Published online Nov 28, 2016. doi: 10.4254/wjh.v8.i33.1471
Peer-review started: June 7, 2016
First decision: August 10, 2016
Revised: August 21, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 28, 2016
Processing time: 172 Days and 11.1 Hours
To investigate serum urokinase-type plasminogen activator receptor (uPAR) and liver stiffness in biliary atresia (BA) and examine the correlation of circulating uPAR, liver stiffness, and clinical outcomes in postoperative BA children.
Eighty-five postKasai BA children and 24 control subjects were registered. Circulating uPAR was measured using enzyme-linked immunosorbent essay. Liver stiffness was analyzed using transient elastography.
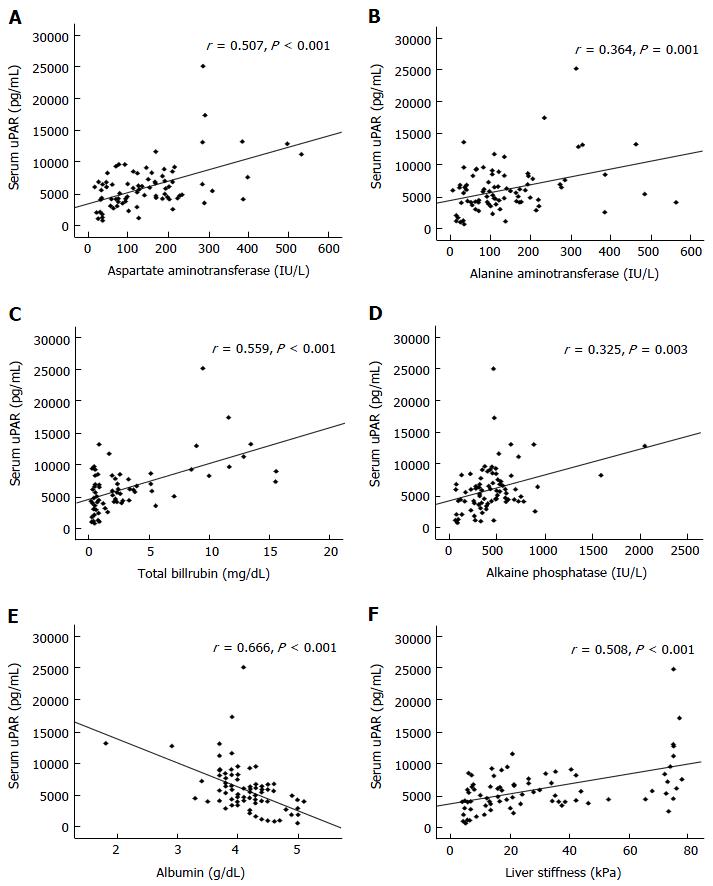
BA children had significantly greater circulating uPAR and liver stiffness scores than control subjects (P < 0.001). Circulating uPAR and liver stiffness were substantially higher in jaundiced BA children than non-jaundiced BA children (P < 0.001). In addition, circulating uPAR was positively associated with serum aspartate aminotransferase (r = 0.507, P < 0.001), alanine aminotransferase (r = 0.364, P < 0.001), total bilirubin (r = 0.559, P < 0.001), alkaline phosphatase (r = 0.325, P < 0.001), and liver stiffness scores (r = 0.508, P < 0.001).
Circulating uPAR and liver stiffness values were greater in BA children than healthy controls. The increased circulating uPAR was associated with liver dysfunction in BA. As a consequence, serum uPAR and liver stiffness may be used as noninvasive biomarkers indicating the progression of liver fibrosis in postKasai BA.
Core tip: Urokinase plasminogen activator receptor (uPAR) is known to be a substantial factor in the etiopathogenesis of hepatic inflammation and liver fibrogenesis. This study is the first to show that circulating uPAR is more elevated in biliary atresia (BA) children than in control subjects, and that circulating uPAR is correlated with the degree of jaundice and liver fibrosis in biliary atresia. Elevated serum uPAR is positively correlated with the severity of liver stiffness in postKasai BA children. Hence, serum uPAR could be used as a biological parameter indicating the progression and prognosis of liver fibrosis in BA children.
- Citation: Udomsinprasert W, Honsawek S, Jirathanathornnukul N, Chongsrisawat V, Poovorawan Y. Elevation of serum urokinase plasminogen activator receptor and liver stiffness in postoperative biliary atresia. World J Hepatol 2016; 8(33): 1471-1477
- URL: https://www.wjgnet.com/1948-5182/full/v8/i33/1471.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i33.1471
Biliary atresia (BA) is a severe chronic cholestatic liver disease of unknown etiology in young infants. The estimated incidence of BA varies from 1 in 8000 to 1 in 20000 live births, with a high frequency in Asians[1]. Affected newborns exhibit evidence of biliary obstruction within the first few months of life. BA is manifested by impaired liver function and fibroinflammatory obliterative cholangiopathy of both intrahepatic and extrahepatic bile ducts[2,3]. Extrahepatic BA is the most common form of ductal cholestasis. BA patients initially develop neonatal jaundice due to hepatic cholestasis and progress to hepatic fibrosis, which result in biliary cirrhosis[1-3]. Even though no medical therapies exist, sequential treatment strategy involving surgical Kasai portoenterostomy and liver transplantation is the only option for the most affected children. Nonetheless the precise pathogenesis of BA has yet to be determined, a number of theories regarding the etiology of BA include toxin exposure, virus-mediated inflammation, abnormal inflammatory response, defective morphogenesis, genetic mutation, and immunological dysregulation[4].
Urokinase-type plasminogen activator receptor (uPAR, CD87) is a cellular membrane receptor that attachs to urokinase-type plasminogen activator (uPA) with high affinity, through promoting the pericellular activation of plasminogen[5]. The involvement of uPA, its receptor (uPAR), and plasminogen activator inhibitor-1 (PAI-1) in regulation of cell adhesion, migration, proliferation, differentiation, and cell survival has recently demonstrated[6]. uPAR is expressed by a wide range of immune cells and endothelial cells, which contribute to the etiopathogenesis of hepatic inflammation and liver fibrogenesis[7,8]. Once inflammation is activated, uPAR is released from the cell membrane by proteolytic enzymes to produce soluble uPAR[9]. In recent years, previous studies have investigated that elevated circulating uPAR levels have been observed in acute liver failure, chronic liver diseases, and nonalcoholic fatty liver diseases [10-12].
It has been previously shown that certain cytokines and growth factors play possible parts in the etiopathology of biliary atresia[13-16]. The measurements on circulating uPAR and liver stiffness of BA have never been documented. We hypothesized that circulating uPAR and liver stiffness could be more elevated in BA patients than in control subjects and circulating uPAR would be associated with the disease severity and clinical outcomes in postKasia biliary atresia. Hence, the purpose of the current research is to determine circulating uPAR and liver stiffness measurements and to investigate the plausible correlation of circulating uPAR, liver stiffness, and clinical outcomes in postoperative biliary atresia children.
The present study was approved by the Institutional Review Board on Human Research of the Faculty of Medicine, Chulalongkorn University, and was conducted in compliance with the ethical guidelines of the Declaration of Helsinki. All parents of children were informed of the study’s purpose and of any interventions involved in the current study. Written informed consent was derived from the parents prior to the subjects entering the study.
Eighty-five BA children (39 girls and 46 boys with mean age of 9.0 ± 0.6 years) and 24 normal control subjects (11 girls and 13 boys with mean age of 8.5 ± 0.5 years) were enrolled in the study. None of them had undergone liver transplantation. Healthy controls attending the Well Baby Clinic at our institution for vaccination had normal physical findings and no underlying disease. BA children were classified into two groups according to their serum total bilirubin (TB): Non-jaundiced BA children (TB < 2 mg/dL, n = 46) and persistent jaundiced BA children (TB ≥ 2 mg/dL, n = 39).
Samples of peripheral venous blood were collected from every participant, and were kept at -80 °C for subsequent measurement. The quantitative assessment of serum uPAR was performed by using commercially available enzyme-linked immunosorbent essay (Quantikine, R and D Systems, Minneapolis, MN, United States). According to the manufacturer’s protocol, recombinant human uPAR standards and serum samples were added into each well, which has been pre-coated with specific antibody to uPAR. After incubating for 2 h at room temperature, every well was washed thoroughly with wash buffer. Then, uPAR conjugate was pipetted into each well and incubated for 2 h at room temperature. After 4 washes, substrate solution was added into the wells and the microplate was incubated for 30 min at room temperature with protection from light. Lastly, the reaction was stopped by the stop solution and the optical density was determined using an automated microplate reader at 450 nm. A standard optical density-concentration curve was drawn for the determination of uPAR concentration. The liver function tests including serum albumin,TB, direct bilirubin, aspatate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were measured using a Hitachi 912 (Roche Diagnostics, Basel, Switzerland) automated machine at the central laboratory of our hospital.
Transient elastography (Fibroscan, Echosens, Paris, France) measured the liver stiffness between 25 to 65 mm from the skin surface, which is approximately equivalent to the volume of a cylinder of 1 cm diameter and 4 cm length. The measurements were performed by placing a transducer probe of Fibroscan on the intercostal space at the area of the right lobe of the liver with patients lying in a dorsal decubitus position with maximum abduction of the right arm. The target location for measurement was a liver portion that was at least 6 cm thick, and devoid of major vascular structures. The measurements were performed until 10 validated results had been obtained with a success rate of at least 80%. The median value of 10 validated scores was considered the elastic modulus of the liver, and it was expressed in kilopascals (kPa).
Statistical analysis was executed by using the SPSS version 22.0 statistical software package (SPSS Inc., Chicago, IL, United States). Comparisons of demographic and clinical outcomes between groups were performed using χ2 and Student’s unpaired t-test when appropriate. Correlation between numerical data was obtained using Pearson’s correlation coefficient (r). Data were presented as mean ± SEM of the mean. A two-tailed P-value of less than 0.05 was taken to indicate statistical significance.
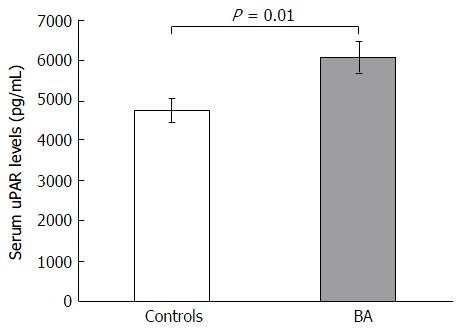
Eighty-five postoperative biliary atresia children and 24 ethnically matched unaffected volunteers were prospectively recruited in the current work. The baseline features of BA children and control subjects are presented in Table 1. There was no significant difference of age and gender between case and control groups. However, circulating uPAR values were substantially greater in BA children than in control subjects (6085.9 ± 400.7 pg/mL vs 4754.5 ± 294.9 pg/mL, P = 0.01) (Figure 1). Moreover, BA group had notably greater liver stiffness values than control group (28.7 ± 2.7 kPa vs 4.1 ± 0.2 kPa, P < 0.001).
| Variables | BA (n = 85) | Controls (n = 24) | P value |
| Age (yr) | 9.0 ± 0.6 | 8.5 ± 0.5 | 0.2 |
| Gender (female:male) | 39:46 | 11:13 | 0.4 |
| Albumin (g/dL) | 4.2 ± 0.1 | - | NA |
| Total bilirubin (mg/dL) | 2.7 ± 0.4 | - | NA |
| Direct bilirubin (mg/dL) | 2.3 ± 0.4 | - | NA |
| AST (IU/L) | 143.7 ± 11.9 | - | NA |
| ALT (IU/L) | 137.1 ± 12.5 | - | NA |
| ALP (IU/L) | 449.2 ± 34.0 | - | NA |
| Liver stiffness (kPa) | 28.7 ± 2.7 | 4.1 ± 0.2 | < 0.001 |
| uPAR (pg/mL) | 6085.9 ± 400.7 | 4754.5 ± 294.9 | 0.01 |
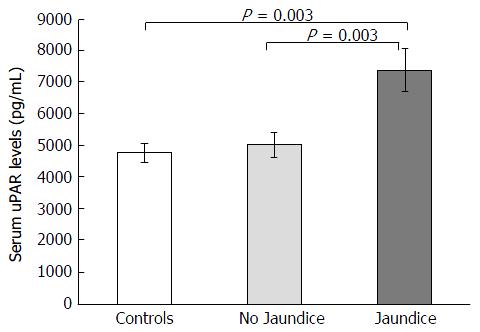
BA children were subdivided into jaundiced group (n = 39) and non-jaundiced group (n = 46). The clinical characteristics and biochemical features of patients according to jaundice status are illustrated in Table 2. Jaudiced BA children exhibited remarkably greater serum uPAR levels than non-jaundiced BA children (7373.5 ± 684.6 pg/mL vs 4994.2 ± 400.9 pg/mL, P = 0.003) (Figure 2). Furthermore, mean liver stiffness measurement of jaundiced BA group was greatly increased compared with that of non-jaundiced BA group (46.2 ± 3.7 kPa vs 13.9 ± 2.0 kPa, P < 0.001).
| Variables | BA patients with jaundice(n = 39) | BA patients without jaundice(n = 46) | P-value |
| Age (yr) | 9.5 ± 0.9 | 8.6 ± 0.9 | 0.4 |
| Gender (female:male) | 18:21 | 21:25 | 0.5 |
| Albumin (g/dL) | 3.8 ± 0.1 | 4.5 ± 0.1 | < 0.001 |
| Total bilirubin (mg/dL) | 5.1 ± 0.7 | 0.5 ± 0.1 | < 0.001 |
| Direct bilirubin (mg/dL) | 4.5 ± 0.6 | 0.2 ± 0.1 | < 0.001 |
| AST (IU/L) | 210.4 ± 17.2 | 84.7 ± 10.2 | < 0.001 |
| ALT (IU/L) | 195.9 ± 19.9 | 85.1 ± 10.7 | < 0.001 |
| ALP (IU/L) | 599.7 ± 52.8 | 313.0 ± 32.0 | < 0.001 |
| Liver stiffness (kPa) | 46.2 ± 3.7 | 13.9 ± 2.0 | < 0.001 |
| uPAR (pg/mL) | 7373.5 ± 684.6 | 4994.2 ± 400.9 | 0.003 |
Subsequent investigation revealed that circulating uPAR was directly associated with serum AST (r = 0.507, P < 0.001), ALT (r = 0.364, P < 0.001), TB (r = 0.559, P < 0.001), ALP (r = 0.325, P < 0.001), and liver stiffness values (r = 0.508, P < 0.001) in BA children (Figure 3). However, circulating uPAR concentration was negatively associated with serum albumin level (r = -0.666, P < 0.001) (Figure 3).
Biliary atresia is a chronic progressive fibroinflammatory liver disorder with mysterious etiology. The etiopathology of BA currently remains elusive and it seems that multiple factors may contribute to the development of BA. Yet today, Kasai operation has been proved as the most effective option of surgical treatment. Without surgery, children with biliary atresia will finally die due to biliary cirrhosis and liver failure[1]. Recently, circulating uPAR levels have been shown to be involved in chronic liver disorders, including chronic hepatitis B and C, liver cirrhosis, and hepatocellular carcinoma[17-20]. Based on our experience, there is no report about circulating uPAR and hepatic fibrosis in various degrees of postoperative biliary atresia.
The present study is the first to show that circulating uPAR and liver fibrosis values were significantly higher in children suffering from BA than in control subjects. Additionally, circulating uPAR in jaundiced BA children was markedly increased with respect to that in non-jaundiced BA children. Elevated circulating uPAR levels were directly associated with total bilirubin, AST, ALT, ALP in post Kasai BA children, suggesting that circulating uPAR is related to degree of jaundice BA children. Furthermore, the degree of jaundice is possibly linked to the severity of intrahepatic biliary obliteration. Both AST and ALT are extensively used as biochemical parameters of hepatic abnormality indicating liver cell injury. Hence, the findings imply that uPAR could have a plausible role in the mechanism of liver cell injury in postoperative biliary atresia, and it would be associated with the severity of bile duct obliteration.
The present investigation demonstrated that circulating uPAR was more pronounced in biliary atresia children than control subjects. In accordance with this observation, Sjöwall et al[10] reported that circulating uPAR was increased in subjects with non-alcoholic fatty liver disease and associated with the severity of fibrosis. Moreover, uPAR expressions in liver tissue samples have been documented in subjects with hepatocellular carcinoma as shown by Morita et al[21]. In addition, Zimmermann et al[12] reported that circulating uPAR was substantially elevated in subjects with chronic liver diseases compared with controls and were closely correlated with liver function and fibrosis.
In light of our findings, certain hypotheses could explain high circulating uPAR in jaundiced biliary atresia children. Firstly, the release of uPAR in the injured liver could be accountable for the increased circulating uPAR. Secondly, the elevation of circulating uPAR may be ascribed to the unbalance between uPAR synthesis and uPAR clearance. The reduction of uPAR destruction in BA children with liver fibrosis may lead to the elevated circulating uPAR. Decreased pre-systemic hepatic metabolism might explain the increased uPAR levels in serum BA children with hepatic dysfunction. Besides, other tissues outside the liver could synthesize and release uPAR into the blood. The rising serum level of uPAR is likely attributable to the results of hepatocellular injury and further liver fibrosis. Whether increment of serum uPAR in BA children indicates low destruction, high production, or both remain obscure. Additional research will be needed to clarify the molecular basis leading to increased circulating uPAR.
Several caveats need to be acknowledged in this study. First, relatively small sample size of enrolled subjects limits the statistical power of our findings. Second, the cross-sectional study precludes definite information regarding causal relationships. In addition, inadequate assessment of various confounders such as comorbidity must be considered. To address these challenges, future studies should collect prospective measurements of these data to preclude bias and reverse causation. Moreover, the present investigation was restricted to the subjects under follow-up at our institution. Accordingly, our results may not be generalized across different populations. Finally, hepatic expression of uPAR has not been investigated. Further studies on immunohistochemistry of uPAR from liver tissues might provide better knowledge on molecular mechanisms of uPAR in biliary atresia.
To sum up, our study illustrated that circulating uPAR and liver stiffness measurement were markedly higher in biliary atresia children than in control subjects. Circulating uPAR was more elevated in jaundiced BA children compared to non-jaundiced BA children. Furthermore, elevated serum uPAR was correlated with hepatic dysfunction and outcome parameters. Circulating uPAR and liver stiffness values might be used as noninvasive biological markers indicating the progression and prognosis of hepatic fibrosis in postoperative biliary atresia children. Although underlying mechanisms of the cause and effect relationships remain elusive, there is abundant room for the definite role of uPAR in the etiopathogenesis of hepatic fibrosis in BA.
The authors thank the Thailand Research Fund (RSA5880019), the Research Chair Grant from the National Science and Technology Development Agency, and the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship to WU, National Research University Project, through the Ageing Cluster (NRU59-056-AS), Chulalongkorn University.
Biliary atresia (BA) is a severe chronic cholestatic liver disease of unknown etiology in young infants. The exact pathogenesis of BA remains a matter of debate. Circulating urokinase plasminogen activator receptor (uPAR) has arisen as a promising biochemical marker of certain disorders, such as liver injury and fibrosis. Although recent reports suggest a potential applicability for the measurement of circulating uPAR in liver fibrosis, the assessments on circulating uPAR and liver stiffness of BA have never been documented.
Recent evidences demonstrate the significance of urokinase plasminogen activator receptor in hepatitis, liver fibrosis, and liver failure. The current study shows that circulating uPAR levels are more elevated in BA children than in control subjects. Moreover, uPAR level is correlated with liver stiffness, and clinical outcomes in postoperative BA.
BA children exhibited significantly higher circulating uPAR and liver stiffness values than control subjects. Circulating uPAR and liver stiffness values were more pronounced in jaundiced BA children than in non-jaundiced BA children. Additionally, elevated circulating uPAR levels were associated with hepatic dysfunction and clinical outcomes.
Increased circulating uPAR and liver stiffness values were was associated with hepatocellular dysfunction in postKasai children affected with BA. As a consequence, circulating uPAR and liver stiffness measurements could be used as noninvasive biological markers indicating the progression and prognosis of liver fibrogenesis in BA children.
uPAR also known as CD87, is a multidomain membrane protein that has a role in the regulation of cell migration, proliferation, and survival and is expressed by diverse immune cells and endothelial cells, which contribute to the etiopathogenesis of hepatic inflammation and liver fibrogenesis.
Great paper that needs to be published. uPAR is known to be a substantial factor in the etiopathogenesis of hepatic inflammation and liver fibrogenesis.
| 1. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 669] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 2. | Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Erlichman J, Hohlweg K, Haber BA. Biliary atresia: how medical complications and therapies impact outcome. Expert Rev Gastroenterol Hepatol. 2009;3:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | A-Kader HH, Abdel-Hameed A, Al-Shabrawi M, Mohsen N, El-Karaksy H, Hassanein B, Elsayed B, Abdel-Khalik MK, Karjoo M. Is biliary atresia an autoimmune disease? Eur J Gastroenterol Hepatol. 2003;15:447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Dear AE, Medcalf RL. The urokinase-type-plasminogen-activator receptor (CD87) is a pleiotropic molecule. Eur J Biochem. 1998;252:185-193. [PubMed] |
| 6. | Blasi F. uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today. 1997;18:415-417. [PubMed] |
| 7. | Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Zhang LP, Takahara T, Yata Y, Furui K, Jin B, Kawada N, Watanabe A. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J Hepatol. 1999;31:703-711. [PubMed] |
| 9. | Zimmermann HW, Reuken PA, Koch A, Bartneck M, Adams DH, Trautwein C, Stallmach A, Tacke F, Bruns T. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J Intern Med. 2013;274:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sjöwall C, Martinsson K, Cardell K, Ekstedt M, Kechagias S. Soluble urokinase plasminogen activator receptor levels are associated with severity of fibrosis in nonalcoholic fatty liver disease. Transl Res. 2015;165:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Koch A, Zimmermann HW, Gassler N, Jochum C, Weiskirchen R, Bruensing J, Buendgens L, Dückers H, Bruns T, Gerken G. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014;34:1330-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Zimmermann HW, Koch A, Seidler S, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012;32:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Udomsinprasert W, Honsawek S, Anomasiri W, Chongsrisawat V, Vejchapipat P, Poovorawan Y. Elevated adiponectin is associated with poor outcome in children with biliary atresia. Asian Biomedicine. 2012;6:369-376. [DOI] [Full Text] |
| 14. | Honsawek S, Chayanupatkul M, Chongsrisawat V, Vejchapipat P, Poovorawan Y. Increased osteopontin and liver stiffness measurement by transient elastography in biliary atresia. World J Gastroenterol. 2010;16:5467-5473. [PubMed] |
| 15. | Chayanupatkul M, Honsawek S, Vejchapipat P, Chongsrisawat V, Poovorawan Y. Elevated serum bone morphogenetic protein 7 levels and clinical outcome in children with biliary atresia. Eur J Pediatr Surg. 2009;19:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Honsawek S, Chongsrisawat V, Vejchapipat P, Thawornsuk N, Poovorawan Y. Association of serum levels of tissue inhibitors of metalloproteinase-1 with clinical outcome in children with biliary atresia. Asian Pac J Allergy Immunol. 2006;24:161-166. [PubMed] |
| 17. | Berres ML, Schlosser B, Berg T, Trautwein C, Wasmuth HE. Soluble urokinase plasminogen activator receptor is associated with progressive liver fibrosis in hepatitis C infection. J Clin Gastroenterol. 2012;46:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Filik L. Soluble urokinase plasminogen activator receptor in chronic hepatitis due to hepatitis C virus. J Clin Gastroenterol. 2012;46:346-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Chounta A, Ellinas C, Tzanetakou V, Pliarhopoulou F, Mplani V, Oikonomou A, Leventogiannis K, Giamarellos-Bourboulis EJ. Serum soluble urokinase plasminogen activator receptor as a screening test for the early diagnosis of hepatocellular carcinoma. Liver Int. 2015;35:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Sevgi DY, Bayraktar B, Gündüz A, Özgüven BY, Togay A, Bulut E, Uzun N, Dökmetaş İ. Serum soluble urokinase-type plasminogen activator receptor and interferon-γ-induced protein 10 levels correlate with significant fibrosis in chronic hepatitis B. Wien Klin Wochenschr. 2016;128:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Morita Y, Hayashi Y, Wang Y, Kanamaru T, Suzuki S, Kawasaki K, Ohta K, Yamamoto M, Saitoh Y, Itoh H. Expression of urokinase-type plasminogen activator receptor in hepatocellular carcinoma. Hepatology. 1997;25:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fernandez-Pineda I S- Editor: Qi Y L- Editor: A E- Editor: Li D