Published online Dec 8, 2016. doi: 10.4254/wjh.v8.i34.1489
Peer-review started: May 4, 2016
First decision: July 4, 2016
Revised: August 6, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 8, 2016
Processing time: 221 Days and 4.1 Hours
Ischemia-reperfusion injury (IRI) continues to be a major contributor to graft dysfunction, thus supporting the need for therapeutic strategies focused on minimizing organ damage especially with growing numbers of extended criteria grafts being utilized which are more vulnerable to cold and warm ischemia. Nitric oxide (NO·) is highly reactive gaseous molecule found in air and regarded as a pollutant. Not surprising, it is extremely bioactive, and has been demonstrated to play major roles in vascular homeostasis, neurotransmission, and host defense inflammatory reactions. Under conditions of ischemia, NO· has consistently been demonstrated to enhance microcirculatory vasorelaxation and mitigate pro-inflammatory responses, making it an excellent strategy for patients undergoing organ transplantation. Clinical studies designed to test this hypothesis have yielded very promising results that includes reduced hepatocellular injury and enhanced graft recovery without any identifiable complications. By what means NO· facilitates extra-pulmonary actions is up for debate and speculation. The general premise is that they are NO· containing intermediates in the circulation, that ultimately mediate either direct or indirect effects. A plethora of data exists explaining how NO·-containing intermediate molecules form in the plasma as S-nitrosothiols (e.g., S-nitrosoalbumin), whereas other compelling data suggest nitrite to be a protective mediator. In this article, we discuss the use of inhaled NO· as a way to protect the donor liver graft against IRI in patients undergoing liver transplantation.
Core tip: Our manuscript assesses the basic and clinical literature of nitric oxide and liver transplantation and creates a scientific/clinical justification for its routine use.
- Citation: Fukazawa K, Lang JD. Role of nitric oxide in liver transplantation: Should it be routinely used? World J Hepatol 2016; 8(34): 1489-1496
- URL: https://www.wjgnet.com/1948-5182/full/v8/i34/1489.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i34.1489
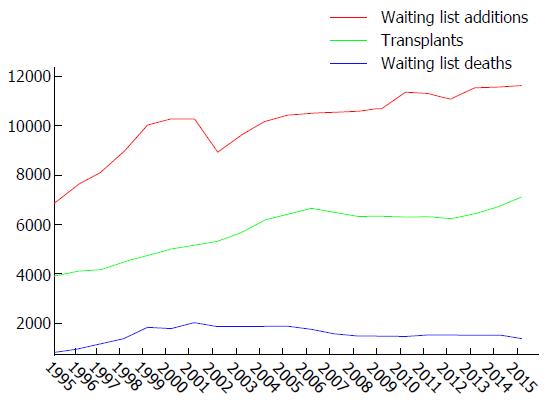
Liver transplantation has become a viable treatment option for recipients suffering from irreversible liver failure for more than three decades. However, the number of recipients on the waiting list continues to grow due to the major mismatch between organ supply and demand, creating tremendous pressure on for the development of techniques to expand the donor pool. There is about 7000 liver transplants performed annually with a trend that in increasing due to demand. As a result, according to national transplant registry database from the Organ Procurement and Transplant Network, about 1000 potential recipients on the waiting list die annually (Figure 1). Therefore, strategies are actively being sought to increase in donor pool. The transplant community has evaluated an option to relax the standard for donors to include donors with suboptimal quality [more damage from anoxic preservation and ischemia-reperfusion injury (IRI)], including the advanced age donor, prolonged cold and warm ischemia time, and hepatic steatosis. Currently, extended criteria donors make up 5%-10% of all donors and this number is increasing. IRI to the liver remains a significant contributor to graft dysfunction, or primary non-function, resulting in increased intensive care unit and hospital stay, increase financial burden, re-transplantation and, in a worst case scenario, death[1].
Nitric oxide (NO·) is an important endogenously produced biological mediator affecting vascular function, metabolic function and host defense mechanisms[2]. It is produced by macrophages, dendritic cells and plays a critical role in host innate and adaptive immunological processes[3,4]. Inhaled NO· has been clinically used to treat pulmonary hypertension due to its vasodilating effect in pulmonary microcirculation without causing any unfavorable systemic hemodynamic changes. More recent evidence has suggested a relative NO· deficiency due to IRI and that the use of preemptive inhaled NO· can attenuate liver IRI during liver transplantation.
Liver graft injury from ischemia-reperfusion is the principal mechanism of liver injury related to procedures involving clamping of hepatic inflow such as hepatectomy and liver transplantation. Cessation of oxygen delivery to sinusoidal microcirculation causes severe ATP depletion which leads to retraction of sinusoidal endothelial cells and Kuppfer cells, bleb formation in the microcirculation[5]. There is obstruction of sinusoid and microcirculatory disturbance due to bleb formation as well as accumulation of leukocytes and platelets leads to prolonged ischemia of hepatocytes, so-called, “no-flow phenomenon”. In addition, due to the upregulation and generation of inflammatory mediators such as oxygen free radicals, cytokines and chemokines from the Kuppfer cells occupying sinusoid there is both a local and systemic inflammatory response after reperfusion[6]. Therefore, IRI of the liver is generally believed to cause severe hepatocellular injury as well as extrahepatic organ inflammation and injury that contributes to perioperative morbidity and mortality.
Reductions of NO· during ischemia-reperfusion of liver aggregates liver injury in both animals and humans[7,8]. In fact, decreased hepatic production of NO· from endothelial nitric oxide synthase or endothelial nitric oxide synthase (eNOS) (responsible for the constitutive production of NO·) within 60 min after reperfusion in human liver transplantation contributes to the ischemia-reperfusion graft injury[8]. In addition to reduced production, NO· is inactivated from the reactions with reactive oxygen species, such as superoxide radical leading to reduced bioavailability[9,10]. As a consequence, reduced NO· bioavailability leads to apoptosis, leukocyte adhesion, increase microcirculatory resistance, and mitochondrial dysfunction[10]. Not surprisingly, the restoration of NO· concentrations lessens liver ischemia injury via reversing the most of the previously mentioned adverse actions. Additional studies support the finding that eNOS is essential for reduction liver graft injury during liver ischemia-reperfusion. Injury to the liver was decreased in the wild type when compared to mice where eNOS was knocked out. When eNOS expression was exogenously increased or NO· donors enhanced protection was realized[11,12]. In addition, established NO· concentrations resulting from inflammation are generally greater due to more robust inducible nitric oxide synthase (iNOS) expression. At the present time the role of iNOS in liver protection is not well known. In a rat liver ischemia-reperfusion model, iNOS enzyme activity was significantly increased in parallel with increased iNOS mRNA expression after reperfusion, which suggests that induction of iNOS has an important role in liver ischemia-reperfusion[13]. Counter to this observation, in a porcine ischemia-reperfusion model, is that portal injection of aminoguanidine, a selective iNOS inhibitor, decreased IRI[14]. Additionally, when iNOS was knocked out in mice and then exposed to warm liver ischemia-reperfusion, they incurred more injury when compared to wild types. While the injury was greater in the iNOS deficient animals, iNOS mRNA was also undetectable in the wild types. While iNOS is crucial in increasing net NO· concentrations and contributing to liver injury resulting from ischemia-reperfusion, further work is needed.
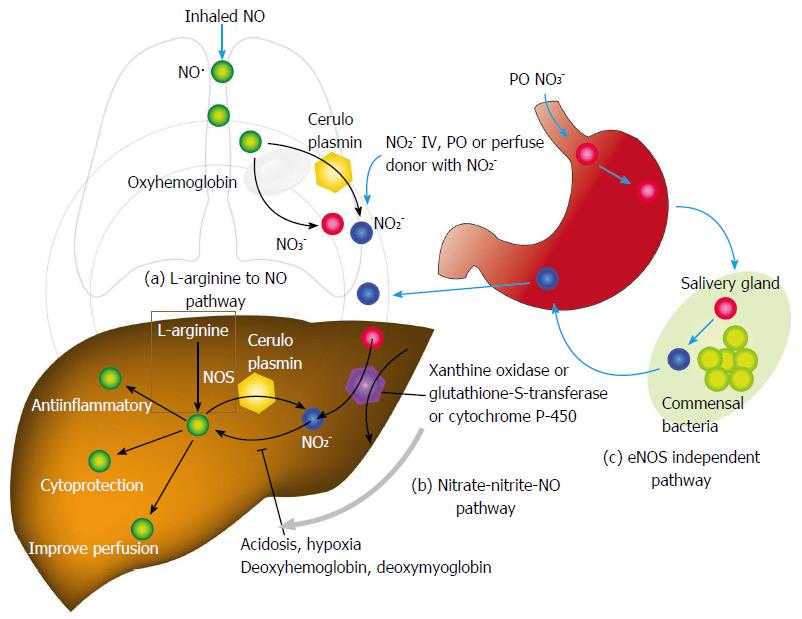
Additional hepatoprotective studies thought to due to endogenous NO· production have been published. Nitric oxide-mediated protection has been shown to inhibit apoptosis depending on concentration via inhibition of caspase proteases via S-nitrosylation[15] (Figure 2). Reduced compartmental concentrations of NO· inhibits apoptosis while increasing concentrations yields toxic reactive nitrogen species such as peroxynitrite or oxygen radicals that have shown to cause apoptosis and necrosis[16]. Other proposed mechanisms of how NO· protects include down-regulation of nuclear factor kappa B[17], mitochondrial complex I inhibition that is reversible, and reductions in mitochondrial calcium accumulation[18]. Controversy continues in regards to how NO· may mitigate inflammation and injury. In fact, Jaeschke et al[19] demonstrated that NOS inhibition did not contribute to hepatic injury at the time of reperfusion. Additionally, the inhibition of NO· did not influence neutrophil function as related to migration or adhesion[20]. Nonetheless, most evidence whether lab-based or clinical, demonstrates favorable effects of NO· during liver ischemia-reperfusion. It is difficult to reconcile the results, but no doubt diversity exists in the role of NO· in different cell types, as well as differing cellular compartmental NO· concentrations, timing of administration, and duration of NO· exposure. Also laboratory methods applied may have some role on this conflicting results.
Administration of inhaled NO· has demonstrated efficacy both in animal and human studies[21-25]. NO· inhalation decreases pulmonary and systemic vascular resistance with resultant improvements in tissue oxygenation increases in renal blood flow, and glomerular filtration rate[26-28]. Moreover, inhaled NO· has been demonstrated to exert extra-pulmonary or peripheral effects to the microvasculature as measured by enhanced perfusion and anti-inflammatory properties during post-reperfusion period[21,22,29,30]. Due to this seminal work, administration of inhaled NO· has undergone more extensive assessment as an anti-inflammatory therapy in humans subjected to predictable ischemia-reperfusion[23,30-32]. How extra-pulmonary effects of inhaled NO· remains unclear but generally it is believed in transformation of unstable NO· to relatively stable, NO·-containing intermediate upon entering in the circulation, and then recycled back to NO· at targeted remote location[31]. Study with a feline ischemia-reperfusion model suggested the intermediate molecule may be plasma S-nitrosothiols (e.g., S-nitrosoalbumin), while studies in both mice and humans points to nitrite as a possible intermediate[31,33,34]. A protective role for nitrite in ischemia-reperfusion is also demonstrated by direct administration of nitrite in murine hepatic ischemia-reperfusion models and together with the demonstration of nitrite conversion to NO· under ischemic location[33,35]. NO·-containing molecules in the blood that are labile under biological conditions and can also be formed with the inhalation of NO· (via nitrosylation or S-nitrosation reactions). These also includes S-nitrosothiols in the red blood cell, ferrous-nitrosylhemoglobin and C- or N-nitrosamines[31,36-38].
Specifically, inhaled NO· (80 ppm, co-administered with 50% oxygen and 50% nitrous oxide, approximately 5 h) was administered preemptively to healthy patients undergoing lower extremity surgery requiring approximately two hours of tourniquet-induced ischemia and continued until the completion of the surgery[32]. Administration of inhaled nitric oxide (80 ppm) reduces the expression of CD11b/CD18, P-selectin, and NF-κB. Also there are associated increase in plasma levels of nitrate/nitrite, and reduced oxidative stress. In this health cohort inhaled NO· administered at 80 ppm significantly reduced inflammation from ischemia-reperfusion of lower extremity. Therefore, under conditions of impaired NO· metabolism, inhaled NO· may be an effective therapy to replenish systemic NO·, thus mitigates injury. A subsequent randomized controlled clinical trial evaluated the effects of preemptive inhaled NO· inrecipients (n = 20) undergoing liver transplantation[39]. Again, inhaled NO· (80 ppm, approximately 4 h) vs placebo was randomly administered to the recipients after patients were anesthetized and stopped upon case completion. Patients who received inhaled NO· significantly demonstrated shorter hospital stay and enhanced recovery of graft function (alanine transaminase and aspartate aminotransferase, prothrombin time and activated partial thromboplastin time) by approximately 2-3 d when compared to the placebo group. The intraoperative transfusion of platelets was reduced by 50% in recipients who received inhaled NO·. As would be expected plasma nitrite levels were significantly increased in inhaled NO· group compare when compared to placebo. Commonly cited untoward side effects such as the formation of critical levels of met hemoglobin, nitrogen dioxide or bleeding complications were not observed. Lang et al[39] then compared a placebo group of patients who received 80 ppm of inhaled NO· during the operative phase of liver transplantation. Patients receiving NO· had better allograft function and reduced hepatobiliary complications 9 mo following the initial operation. Also, inhaled NO· significantly increased concentrations of serum nitrate, nitrite, and nitrosylhemoglobin. Consistent with previous data, nitrite was hypothesized to be critical to the findings of allograft protection. No adverse metabolic or hematologic effects were observed between groups. Mean costs of inhaled NO· was $1020 per transplantation. Use of inhaled NO· has also been tested in models of a non-heart beating donor model and steatotic liver, and demonstrated the injury attenuation and enhanced microcirculatory perfusion[40,41]. These studies are in line with other studies demonstrating inhaled NO· mitigates injury when utilized prior to predictable IRI. Replenishing NO· maybe more important in extended criteria donors which appear even susceptible to ischemia (cold and warm)-reperfusion.
Methemoglobinemia is a well-documented side effect of high dose supplemental inhaled NO·. Methemoglobin (MetHb) is rapidly formed by the oxidation of nitrosylhemoglobin, which is a byproduct from the binding of NO· to hemoglobin. This has been shown to occur in a dose and time-dependent fashion. MetHb has a fewer hemes to bind oxygen despite that methemoglobin has a higher affinity to oxygen compared to hemoglobin (1500 times higher affinity compared to carbon monoxide)[42], thus diminishing the oxygen carrying capacity of the blood. MetHb level of 10% of total hemoglobin cause clinically apparent cyanosis, and MetHb level of 35% cause headache, weakness, and dyspnea, and MetHb level of more than 70% are fatal. As aforementioned, clinically significant methemoglobinemia has not been reported when low-dose inhaled NO· is used. Only few case reports of methemoglobinemia has been reported when inhaled NO· was used in high dose (> 80 ppm)[43-45]. However it is worth mentioning that two cases of methemoglobinemia associated with low dose inhaled NO· due to delivery failure have been reported[46]. In both cases, methemoglobin reductase levels were confirmed to be normal, excluding of heredity methemoglobin reductase deficiency. Authors have speculated that variable (phasic) main flow provided from mechanical ventilator caused periodical accumulation of NO· in the inspiratory limb of airway circuit, leading to variable inhaled NO· concentration. Incorporated slow-response chemiluminescent analyzer was unable to detect this fluctuation of inhaled NO·. This fluctuation of NO· was also shown in lung model. Yamaguchi et al[47] showed that inhaled NO· was more concentrated when it was administered more distally in the inspiratory limb of the circuit as well as administered with lower flow rates. They speculated that delivered NO· was diluted by backflow in the NO· tubing from the higher pressure in the circuit in the early inspiratory phase of ventilation. This concentrated NO· in NO· tubing was delivered in the early expiratory phase, leading to fluctuation in NO· concentration. Therefore, inhaled NO· treatment requires caution during administration and other form of supplementation of NO· may be favored in terms of avoiding life-threatening methemoglobinemia.
Other possible complication is the generation of cytotoxic oxidant, “peroxynitrite” by rapidly reacting with superoxide anion[48]. Peroxynitrite can induce lipid peroxidation and inhibit mitochondrial respiration[49,50]. Indeed lung damage has been reported after inhaled NO· administration[51]. Hydrogen gas discover to have an anti-oxidative effect by scavenging peroxynitrite and other hydroxyl radicals[52]. Hydrogen gas has been shown to ameliorate lipopolysaccharide-induced[53], ventilator-associated[54], and hyperoxia-induced acute lung injury[55]. Therefore co-administration of hydrogen gas has been investigated to enhance lung protection by NO·.
Underlying mechanisms of how inhaled NO· decreases injury remains speculative. Nitrite, an oxidative product of NO· metabolism, seems to play a protectant role, however[33,34,56-58]. Thus, consistent with this line of thinking, sodium nitrite has been shown to alleviate acute injury from ischemia-reperfusion in both murine heart (decreased myocardial infarct size) and liver (decrease apoptosis in hepatocytes)[33]. Nitrite-mediated protection seems to involve biochemical pathways that connect ischemia to nitrite reduction to NO· production, therefore exerting cytoprotection by multiple possible mechanisms. In a model of murine liver transplantation, harvested syngeneic liver grafts were perfused with Lactated Ringers, and University of Wisconsin solution, and sodium nitrite supplemented solution during cold preservation period. Several recipients were treated with or without nitrite via an intraperitoneal injection. Liver injury demonstrated by enzyme release was significantly mitigated with both nitrite-supplemented solutions. The protective role of nitrite against cold ischemic-induced injury was more robust with longer preservation periods. Cell morphology and architecture was better preserved with grafts treated with nitrite. Hepatocyte cell death/necrosis was significantly reduced in the nitrite supplemented liver grafts. Liver grafts with extended cold preservation times demonstrated both improved tissue histology and liver function after reperfusion when treated with either the nitrite-supplemented preservation solution or in just the nitrite-treated recipients. Surprisingly, combination treatment of both liver graft and recipient did not demonstrate protection. Further clinical studies in the use of inhalation of NO· or injection of NO· donors for extended criteria donor may have a large clinical impact given that there is a surge in use of extended criteria donors to expand donor pool and warrants further investigation.
Other potential route of NO· donor administration is per gastrointestinal tract. In fact, dietary intake of nitrate is major source of NO· donor in mammals[59]. Dietary nitrate is abundant in many vegetables and water. Ingested nitrate is absorbed from intestine. One quarter of absorbed nitrate is concentrated in saliva and metabolized to nitrite by commensal bacteria[60]. Inorganic nitrite is metabolized to NO· in the presence of gastric acid[61-63]. This production pathway of nitric oxide is independent of eNOS (eNOS - independent NO· production) and accounts for majority of nitrite and nitrate in mammalian body[62,63]. Absorbed nitrite, nitrate, or NO· from small intestine is directly delivered to liver through portal vein (Figure 2). Therefore, per oral administration of NO· donor can be a potential route of administration, especially post-transplant period.
Additional drugs that donate NO· have been thoroughly assessed[64-67]. Only two types of these drugs feasible to use clinically: Nitrates, and sodium nitroprusside. Nitrates, such as nitroglycerin are widely used to treat patients suffering from coronary ischemia and/or myocardial infarction due to the pronounced venodilatory effect that assists in reducing venous return and myocardial oxygen demand. Several formulations are available commercially including a slow release oral form, an ointment, a transdermal patch, a nebulizer, and lastly intravenous formulations. A major limitation of organic nitrates is tachyphylaxis due to sustained usage. Nitroglycerin releases NO·via the enzyme mitochondrial aldehyde dehydrogenase[68]. Sodium nitroprusside is another commercially available drug that is an NO· donor. Sodium nitroprusside’s release of NO· is complex and but its net effect is to significantly diminish mRNA expression of a few pro-inflammatory mediators that promote hepatic injury[12]. Lastly, enhanced eNOS up-regulation confers hepatoprotection during IRI and may allow for another therapeutic option. When agents that increase eNOS expression such as trimetazidine, 5-amino-4-imidazole carboxamide riboside or activated protein C, are added to liver preservation solutions, hepatic allograft protection is afforded[12].
IRI has been well characterized the liver especially as it relates to liver resections and liver transplantation. The contribution of NO· deficiency is a newer finding and may have a central role in the pathogenesis of this injury. Replenishing the liver with NO·via either by inhalation, inhaled or intravenous nitrate or via other donor drugs represents a pragmatic means of mitigating injury. Clinical studies incorporating inhaled NO· provide solid evidence in mitigating injury from IRI. Inhaled NO· has demonstrated repeated efficacy without any demonstrable metabolic or hematological toxicities. Costs of routine NO· administration during liver transplantation is negligible when the entire spectrum of care is considered. Therefore, NO· has a potential to be a good therapeutic option for organ resuscitation in liver transplantation, especially for the extended criteria (marginal quality) donors, but further investigation is still warranted for routine clinical use.
| 1. | de Rougemont O, Dutkowski P, Clavien PA. Biological modulation of liver ischemia-reperfusion injury. Curr Opin Organ Transplant. 2010;15:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4569] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 3. | Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Chen C, Lee WH, Zhong L, Liu CP. Regulatory T cells can mediate their function through the stimulation of APCs to produce immunosuppressive nitric oxide. J Immunol. 2006;176:3449-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83-95. [PubMed] |
| 6. | Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Köken T, Inal M. The effect of nitric oxide on ischemia-reperfusion injury in rat liver. Clin Chim Acta. 1999;288:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Varadarajan R, Golden-Mason L, Young L, McLoughlin P, Nolan N, McEntee G, Traynor O, Geoghegan J, Hegarty JE, O’Farrelly C. Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation. 2004;78:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 325] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Abe Y, Hines I, Zibari G, Grisham MB. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch Biochem Biophys. 2009;484:232-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980-H2986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Katsumi H, Nishikawa M, Yamashita F, Hashida M. Prevention of hepatic ischemia/reperfusion injury by prolonged delivery of nitric oxide to the circulating blood in mice. Transplantation. 2008;85:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Isobe M, Katsuramaki T, Kimura H, Nagayama M, Matsuno T, Yagihashi A, Hirata K. Role of inducible nitric oxide synthase on hepatic ischemia and reperfusion injury. Transplant Proc. 2000;32:1650-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol. 2005;38:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Kim PK, Billiar TR. Give me iNOS or give me death. Hepatology. 2001;34:436-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci USA. 2004;101:8841-8842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Jaeschke H, Schini VB, Farhood A. Role of nitric oxide in the oxidant stress during ischemia/reperfusion injury of the liver. Life Sci. 1992;50:1797-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 178] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Cannon RO, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379-H384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr Atheroscler Rep. 2004;6:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 691] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 27. | Takahashi Y, Kobayashi H, Tanaka N, Sato T, Takizawa N, Tomita T. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. Am J Physiol. 1998;274:H349-H357. [PubMed] |
| 28. | Troncy E, Francoeur M, Salazkin I, Yang F, Charbonneau M, Leclerc G, Vinay P, Blaise G. Extra-pulmonary effects of inhaled nitric oxide in swine with and without phenylephrine. Br J Anaesth. 1997;79:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 2102] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 30. | Gianetti J, Del Sarto P, Bevilacqua S, Vassalle C, De Filippis R, Kacila M, Farneti PA, Clerico A, Glauber M, Biagini A. Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg. 2004;127:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ng ES, Jourd’heuil D, McCord JM, Hernandez D, Yasui M, Knight D, Kubes P. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circulation Res. 2004;94:559-565. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Mathru M, Huda R, Solanki DR, Hays S, Lang JD. Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans. Anesthesiology. 2007;106:275-282. [PubMed] |
| 33. | Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 515] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 34. | Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, Kelm M, Wink DA, Espey MG. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 453] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Li W, Meng Z, Liu Y, Patel RP, Lang JD. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury. J Transplant. 2012;2012:635179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36:707-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 39. | Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 40. | Srinivasan PK, Yagi S, Doorschodt B, Nagai K, Afify M, Uemoto S, Tolba R. Impact of venous systemic oxygen persufflation supplemented with nitric oxide gas on cold-stored, warm ischemia-damaged experimental liver grafts. Liver Transpl. 2012;18:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Nagai K, Yagi S, Afify M, Bleilevens C, Uemoto S, Tolba RH. Impact of venous-systemic oxygen persufflation with nitric oxide gas on steatotic grafts after partial orthotopic liver transplantation in rats. Transplantation. 2013;95:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Gibson QH, Roughton FJ. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957;136:507-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 300] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Nakajima W, Ishida A, Arai H, Takada G. Methaemoglobinaemia after inhalation of nitric oxide in infant with pulmonary hypertension. Lancet. 1997;350:1002-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Heal CA, Spencer SA. Methaemoglobinaemia with high-dose nitric oxide administration. Acta Paediatr. 1995;84:1318-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Hovenga S, Koenders ME, van der Werf TS, Moshage H, Zijlstra JG. Methaemoglobinaemia after inhalation of nitric oxide for treatment of hydrochlorothiazide-induced pulmonary oedema. Lancet. 1996;348:1035-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Taylor MB, Christian KG, Patel N, Churchwell KB. Methemoglobinemia: Toxicity of inhaled nitric oxide therapy. Pediatr Crit Care Med. 2001;2:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Yamaguchi N, Togari H, Suzuki S. During neonatal mechanical ventilatory support, the delivered nitric oxide concentration is affected by the ventilatory setting. Crit Care Med. 2000;28:1607-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 981] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 49. | Grisham MB, Jourd’Heuil D, Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol. 1999;276:G315-G321. [PubMed] |
| 50. | Liaudet L, Soriano FG, Szabó C. Biology of nitric oxide signaling. Crit Care Med. 2000;28:N37-N52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Hallman M, Bry K, Turbow R, Waffarn F, Lappalainen U. Pulmonary toxicity associated with nitric oxide in term infants with severe respiratory failure. J Pediatr. 1998;132:827-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1796] [Article Influence: 94.5] [Reference Citation Analysis (4)] |
| 53. | Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock. 2010;34:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Huang CS, Kawamura T, Peng X, Tochigi N, Shigemura N, Billiar TR, Nakao A, Toyoda Y. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem Biophys Res Commun. 2011;408:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Sun Q, Cai J, Liu S, Liu Y, Xu W, Tao H, Sun X. Hydrogen-rich saline provides protection against hyperoxic lung injury. J Surg Res. 2011;165:e43-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Gladwin MT. Nitrite as an intrinsic signaling molecule. Nat Chem Biol. 2005;1:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 440] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 58. | Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology. 2010;113:1460-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 485] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 60. | Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med. 2006;41:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 467] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Rocha BS, Gago B, Barbosa RM, Lundberg JO, Radi R, Laranjinha J. Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic Biol Med. 2012;52:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Gago B, Lundberg JO, Barbosa RM, Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic Biol Med. 2007;43:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Aiba M, Takeyoshi I, Ohwada S, Kawashima Y, Iwanami K, Sunose Y, Yamada T, Tsutsumi H, Matsumoto K, Morishita Y. Novel nitric oxide donor (FK409) ameliorates liver damage during extended liver resection with warm ischemia in dogs. J Am Coll Surg. 2001;193:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Kim YM, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 403] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Nilsson B, Delbro D, Wallin M, Friman S. Protective effect of nitric oxide and prostaglandin E(2) in ischemia/reperfusion injury of the liver. Transplant Proc. 2001;33:2518-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Yang SL, Lou YJ. Sodium nitroprusside decreased leukotriene C4 generation by inhibiting leukotriene C4 synthase expression and activity in hepatic ischemia-reperfusion injured rats. Biochem Pharmacol. 2007;73:724-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Katsumi H, Nishikawa M, Yasui H, Yamashita F, Hashida M. Prevention of ischemia/reperfusion injury by hepatic targeting of nitric oxide in mice. J Control Release. 2009;140:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boucek CD, Okumura T, Samhan-Arias AK, Zhao Q S- Editor: Qi Y L- Editor: A E- Editor: Li D