Published online Nov 28, 2016. doi: 10.4254/wjh.v8.i33.1459
Peer-review started: May 31, 2016
First decision: July 20, 2016
Revised: August 4, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 28, 2016
Processing time: 178 Days and 11.8 Hours
To investigated in non-alcoholic-fatty-liver-disease (NAFLD), with ultrasound (US)-detected fatty liver, and in a group of non-alcoholic and otherwise healthy subjects, relationship of neglected features of lifestyle with NAFLD and obesity.
Five hundred and thirty-two NAFLD and 667 non-NAFLD healthy subjects, age 21-60 years were studied. Severity of liver steatosis was assessed by US bright liver score. The adherence to mediterranean diet score (AMDS) was assessed on the basis of a 1-wk recall computerized questionnaire which included a detailed physical activity reports (Baecke questionnaire). The western dietary profile score, as a simplified paradigm of unhealthy diet, a questionnaire quantifying sun exposure score and a sleep habits questionnaires provided a further comprehensive lifestyle assessment.
Body mass index (BMI), insulin resistance (HOMA), and triglycerides, poorer adherence to a mediterranean diet profile, sedentary habits, minor sun exposure and use of “western diet” foods are greater in NAFLD. Multiple linear regression analysis, weighted by years of age, displays BMI, HOMA and AMDS as the most powerful independent predictors of fatty liver severity; however, also the physical activity score, the western diet habit and the sun exposure score are acting inside the model with significant independent effects.
Articulated clinical intervention, according to our results, are justified in NAFLD and can be pursued addressing by focused intervention nutritional profile, physical exercise mainly in open-air subsets for enhancing sun exposure and healthier sleep duration and rhythm.
Core tip: Non-alcoholic-fatty-liver-disease (NAFLD) is a multifactorial condition associated with malnutrition and, mainly, with obesity, sedentary life and insulin resistance; some neglected factor, such as sleep and sun exposure curtailment, along with D vitamin deficiency, are associated with NAFLD; articulated clinical intervention, according to our results, is justified in NAFLD and can be pursued addressing by focused intervention nutritional profile, open-air physical exercise for enhancing sun exposure and healthy behaviour targeted to improved sleep duration and rhythm.
- Citation: Trovato FM, Martines GF, Brischetto D, Trovato G, Catalano D. Neglected features of lifestyle: Their relevance in non-alcoholic fatty liver disease. World J Hepatol 2016; 8(33): 1459-1465
- URL: https://www.wjgnet.com/1948-5182/full/v8/i33/1459.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i33.1459
Liver diseases, already in the past, were considered at least partly a consequence of unhealthy lifestyles and adverse environmental conditions, a concept that was very well addressed also by pathologists[1]. Lifestyle regards the use of the body functions related to physical exercise, exerted in work, love, leisure or sport, the quality and quantity of food, the sleep and rest rhythms, the exposure to hostile or unhealthy environmental factors, and other aspects, including fashion, clothing and non-sport leisure activity[2,3]. As in the past, the impact of the fashions and of beliefs based on alleged scientific statements and commercial information, namely publicity, is the key factor[4]. This framework, also by conditioning different lifestyles, reasonably affects the “establishment and maintenance of several diseases, including liver disease”[5]. In a very simplified manner today we tend to describe the lifestyles in medicine especially in terms of diet and physical inactivity or sedentary life, with a synergistic effect on body size - obesity - and on disease related with excessive food intake (atherosclerosis and liver disease)[6]. Marketing strategies focus much on some related aspects that have an influence on nutrition and physical activity, but also with trade repercussions, while neglecting and avoiding other modes of social behavior. Some of these factors, such as sleep duration[7,8], the sleeping patterns[9-12], including shift-work related effects[7], exposure to noise[13,14], the level of social alarm about events or situations[15], the possibility of urban mobility[16,17], may have determinant effects on nutritional profiles and exercise implementation. Communication and perception of risks, as traditionally recognized, are flanked by communication and induction of apparently neutral behavior that can behave as true risk factors for disease. The strong pressure towards practices aimed at optimizing physical fitness and dietary methods aimed at healthy foods often involves forms of orthorexia[18]; such strategies are widely used to gain and maintain niches of food and fitness markets. All this would be irrelevant, except that, as in the case of prevention of obesity and fatty liver, and probably also in the field of atherosclerotic, neurodegenerative and cancer diseases, dietary caloric intake and a sedentary lifestyle are not the only factors exerting independent synergistic effects[6]. In fact, even the dietary profiles[19], methods of exercise implementation[20,21], and other related factors, such as sleep deprivation[4], D vitamin deficiency and exposure to sunlight[22], environmental noise[16], and reasonably also others, seem to be part of an interrelated group of neglected risk factors, which only now are beginning to be studied more methodically.
Aim of our research is to investigate if some of the above mentioned neglected behavioural factors, concurrently with nutritional and physical exercise profile, may be associated or contribute independently as factors related to fatty liver in a group of non-alcoholic and otherwise healthy subjects with ultrasound (US)-detected fatty liver.
Five hundred thirty-two non-alcoholic-fatty-liver-disease (NAFLD) and 667 non-NAFLD subjects (women 749, men 450, total 1199), age 21-60 years, without relevant acute or chronic disease, as below detailed in the exclusion criteria, were studied. These patients were consecutively referred to the same out-patients public medical unit (day-hospital) for lifestyle-nutritional prescription addressed to the management of minor digestive disease (mainly gastro-esophageal reflux syndrome or irritable bowel syndrome), overweight or obesity. The subjects were enrolled throughout January 2008-December 2015, were all patients first-time visitors, had not had previous referral or intervention in our unit, and were studied by a comprehensive US assessment (liver-abdomen, heart, thyroid and lung), according to our current practice[3]. Exclusion criteria: (1) all patients with signs of moderate-severe congestive heart failure, previous myocardial infarction, idiopatic myocardiopathy, pericarditis, malignancies; (2) severe chronic liver disease, apart from the lone finding of bright liver; abnormal aminotransferase levels at the beginning of this study, defined as alanine transaminase (ALT) > 30 IU/L in men and ALT > 19 IU/L in women; acute or chronic virus hepatitis, which were excluded by concurrent laboratory assays, as below detailed; (3) any history of diabetes mellitus (fasting glucose ≥ 126 mg/dL or HbA1c ≥ 6.5%) or drug intake of anti-diabetic drugs, particularly metformin; (4) extreme obesity [body mass index (BMI) ≥ 40] and underweight bad-nourished profile (BMI < 18.5 or serum albumin < 3 g/dL); (5) acute and/or chronic infectious, rheumatic or autoimmune disease; and (6) alcohol abuse (exceeding 20 g/d on a weekly base); renal insufficiency, i.e., glomerular filtration rate < 90 mL/min per 1.73 m2 and/or proteinuria > 0.10 g/d. According to these exclusion criteria 1508 further subjects, potentially but only partially eligible, are excluded by this study.
The severity of liver steatosis was assessed by US bright liver score (BLS), graded 1-3: grade 0 was the absence of bright liver, i.e., a normal pattern[23], BLS was and previously validated by US-guided fine needle aspirate biopsy by 20 Gauge Menghini’s needles[3]; GE echo color Doppler machines (GE Logiq 5/Vivid7 Expert US manufactured by GE Medical Systems, Milwaukee, WI, United States), high resolution, equipped with real-time convex, phased array and linear scan transducers, were used throughout this study.
Routine laboratory tests included virus hepatitis (hepatitis A, B and C virus, i.e., HAV, HBV and HCV) and cancer biomarkers (Alpha-fetoprotein, CEA, Ca125, Ca 19-9, Ca15-3), thyroid hormones FT3 FT4, thyroid-stimulating hormone, aspartate aminotransferase, ALT, γ-glutamyl transpeptidase, ferritin, total protein, and albumin. Mediterranean diet adherence profile was assessed by the adherence to mediterranean diet score (AMDS) on the basis of a 1-wk recall computerized questionnaire[3,5] using a pictogram-based method of visualizing dietary intake, descriptive also of the size of the single portion; pictograms includes also items for the quantification of physical activity, which is otherwise quantified by detailed physical activity reports (Baecke questionnaire)[5]. The Western Dietary Profile score, as a simplified paradigm of unhealthy diet, was assessed submitting a specific questionnaire, which is reported in Appendix; also the Baecke’s physical activity questionnaire is briefly described in appendix, and subsequently the total score was studied by statistical analysis. The questionnaires submitted for quantifying sun exposure score, used mainly as an index of the open air activity and sleep habits questionnaires are routinely included within the context of a comprehensive lifestyle assessment, and detailed in appendix. The study and the manuscript were approved by the institutional review board of the project office. No conflict of interest is to be declared for this invited manuscript. Written informed consent was obtained from each patient prior to the clinical data recording and before the US procedure, allowing the use of information for teaching and clinical research. Detail that might disclose the identity of the subjects under study is carefully omitted in any part of the study.
Comparison of data between the two groups of patients, NAFLD vs controls, is reported and differences assessed by Student’s t test. Subsequently: (1) receiver operating characteristic (ROC) curve analysis of data of controls vs NAFLD subjects is used for defining the optimal cut-offs which may distinct the two group. The performance of each measure in prediction of NAFLD was evaluated by ROC curve. The area under the ROC curve and the 95%CI were used as indexes of accuracy. The optimal cut-off value was determined with maximum sum of sensitivity and specificity. For the purpose of identifying such thresholds, the measures used were BMI, HOMA, AMDS, western diet score (WDS), Physical activity Baecke’s total score, sun exposure score, and sleep daily hours, calculated on a weekly base; (2) contingency tables and odds ratio of NAFLS vs non-NAFLD were calculated, according to each defined cut-off; and (3) MLR analysis, weighted by age, using BMI, HOMA, AMDS, WDS, physical activity baecke’s total score, Sun exposure score, sleep hours vs BLS score of fatty liver was at last performed.
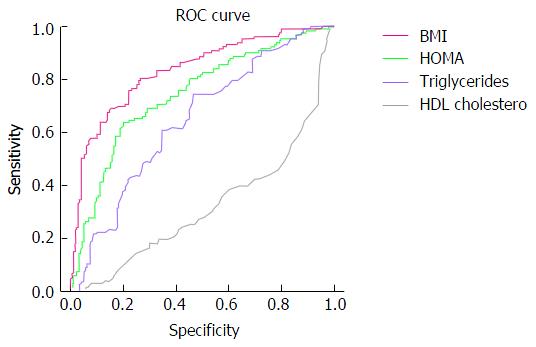
The two groups of patients were comparable for age (Table 1), while other measures, such as BMI, HOMA and Triglycerides are greater in NAFLD. Comparison of data between the two groups of patients, NAFLD vs controls, is reported in detail (Table 1): A poorer adherence to a mediterranean diet profile, greater sedentary habits and greater use of “western diet” foods are the main differences. Moreover, liver size and, obviously, detection of fatty liver are the main US feature distinctive of the two groups. The ROC curve analysis graph of the data of controls vs NAFLD subjects for BMI, HOMA, HDL Cholesterol is displayed in Figure 1.
| NAFLD (n = 532) | Controls (n = 667) | P vaule | |
| Age, yr | 48.11 ± 9.00 | 48.60 ± 8.70 | 0.343 |
| Systolic blood pressure (mmHg) | 124.53 ± 9.71 | 121.21 ± 10.80 | < 0.0001 |
| Diastolic blood pressure (mmHg) | 78.84 ± 6.72 | 76.50 ± 6.73 | < 0.0001 |
| BMI, kg/m2 | 30.49 ± 5.55 | 24.44 ± 3.72 | < 0.0001 |
| HOMA | 2.89 ± 1.76 | 1.80 ± 1.28 | < 0.0001 |
| eGFR | 82.49 ± 14.15 | 82.15 ± 17.44 | 0.714 |
| Total cholesterol, mg/dL | 205.17 ± 37.16 | 207.09 ± 38.82 | 0.387 |
| HDL cholesterol, mg/dL | 51.67 ± 15.85 | 61.45 ± 16.41 | < 0.0001 |
| Triglycerides, mg/dL | 109.08 ± 42.41 | 95.23 ± 58.59 | < 0.0001 |
| LDL cholesterol, mg/dL | 131.98 ± 33.45 | 126.59 ± 37.29 | 0.009 |
| γ-GT (U/L) | 33.24 ± 29.40 | 26.03 ± 21.95 | < 0.0001 |
| AST (U/L) | 20.77 ± 5.91 | 21.01 ± 7.10 | 0.530 |
| ALT (U/L) | 15.65 ± 4.60 | 10.40 ± 4.88 | < 0.0001 |
| Alkaline phosphatase (U/L) | 68.37 ± 18.49 | 72.75 ± 43.42 | 0.030 |
| Serum albumin (g/dL) | 4.62 ± 0.39 | 4.53 ± 0.40 | < 0.0001 |
| Lifestyle items | |||
| AMDS | 32.21 ± 0.91 | 34.91 ± 0.61 | < 0.0001 |
| Baecke - physical activity total score | 39.82 ± 3.60 | 41.43 ± 3.32 | < 0.0001 |
| Western diet score | 22.84 ± 7.87 | 12.73 ± 2.48 | < 0.0001 |
| Sun exposure score | 31.43 ± 3.89 | 35.73 ± 5.25 | < 0.0001 |
| Sleep hours | 7.86 ± 1.31 | 7.90 ± 1.23 | 0.552 |
| NAFLD | Controls | χ2 | P value | OR | 95%CI | |
| BMI ≥ 26.40 | 408 | 167 | 316.3851 | < 0.0001 | 9.851 | 7.546-12.861 |
| BMI < 26.40 | 124 | 500 | ||||
| HOMA ≥ 1.87 | 368 | 211 | 167.0111 | < 0.0001 | 4.849 | 3.792-6.202 |
| HOMA < 1.87 | 164 | 456 | ||||
| HDL ≥ 54.50 | 204 | 400 | 55.3581 | < 0.0001 | 0.415 | 0.329-0.524 |
| HDL < 54.50 | 328 | 267 | ||||
| TGL ≥ 94 | 324 | 240 | 73.7751 | < 0.0001 | 2.771 | 2.191-3.506 |
| TGL < 94 | 208 | 427 | ||||
| AMDS ≥ 34 | 32 | 650 | 1008.8311 | < 0.0001 | 0.002 | 0.001-0.003 |
| AMDS < 34 | 500 | 17 | ||||
| BAECKE ≥ 41.5 | 181 | 354 | 43.4681 | < 0.0001 | 0.456 | 0.360-0.577 |
| BAECKE < 41.5 | 351 | 313 | ||||
| WDS ≥ 15.5 | 399 | 97 | 445.9811 | < 0.0001 | 17.629 | 13.174-23.590 |
| WDS < 15.5 | 133 | 570 | ||||
| SES ≥ 34.5 | 111 | 348 | 122.7881 | < 0.0001 | 0.242 | 0.187-0.313 |
| SES < 34.5 | 421 | 319 | ||||
| Sleep hours ≥ 8 | 319 | 370 | 2.5921 | 0.107 | 1.210 | 0.959-1.527 |
| Sleep hours < 8 | 208 | 292 |
The most suitable thresholds distinctive of NAFLD vs controls are, in our population: BMI ≥ 26.40, HOMA ≥ 1.87, HDL < 54.50, TGL ≥ 94, AMDS < 34, WDS ≥ 15.5, physical activity Baecke’s total score < 41.5, Sun exposure score SES < 34.5, and sleep daily hours, calculated on a weekly base sleep hours < 8.0. Contingency tables and Odds ratio were calculated for NAFLD vs controls, according to above defined thresholds. Greater prevalence of overweight-obesity, insulin resistance, increased triglycerides and low HDL cholesterol, poor adherence to mediterranean diet profile, greater use of Western diet food, greater sedentary life habits and minor sun exposure, open air time were observed (Table 2).
Multiple Linear regression analysis (Table 3), weighted by years of age for avoiding age as a potential confounding factor, using the same items as predictors of the severity of fatty liver, assessed by US as BLS, confirms the significance of the chosen model, displaying BMI, HOMA and AMDS as the most powerful predictors of fatty liver severity; also the physical activity score, the western diet habit and the sun exposure score are still inside the model, with significant independent effects. The number of sleep hours does not show any significant linear effect in the model. Nonetheless, in a separate analysis, sleep hours display a U shaped behaviour, showing a greater relationship with more severe fatty liver at the two extremes of the curve: Few and many hours of sleep are both associated with more severe fatty liver.
| Predictors | R | R2 | F | Sig. | β | P value |
| 0.965 | 0.932 | 2309.1 | < 0.0001 | |||
| BMI, kg/m2 | -0.448 | < 0.0001 | ||||
| HOMA | -0.393 | < 0.0001 | ||||
| AMDS | -1.398 | < 0.0001 | ||||
| Baecke | -0.074 | < 0.0001 | ||||
| WDS | 0.069 | < 0.0001 | ||||
| Sun exposure score | -0.044 | < 0.0001 | ||||
| Sleep hours | -0.008 | 0.296 |
Currently, overweight and obesity are the most established associated factors of NAFLD, and are considered, even with some limitation, actual risk factors and putative, indirect causative factors[2,3]. Nonetheless, other and quite neglected factors were and are studied: Most of them are related to behaviour, such as physical activity[5], sleep habits[4] and Sun exposure, this last with a likely effects on vitamin D status[22]. Nutrition has a qualitative profile, and not only a quantitative one, i.e., not only caloric intake, so that the association of unhealthy dietary habits, apart the abuse of alcohol, is associated with unhealthy liver and, notably, NAFLD. This is confirmed in our study in which we observe that, apart the greater BMI, also a poorer adherence to mediterranean diet profile[5], widely and since several years used as a proxy of healthy diet, strongly predicts the occurrence of NAFLD, independently from overweight. Also the almost reciprocal western diet profile displays an unfavourable relationship for the occurrence of NAFLD. This is confirmed in our study by the significant difference of averages, with a greater WDS in NAFLD (Table 1), by the greater odds of NAFLD associated with greater BMI and western diet habits, and with lower adherence to mediterranean diet (Table 2). Moreover, by a model of multivariate analysis (Table 3) the effects of BMI, mediterranean diet and western diet are independently operating, addressing clearly to the opposite effects of mediterranean diet (favourable) and of western diet and overweight (detrimental). Concurrently with nutritional profiles and BMI, sedentary life, assessed quantitatively as physical activity score, displays the same effects: A better physical exercise profile is associated with a lower prevalence (Table 2) and severity of bright liver score (Table 3), as assessed in NAFLD by liver US. Physical activity score is overall poorer in NAFLD vs controls (Table 1). The same association is observed for the sun exposure score, which is greater in controls (Table 1) and which may indicate, apart a greater open air life, also a better D vitamin status, important because vitamin D deficiency is associated with NAFLD[22]. Differently from the observation reported in youngsters[4], sleep hours do not show any significant relationship with NAFLD.
We must acknowledge several limitations of our study. First, the overall, comparison between NAFLD patients and controls (Table 1) does not display extreme differences, even if they are statistically significant, when considering sleep hours, sun exposure, AMDS and physical activity. There are very different features considering the greater score of Western Diet profile pattern in NAFLD. These even small differences between NAFLD and controls become more relevant within the model that takes into account all the co-variates, so that we must still consider them as relatively important features regarding NAFLD, even envisaging a size effect in the group studied.
Second limitation is that our eligibility criteria were rather strict, resulting in a population without significant co-morbidities, since all patients with diabetes and/or even minimally elevated ALT levels were excluded. It is possible that the analyzed lifestyle measures might work differently in a more comprehensive NAFLD cohort that includes other associated diseases. Scope of the study was to investigate NAFLD as an almost-isolated disease, and even with these restrictions association of recognized and neglected aspects of lifestyle are seemingly operating.
Modification over the time of healthier nutritional and behavioural profiles is a very articulated topic of investigation, which includes also the need of assessing the process of erosion of traditionally cohesive family and community relationships[24] with effects on health and mortality. Such studies have a counterpart in the current societal efforts aimed at the preservation of traditional habits, and even clinical conditions, such as high hemoglobin levels[25] which often are credited as healthier. Many animal models have been studied in which dietary variations produce liver injury, and by extrapolation, malnutrition, particularly deficiencies of protein and vitamins has long been considered an important factor in human cirrhosis when no evidence existed for another aetiology; by contrast, weight reduction through low-calorie diets or starvation reduces the steatosis resulting from obesity[1]. Malnutrition was in the last century, and now again, the key of many disease and, notably of liver disease, with its paradigm of fatty liver evolving toward fibrosis. Apart the pioneering studies on lifestyle changes[26] we are still on the starting blocks because each aspect of lifestyle is studied, and thereafter assessed and managed as an individual factor. Despite the great attention which is devoted in Europe to healthier environment and to urban mobility, using the paradigm of smart city, few or no research are at the moment published and available, even if elsewhere there is already a move in this sense also by comprehensive approach focused to clinical risk assessment and management[2]. The important most recent reviews appropriately address benefits of healthy diet and exercise on NAFLD[27] both in adults[28] and in children[29], even if other factors, genetic[30], behavioural and environmental should not be neglected[31,32]. The opportunity for the medicine are relevant since articulated clinical intervention, which, according to our results, are justified, can be pursued with a focus on nutritional profile, physical exercise mainly open-air for enhancing sun exposure and improving sleep duration and rhythm[33], cultural and traditional medicine issues and, comprehensively, the quality of life[34-39]. The pre-requisite is that both medical doctor and patient should not be mucking around in search of the magic bullet, and instead try to take seriously and with a strategy the road of lasting lifestyle change. Individual, professional and societal benefits are the outcomes that can be reached[2].
In a very simplified manner today the authors tend to describe the lifestyles in medicine especially in terms of diet and physical inactivity or sedentary life, with a synergistic effect on body size - obesity - and on disease related with excessive food intake (atherosclerosis and liver disease).
Many animal models have been studied in which dietary variations produce liver injury, and by extrapolation, malnutrition; particularly deficiencies of protein and vitamins has long been considered an important factor in human cirrhosis when no evidence existed for another aetiology; by contrast, weight reduction through low-calorie diets or starvation reduces the steatosis resulting from obesity.
This is confirmed in their study in which they observe that, apart the greater BMI, also a poorer adherence to mediterranean diet profile, widely and since several years used as a proxy of healthy diet, strongly predicts the occurrence of non-alcoholic-fatty-liver-disease (NAFLD), independently from overweight. Also the almost reciprocal western diet profile displays an unfavourable relationship for the occurrence of NAFLD. This is confirmed in our study by the significant difference of averages, with a greater western diet score in NAFLD, by the greater odds of NAFLD associated with greater body mass index and western diet habits, and with lower adherence to mediterranean diet.
The opportunity for the medicine is relevant since articulated clinical intervention, which, according to their results, are justified, can be pursued with a focus on nutritional profile, physical exercise mainly open-air for enhancing sun exposure and improving sleep duration and rhythm, cultural and traditional medicine issues and, comprehensively, the quality of life. The pre-requisite is that both medical doctor and patient should not be mucking around in search of the magic bullet, and instead try to take seriously and with a strategy the road of lasting lifestyle change. Individual, professional and societal benefits are the outcomes that can be reached.
The manuscript of “Neglected features of lifestyle: Their relevance in non-alcoholic fatty liver disease” is very interesting.
| 1. | Popper H, Schaffner F. Nutritional cirrhosis in man? N Engl J Med. 1971;285:577-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Trovato FM, Catalano D, Musumeci G, Trovato GM. 4Ps medicine of the fatty liver: the research model of predictive, preventive, personalized and participatory medicine-recommendations for facing obesity, fatty liver and fibrosis epidemics. EPMA J. 2014;5:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Catalano D, Trovato GM, Martines GF, Randazzo M, Tonzuso A. Bright liver, body composition and insulin resistance changes with nutritional intervention: a follow-up study. Liver Int. 2008;28:1280-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Trovato FM, Catalano D, Martines GF, Pace P, Trovato GM. Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin Nutr. 2015;34:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Trovato GM. Clinical research and methodology. The paradigm of fatty liver and atherosclerosis behind the chicken or the egg dilemma. Atherosclerosis. 2016;249:228-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Imaizumi H, Takahashi A, Tanji N, Abe K, Sato Y, Anzai Y, Watanabe H, Ohira H. The Association between Sleep Duration and Non-Alcoholic Fatty Liver Disease among Japanese Men and Women. Obes Facts. 2015;8:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Bernsmeier C, Weisskopf DM, Pflueger MO, Mosimann J, Campana B, Terracciano L, Beglinger C, Heim MH, Cajochen C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS One. 2015;10:e0143293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Yu JH, Ahn JH, Yoo HJ, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Shin C, Kim NH. Obstructive sleep apnea with excessive daytime sleepiness is associated with non-alcoholic fatty liver disease regardless of visceral fat. Korean J Intern Med. 2015;30:846-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Miyake T, Kumagi T, Furukawa S, Hirooka M, Kawasaki K, Koizumi M, Todo Y, Yamamoto S, Tokumoto Y, Ikeda Y. Short sleep duration reduces the risk of nonalcoholic fatty liver disease onset in men: a community-based longitudinal cohort study. J Gastroenterol. 2015;50:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V, Verrillo E, Baviera G, Musso G. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Oliveira MJ, Freitas D, Carvalho AP, Guimarães L, Pinto A, Águas AP. Exposure to industrial wideband noise increases connective tissue in the rat liver. Noise Health. 2012;14:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Xi YP. [Histologic and ultrastructural changes in the liver in ageing rats and the effects due to food restriction and noise]. Zhonghua Bing Li Xue Za Zhi. 1989;18:118-120. [PubMed] |
| 15. | Trovato G, Pace P, Martines GF, Brischetto D. Mala-movida: late bed-timing and wake-up induce malnutrition and underweight in youngsters. Chronobiol Int. 2014;31:945-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Trovato G, Brischetto D, Martines GF. Teens’ obesity, noise and sleep deprivation: a perverse liaison. Let’s move beyond “movida”. Obesity (Silver Spring). 2014;22:1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Trovato G, Brischetto D, Pace P, Fabio Martines G. Perceived body weight status of youngsters interferes with headache in obese and non-obese subjects. Headache. 2014;54:1062-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Musolino C, Warin M, Wade T, Gilchrist P. ‘Healthy anorexia’: The complexity of care in disordered eating. Soc Sci Med. 2015;139:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Trovato GM, Catalano D, Martines GF, Pirri C, Trovato FM. Western dietary pattern and sedentary life: independent effects of diet and physical exercise intensity on NAFLD. Am J Gastroenterol. 2013;108:1932-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of Aerobic and Resistance Exercise Training on Liver Enzymes and Hepatic Fat in Iranian Men With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2015;15:e31434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Whitsett M, VanWagner LB. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J Hepatol. 2015;7:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Lee SM, Jun DW, Cho YK, Jang KS. Vitamin D deficiency in non-alcoholic fatty liver disease: The chicken or the egg? Clin Nutr. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Mathiesen UL, Franzén LE, Aselius H, Resjö M, Jacobsson L, Foberg U, Frydén A, Bodemar G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Egolf B, Lasker J, Wolf S, Potvin L. The Roseto effect: a 50-year comparison of mortality rates. Am J Public Health. 1992;82:1089-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Tanoglu A, Kara M. Nonalcoholic fatty liver disease-related cardiovascular risk: Is there an association with blood hemoglobin levels? Eur J Gastroenterol Hepatol. 2015;27:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Bruhn JG, Philips BU, Wolf S. Social readjustment and illness patterns: comparisons between first, second and third generation Italian-Americans living in the same community. J Psychosom Res. 1972;16:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Fan M, Sun J, Zhou B, Chen M. The Smart Health Initiative in China: The Case of Wuhan, Hubei Province. J Med Syst. 2016;40:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Hannah WN, Harrison SA. Lifestyle and Dietary Interventions in the Management of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (4)] |
| 29. | Africa JA, Newton KP, Schwimmer JB. Lifestyle Interventions Including Nutrition, Exercise, and Supplements for Nonalcoholic Fatty Liver Disease in Children. Dig Dis Sci. 2016;61:1375-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology. 2016;150:1778-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 31. | Karrar A, Stepanova M, Alaparthi L, Lingam S, Younoszai Z, Zheng L, Malik KS, Younossi E, Monge F, Hunt SL. Anti-adipocyte antibody response in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2015;30:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Estep JM, Goodman Z, Sharma H, Younossi E, Elarainy H, Baranova A, Younossi Z. Adipocytokine expression associated with miRNA regulation and diagnosis of NASH in obese patients with NAFLD. Liver Int. 2015;35:1367-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of Sleep Disorders with Nonalcoholic Fatty Liver Disease (NAFLD): A Population-based Study. J Clin Exp Hepatol. 2013;3:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, Younossi ZM. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Kim MS, Ong M, Qu X. Optimal management for alcoholic liver disease: Conventional medications, natural therapy or combination? World J Gastroenterol. 2016;22:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Yao H Qiao YJ, Zhao YL, Tao XF, Xu LN, Yin LH, Qi Y, Peng JY. Herbal medicines and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;6890-6905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Danielsson J, Kangastupa P, Laatikainen T, Aalto M, Niemelä O. Impacts of common factors of life style on serum liver enzymes. World J Gastroenterol. 2014;20:11743-11752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9338-9344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 39. | Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813-5819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Tanoglu A, Zielinski J S- Editor: Qi Y L- Editor: A E- Editor: Li D