test (P = 0.14).
Association of PNPLA3 polymorphisms with advanced fibrosis and cirrhosis in CHC patients:Among seven studies on 3377 patients, the pooled OR for rs738409 GG genotype compared to CC and CG genotypes in CHC was 1.762 (CI: 1.258-2.469) (Figure 2B). The data was heterogeneous (I2 = 65.9%, P = 0.081), with evidence of publication bias as assessed by Begg-Mazumdar test (P = 0.036) and tendency for publication bias as assessed by Egger test (P = 0.059). Sensitivity analysis after excluding studies with lowest[14] and highest[49] OR revealed similar effect size: 1.82 (95%CI: 1.41-2.34) with I2 = 18.8%, P = 0.30. Additionally, when Dual and Tweedie trim and fall test was used to assess publication bias, 3 studies were trimmed with no change in effect size (OR = 1.39, 95%CI: 1.01-1.92).
Association of PNPLA3 polymorphisms and HCC in CHC patients: Among seven studies on 2274 patients, the pooled OR for rs738409 GG genotype compared to CC and CG genotypes in CHC was 2.002 (95%CI: 1.519-2.639) (Figure 2C). The data was homogenous (I2 = 30%, P = 0.19), without publication bias as assessed by Egger test (P = 0.91) and Begg-Mazumdar test (P = 0.99).
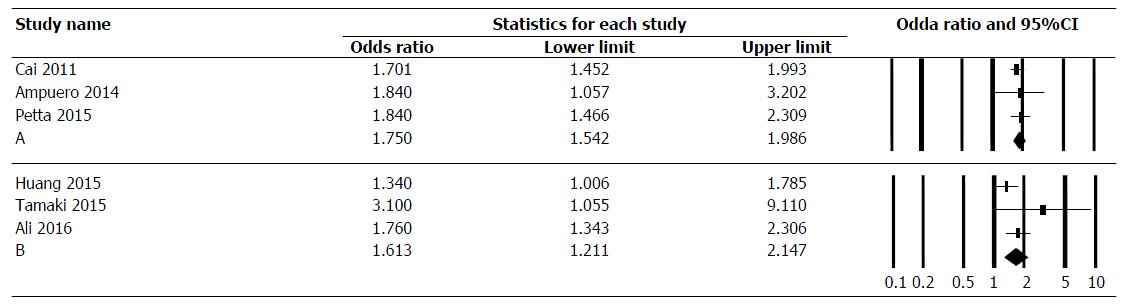
Association of PNPLA3 polymorphisms with FL in CHC patients: Among three studies on 1794 patients, the pooled OR for rs738409 GG and CG genotypes compared to CC genotype in CHC was 1.750 (95%CI: 1.542-1.986) (Figure 3A). The data was homogeneous (I2 = 0.0%, P = 0.84), without publication bias as assessed by Egger test (P = 0.57) and Begg-Mazumdar test (P = 0.99).
Association of PNPLA3 polymorphisms with advanced fibrosis, and cirrhosis in CHC patients: Among three studies on 2131 patients, the pooled OR for rs738409 GG and CG genotypes compared to CC genotype in CHC was 1.613 (95%CI: 1.211-2.147) (Figure 3B). The data was homogeneous (I2 = 41%, P = 0.18), without publication bias as assessed by Egger test (P = 0.056) and Begg-Mazumdar test (P = 0.99).
We have previously described that PNPLA3 polymorphism is a modifier in the natural history of ALD[5] and NAFLD[6-8]. In this meta-analysis, we found a clear association between PNPLA3 polymorphisms and the entire spectrum (steatosis/fatty liver, cirrhosis, and HCC) of liver disease in CHC patients.
It was previously reported that PNPLA3 polymorphisms were an independent predictor of more rapid fibrosis progression in patients with chronic hepatitis C[50]. The mechanism whereby rs738409 influences the development of fatty liver likely involves a decreased ability of the 148M PNPLA3 variant to regulate hepatic lipid metabolism[54]. It is not known whether the rs738409 SNP influences the steatogenic effect of HCV and the progression of CHC. However, if steatosis causes fibrosis progression in CHC, then it may be assumed the rs738409 SNP should also be associated with advanced fibrosis and HCC[40].
Like any other meta-analysis, our study had to face the possibility of publication bias. In order to minimize this possibility, and the subsequent overestimation of the true effect size due to negative study identification failure[55], we combined searches from PubMed/Medline, Embase and Cochrane with manual searches. Although we used procedures in agreement with current guidelines, we cannot formally rule out the possibility that we overlooked studies that were not accessible[55]. Another limitation of this meta-analysis is the inclusion of case-control studies in which the potential for biases (e.g., selection and reporting) is higher when compared to randomized trials, and they are more inherent to confounding factors. In contrary to the previous meta-analysis on PNPLA3 polymorphisms in alcoholic and non-alcoholic liver diseases that compared different genotypes[5-8], our current analysis used the recessive model when comparing GG genotype vs CC and CG genotypes, and the dominant model when comparing GG and CG genotypes related to the CC genotype. Finally, no pooled data were provided on steatohepatitis in chronic HCV patients as only one study had reported such an association[14], while the studies by Ezzikouri et al[44] and Yasui et al[48] either did not have biopsies performed or reported “necroinflammatory changes”. Lack of standard definition amongst these studies prevented pooling them together.
The PNPLA3 GG genotype was negatively associated with sustained virological response and early viral kinetics in patients receiving peginterferon and ribavirin[15]. Also, in patients with chronic hepatitis C who failed to achieve sustained virologic response following interferon-based therapy, IL28B and PNPLA3 were independent predictors of rapid fibrosis progression[50]. Tamaki et al[50] developed a fibrosis progression-score by combining IL28B and PNPLA3 genotypes and ALT values, which stratified patients into low, intermediate, and high-risk groups for fibrosis progression. However, this fibrosis progression score needs external validation. In the era of direct-acting antiviral therapy, the question that remains unanswered is whether or not PNPLA3 polymorphisms identify high-risk CHC patients that are responsive to new treatment regimens. In summary, this meta-analysis provides strong evidence for the association of PNPLA3 polymorphisms and the spectrum of liver disease in patients with CHC, beginning with fatty liver disease and extending as far as cirrhosis and even HCC in patients with CHC. Further studies on treatment response are needed in this group of patients who carry a higher risk for more rapidly progressive liver disease.
Hepatitis C virus (HCV) infection is one of the most important causes of chronic liver disease in the United States. About 27% of cases of cirrhosis and 25% of hepatocellular carcinoma (HCC) worldwide are secondary to HCV infection.
Given that the association between PNPLA3 polymorphism and liver disease spectrum in chronic hepatitis C (CHC) patients has not been consistent, especially for HCC and cirrhosis, the authors performed this meta-analysis to further examine the association of PNPLA3 polymorphisms with the predisposition to the entire spectrum of liver disease among patients with CHC.
In this meta-analysis, they found a clear association between PNPLA3 polymorphisms and the entire spectrum (steatosis/fatty liver, cirrhosis, and HCC) of liver disease in CHC patients.
This manuscript is very well designed; the authors did a great effort in selecting the articles to be included in the meta-analysis with a proper quality scoring of selected articles.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kohla MAS, Pekgoz M, Yang SS S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 794] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 2. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 838] [Cited by in RCA: 833] [Article Influence: 43.8] [Reference Citation Analysis (6)] |
| 3. | Labib HA, Ahmed HS, Shalaby SM, Wahab EA, Hamed EF. Genetic polymorphism of IL-23R influences susceptibility to HCV-related hepatocellular carcinoma. Cell Immunol. 2015;294:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Salameh H, Raff E, Erwin A, Seth D, Nischalke HD, Falleti E, Burza MA, Leathert J, Romeo S, Molinaro A. PNPLA3 Gene Polymorphism Is Associated With Predisposition to and Severity of Alcoholic Liver Disease. Am J Gastroenterol. 2015;110:846-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 6. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 756] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 7. | Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: a HuGE review and meta-analysis. Sci Rep. 2015;5:9284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Zhang L, You W, Zhang H, Peng R, Zhu Q, Yao A, Li X, Zhou Y, Wang X, Pu L. PNPLA3 polymorphisms (rs738409) and non-alcoholic fatty liver disease risk and related phenotypes: a meta-analysis. J Gastroenterol Hepatol. 2015;30:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Salameh H, Al Hanayneh M, Masadeh M, Nasseemuddin M, Matin T, Erwin A, singal A. PNPLA3 as a Genetic Determinant of Risk for and Severity of Non-alcoholic Fatty Liver Disease Spectrum. J Clin Trans Hepatol. 2016;4:175-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2718] [Article Influence: 123.5] [Reference Citation Analysis (3)] |
| 11. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2682] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 12. | Guyot E, Sutton A, Rufat P, Laguillier C, Mansouri A, Moreau R, Ganne-Carrié N, Beaugrand M, Charnaux N, Trinchet JC. PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J Hepatol. 2013;58:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 13. | Nischalke HD, Berger C, Luda C, Berg T, Müller T, Grünhage F, Lammert F, Coenen M, Krämer B, Körner C. The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis C cirrhosis. PLoS One. 2011;6:e27087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Petta S, Vanni E, Bugianesi E, Rosso C, Cabibi D, Cammà C, Di Marco V, Eslam M, Grimaudo S, Macaluso FS. PNPLA3 rs738409 I748M is associated with steatohepatitis in 434 non-obese subjects with hepatitis C. Aliment Pharmacol Ther. 2015;41:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Valenti L, Aghemo A, Stättermayer AF, Maggioni P, De Nicola S, Motta BM, Rumi MG, Dongiovanni P, Ferenci P, Colombo M. Implications of PNPLA3 polymorphism in chronic hepatitis C patients receiving peginterferon plus ribavirin. Aliment Pharmacol Ther. 2012;35:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] |
| 17. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 18. | Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 19. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 20. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48315] [Article Influence: 2100.7] [Reference Citation Analysis (4)] |
| 21. | Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. 2nd edition ed. London: BMJ Books 2005; 285-312. |
| 22. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12519] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 23. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 42300] [Article Influence: 1458.6] [Reference Citation Analysis (4)] |
| 24. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9466] [Article Influence: 364.1] [Reference Citation Analysis (0)] |
| 25. | Trim and Fill Analysis for ‘rma.uni’ Objects. Available from: http://handbook.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm. |
| 26. | Hamza S, Petit JM, Masson D, Jooste V, Binquet C, Sgro C, Guiu B, Bronowicki JP, Thieffin G, Di Martino V. PNPLA3 rs738409 GG homozygote status is associated with increased risk of hepatocellular carcinoma in cirrhotic patients. J Hepatol. 2012;S281-S282. [DOI] [Full Text] |
| 27. | Way M, McQuillin A, Gurling HMD, Morgan MY. The PNPLA3 I148M mutation significantly increases the risk of developing alcohol-related cirrhosis in alcohol-dependent individuals. J Hepatol. 2013;58:S563-S564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Dutta AK. Genetic factors affecting susceptibility to alcoholic liver disease in an Indian population. Ann Hepatol. 2013;12:901-907. [PubMed] |
| 29. | Nguyen-Khac E, Houchi H, Dreher M-L, Herpe Y-E, Naassila M. Is PNPLA3 polymorphism involved in severe acute alcoholic hepatitis. Hepatology. 2011;976A. |
| 30. | Bhatt SP, Nigam P, Misra A, Guleria R, Pandey RM, Pasha MA. Genetic variation in the patatin-like phospholipase domain-containing protein-3 (PNPLA-3) gene in Asian Indians with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2013;11:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Burza MA, Pirazzi C, Maglio C, Sjöholm K, Mancina RM, Svensson PA, Jacobson P, Adiels M, Baroni MG, Borén J. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, Calí AM, Narayan D, Shaw MM, Pierpont B. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Zampino R, Pisaturo MA, Cirillo G, Marrone A, Macera M, Rinaldi L, Stanzione M, Durante-Mangoni E, Gentile I, Sagnelli E. Hepatocellular carcinoma in chronic HBV-HCV co-infection is correlated to fibrosis and disease duration. Ann Hepatol. 2015;14:75-82. [PubMed] |
| 35. | Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, Abu-Asab M, Orenstein A, Engle RE, Hu X. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis. 2015;60:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Mandorfer M, Payer BA, Schwabl P, Steiner S, Ferlitsch A, Aichelburg MC, Stättermayer AF, Ferenci P, Obermayer-Pietsch B, Grabmeier-Pfistershammer K. Revisiting liver disease progression in HIV/HCV-coinfected patients: the influence of vitamin D, insulin resistance, immune status, IL28B and PNPLA3. Liver Int. 2015;35:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Huang CF, Dai CY, Yeh ML, Huang CI, Tai CM, Hsieh MH, Liang PC, Lin YH, Hsieh MY, Yang HL. Association of diabetes and PNPLA3 genetic variants with disease severity of patients with chronic hepatitis C virus infection. J Hepatol. 2015;62:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Wong GL, Chan HL, Tse CH, Chan PO, Cheng JC, Cheng JS, Lau SH, Lee EK, Ma JM, Chan AW. Impact of IL28B and PNPLA3 polymorphisms on treatment outcomes in patients infected with genotype 6 hepatitis C virus. J Gastroenterol Hepatol. 2015;30:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Cai T, Dufour JF, Muellhaupt B, Gerlach T, Heim M, Moradpour D, Cerny A, Malinverni R, Kaddai V, Bochud M. Viral genotype-specific role of PNPLA3, PPARG, MTTP, and IL28B in hepatitis C virus-associated steatosis. J Hepatol. 2011;55:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, Dongiovanni P, Maggioni M, Fracanzani AL, Rametta R. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | Trépo E, Pradat P, Potthoff A, Momozawa Y, Quertinmont E, Gustot T, Lemmers A, Berthillon P, Amininejad L, Chevallier M. Impact of patatin-like phospholipase-3 (rs738409 C& gt; G) polymorphism on fibrosis progression and steatosis in chronic hepatitis C. Hepatology. 2011;54:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Corradini SG, Burza MA, Molinaro A, Romeo S. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology. 2011;53:1776; author reply 1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 43. | Valenti L, Aghemo A, Stättermayer AF. Interaction between IL28B and PNPLA3 genotypes in the pathogenesis of steatosis in chronic hepatitis C non genotype-3 patients. J Hepatol. 2012;56:1209-1210; author reply 1210-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Ezzikouri S, Alaoui R, Tazi S, Nadir S, Elmdaghri N, Pineau P, Benjelloun S. The adiponutrin I148M variant is a risk factor for HCV-associated liver cancer in North-African patients. Infect Genet Evol. 2014;21:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Stättermayer AF, Rutter K, Beinhardt S, Wrba F, Scherzer TM, Strasser M, Hofer H, Steindl-Munda P, Trauner M, Ferenci P. Role of FDFT1 polymorphism for fibrosis progression in patients with chronic hepatitis C. Liver Int. 2014;34:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Ampuero J, Del Campo JA, Rojas L, García-Lozano JR, Solá R, Andrade R, Pons JA, Navarro JM, Calleja JL, Buti M. PNPLA3 rs738409 causes steatosis according to viral & amp; IL28B genotypes in hepatitis C. Ann Hepatol. 2014;13:356-363. [PubMed] |
| 47. | Sato M, Kato N, Tateishi R, Muroyama R, Kowatari N, Li W, Goto K, Otsuka M, Shiina S, Yoshida H. Impact of PNPLA3 polymorphisms on the development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Hepatol Res. 2014;44:E137-E144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Yasui K, Kawaguchi T, Shima T, Mitsuyoshi H, Seki K, Sendo R, Mizuno M, Itoh Y, Matsuda F, Okanoue T. Effect of PNPLA3 rs738409 variant (I148 M) on hepatic steatosis, necroinflammation, and fibrosis in Japanese patients with chronic hepatitis C. J Gastroenterol. 2015;50:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Nakaoka K, Hashimoto S, Kawabe N, Nitta Y, Murao M, Nakano T, Shimazaki H, Kan T, Takagawa Y, Ohki M. PNPLA3 I148M associations with liver carcinogenesis in Japanese chronic hepatitis C patients. Springerplus. 2015;4:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Tamaki N, Kurosaki M, Higuchi M, Takada H, Nakakuki N, Yasui Y, Suzuki S, Tsuchiya K, Nakanishi H, Itakura J. Genetic Polymorphisms of IL28B and PNPLA3 Are Predictive for HCV Related Rapid Fibrosis Progression and Identify Patients Who Require Urgent Antiviral Treatment with New Regimens. PLoS One. 2015;10:e0137351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Huang CF, Chen JJ, Yeh ML, Huang CI, Hsieh MY, Yang HL, Dai CY, Huang JF, Lin ZY, Chen SC. PNPLA3 genetic variants determine hepatic steatosis in non-obese chronic hepatitis C patients. Sci Rep. 2015;5:11901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Petta S, Maida M, Grimaudo S, Pipitone RM, Macaluso FS, Cabibi D, Cammà C, Di Marco V, Sferrazza S, Craxì A. TM6SF2 rs58542926 is not associated with steatosis and fibrosis in large cohort of patients with genotype 1 chronic hepatitis C. Liver Int. 2016;36:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Ali M, Yopp A, Gopal P, Beg MS, Zhu H, Lee W, Singal AG. A Variant in PNPLA3 Associated With Fibrosis Progression but not Hepatocellular Carcinoma in Patients With Hepatitis C Virus Infection. Clin Gastroenterol Hepatol. 2016;14:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706-6715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 503] [Article Influence: 29.6] [Reference Citation Analysis (2)] |
| 55. | Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 715] [Article Influence: 27.5] [Reference Citation Analysis (0)] |