Published online Nov 28, 2016. doi: 10.4254/wjh.v8.i33.1419
Peer-review started: April 5, 2016
First decision: June 12, 2016
Revised: July 26, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: November 28, 2016
Processing time: 239 Days and 21.2 Hours
Primary biliary cholangitis (PBC), formerly referred to as primary biliary cirrhosis, is an infrequent progressive intrahepatic cholestatic autoimmune illness that can evolve into hepatic fibrosis, hepatic cirrhosis, hepatic failure, and, in some cases, hepatocellular carcinoma. The disease itself is characterized by T-lymphocyte-mediated chronic non-suppurative destructive cholangitis and elevated serum levels of extremely specific anti-mitochondrial autoantibodies (AMAs). In this article, we will not only review epidemiology, risk factors, natural history, predictive scores, radiologic approaches (e.g., acoustic radiation force impulse imaging, vibration controlled transient elastography, and magnetic resonance elastography), clinical features, serological characteristics covering biochemical markers, immunoglobulins, infections markers, biomarkers, predictive fibrosis marker, specific antibodies (including AMAs such as AMA-M2), anti-nuclear autoantibodies [such as anti-multiple nuclear dot autoantibodies (anti-sp100, PML, NDP52, anti-sp140), anti-rim-like/membranous anti-nuclear autoantibodies (anti-gp210, anti-p62), anti-centromere autoantibodies, and some of the novel autoantibodies], histopathological characteristics of PBC, diagnostic advances, and anti-diastole of PBC. Furthermore, this review emphasizes the recent advances in research of PBC in terms of therapies, including ursodeoxycholic acid, budesonide, methotrexate, obeticholic acid, cyclosporine A, fibrates such as bezafibrate and fenofibrate, rituximab, mesenchymal stem cells transplant, and hepatic transplant. Currently, hepatic transplant remains the only optimal choice with acknowledged treatment efficiency for end-stage PBC patients.
Core tip: Primary biliary cholangitis (PBC), previously called primary biliary cirrhosis, is an autoimmune non-suppurative inflammatory disease of the bile duct that is usually complicated by intrahepatic cholestasis and intrahepatic bile ductule damage, and eventually leads to liver fibrosis and cirrhosis. This review will focus on the clinical, serological and histopathological characteristics of PBC, as well as the advances in the diagnosis and treatment of the disease.
- Citation: Huang YQ. Recent advances in the diagnosis and treatment of primary biliary cholangitis. World J Hepatol 2016; 8(33): 1419-1441
- URL: https://www.wjgnet.com/1948-5182/full/v8/i33/1419.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i33.1419
Primary biliary cholangitis (PBC)[1-8] is a relatively rare chronic intrahepatic cholestatic illness characterized by a T-lymphocyte-mediated attack on small intralobular biliary ducts and the presence of elevated plasma concentrations of specific anti-mitochondrial antibodies (AMAs), resulting in hepatic fibrosis and, ultimately, hepatic cirrhosis or hepatic failure, with the potential for hepatic cellular carcinoma via complications[9-11]. PBC predominantly affects women, at a ratio of approximately 12:1 of women to men, who are normally diagnosed at middle-age, primarily in an initial symptomless early stage[9-12]. There is positive association between the national incidence of PBC and socioeconomic status, as estimated by the Human Development Index (HDI)[13]. Moreover, in less-developed countries, the incidence of PBC might be less common[13]. Fatigue and pruritus are incipient clinical manifestations that appear in approximately 20% of PBC patients[14]. Although the clinical presentation and natural disease history of PBC patients have progressively improved over the years due to the recognition of earlier widespread use of ursodeoxycholic acid (UDCA), about 1/3 of PBC patients display suboptimal biochemical responses to UDCA and a poor prognosis[9-12]. At present, hepatic transplant remains the most beneficial therapeutic modality for patients with end-stage PBC[9-12]. This article will focus on the epidemiology, risk factors, clinicopathologic characteristics, serological features, histopathological characteristics, radiologic evaluation approaches, diagnosis, and differential diagnosis, as well as recent advances in the therapy of PBC.
The disorder generally referred to currently as “primary biliary cirrhosis” was primitively depicted in 1851, but not formally named until 1950[1-8]. However, it was later rightly recognized that the application of the terminology “primary biliary cirrhosis” is for a catachresis in patients in the presence of early-stage disease and histopathological characteristics of non-suppurative destructive cholangitis that are usually complicated with intrahepatic cholestasis and intrahepatic bile ductule damage. In recent decades, the prognosis of PBC patients has been observably ameliorated since the disease entity was first described more than 150 years ago due to the application of UDCA. Since a great number of PBC patients do not suffer from hepatic cirrhosis, this tag has perceptibly disrupted many PBC patients, who strive for more accurate nomenclature[1-8]. At the second European Association for the Study of the Liver (EASL) monothematic conference on “primary biliary cirrhosis” in 2014, representatives of multitudinous patient cohorts from a variety of countries worldwide requested altering the eponym “cirrhosis” to another that would more precisely represent the characteristics of the disorder[1-8]. From the point of view of the patient, the eponym “cirrhosis” is misdirecting in some ways, and may result in stigmatization and confusion with alcoholic cirrhosis, as well as a shortage of transparency with regards to the stage and prognosis of the disease. From the physician’s perspective, misapplication of the terminology “cirrhosis” is counter-productive to their job. In order to assist and cure patients both within and without the hospital setting who are trying to balance their private lives with their medical demands, it is vital that the term “cirrhosis” be changed[1-8]. The suggested change of “cirrhosis” to “cholangitis” was ratified by the EASL in November 2014, by the American Association for the Study of Liver Diseases in April 2015, and by the AGA in July 2015, respectively[1-8]. In order to inform more people worldwide regarding this change, an article was published in 2015 titled “Changing nomenclature for PBC: From “cirrhosis” to “cholangitis”” in various well-known international medical journals, such as Gastroenterology, Am J Gastroenterol, Gut, Hepatology, J Hepatol, Dig Liver Dis, Clin Res Hepatol Gastroenterol, and Clin Gastroenterol Hepatol[1-8]. Adopting the terminology “primary biliary cholangitis” for the illness known by the acronym PBC is therefore long overdue, so as to bring it into correspondence with a very recent global consensus.
Epidemiology provides significant clues towards our comprehension of the unsearchable etiopathogenesis of PBC. In the past two decades, there have been a chain of epidemiological retrospective investigations concerning patients with PBC[15-22]. The epidemiology of PBC has not only changed significantly over the past twenty years, with a trend towards increasing prevalence in many places around the world[15-22], but is also positively correlated with the national HDI[13]. There is a positive, but not significant, correlation between PBC incidence and HDI on a global level (r = 0.348, P = 0.082)[13]. However, in Europe, a significantly positive correlation exists between PBC incidence and HDI (r = 0.455, P = 0.044)[13]. Moreover, the PBC incidence is positively related to the health index (r = 0.422, P = 0.036), but negatively related to the education index (r = -0.650, P < 0.01)[13]. The prevalence and incidence rates of PBC patients have been reportedly augmenting annually worldwide, making changing the name “cirrhosis” vital[15-22]. A study in the United States showed that, during the period of 1975-1995, the overall age and sex-adjusted incidence rate of PBC was 27/1000000 per year, with the incidence in female and male populations being 45/1000000 and 7/1000000 per year, respectively[15]. In 1995, the age- and sex-adjusted prevalence was 654/1000000 for women, 121/1000000 for men, and 402/1000000 overall[15]. A study in Canada revealed that, from 1996 to 2002, the overall age and sex-adjusted incidence rate of PBC was 30.3/1000000 per year (female: 48.4/1000000, male: 10.4/1000000); the prevalence was 100/1000000 in 1996 and 227/1000000 in 2002[16]. A study in Lombardy, Italy and in Denmark suggested that during 2000-2009, the overall age and sex-adjusted incidence rate of PBC in Lombardy was 16.7/1000000 per year (female-to-male ratio 2.3:1), the point prevalence in Lombardy was 160/1000000 in 2009, the incidence of PBC in Denmark was 11.4/1000000 per year (female-to-male ratio 4.2:1), and the point prevalence in Denmark was 115/1000000 in 2009[17]. A study in Crete, Greece showed that, from 1990 to 2010, the incidence of PBC was 20.88/1000000, and the prevalence was 365/1000000[18]. A study in the Netherlands demonstrated that, between 2000 and 2008, the incidence of PBC was 11/1000000 (3/1000000 in men and 19/1000000 in women) and the point prevalence in 2008 was 132/1000000[19]. A study in Iceland indicated that the point prevalence in 2010 was 383/1000000, while the age-standardized rate of incidence for female patients in the 1st (1991-2000) and 2nd phases (2001-2010) were 34/1000000 and 41/1000000, respectively[20]. Overall incidence rates in the 1st and 2nd phases were 20/1000000 and 25/1000000, respectively[20]. Although the prevalence of PBC was higher in some regions of North America and northern Europe, it was rarely seen in Australia. A study in Australia demonstrated that the age-adjusted prevalence rate of PBC was 51/1000000[21]. A study in South Korea revealed that, between 2009 and 2013, the age and sex-adjusted incidence of PBC from 2011 to 2013 was 8.57/1000000 per year (ratio of female to male was 6.2:1), while the age and sex-adjusted prevalence rate from 2009 to 2013 was 47.50/1000000[22]. At the time of writing, there is still a notable lack of large, nationwide population-based solid epidemiological information concerning PBC in China. One study in China indicated that the point prevalence rate of adult PBC patients who received a health examination in southern China was 492/1000000, with the prevalence in women over 40 years old being up to 1558/1000000 (ELISA method)[23]. The overall prevalence of PBC reported by Liu et al[23] was much higher than previously reported in the literature, which was likely due to the methodology used in the study. In general, the different prevalence and incidence rates of PBC reported in the aforementioned literature were mainly due to differences in assay methods, gender and age distributions of population groups, and geographic regions. There is presently no precise epidemiologic data on the prevalence of PBC in Africa, but it is speculated to be one of the lowest in the world.
To date, the pathogenesis of PBC remains largely unknown, although geographical distributions, genetic susceptibility, and environmental factors may be some potential risk factors for the disease[9-10]. Both familial clustering and monozygotic twins with an identical DNA sequence provided good evidence for its genetic susceptibility and high degree of consistency[11]. Environmental factors, such as smoking, drug abuse, and microbiome complexities, may play a vital role in breaking the immune tolerance of individuals with genetic susceptibility[9-11]. Furthermore, recent novel hypotheses on latent environmental triggers, such as chemical xenobiotics, which result in the breaking of self-tolerance within the unparalleled immunological environment of the liver, have also been suggested[11,12]. As PBC overwhelmingly affects females, factors such as major defects in sex chromosomes, abnormal genetic architecture, and epigenetic abnormalities strongly suggest an effect of genetic and epigenetic factors in the triggering and perpetuation of autoimmune aggression in PBC[9-12]. Several human leukocyte antigen (HLA) risk loci that provide prognostic information and a few non-HLA risk loci associated with the development of PBC have recently been confirmed by means of genome-wide association studies (GWAS)[24-31]. GWAS showed that HLA-DQB1 (*0402), HLA-DRB1 genes (*08,*14), and HLA-DPB1 gene (*03:01) were predisposing risk alleles for PBC susceptibility[24,25]. Aside from the HLA locus, a number of non-HLA genes including IL-12A (rs6441286, rs574808), IL-12RB2 (rs3790567), STAT4, CD80, DENND1B, CXCR5, IL-7R, TNFRSF1A, NFKB1 and CLEC16A were also closely related to PBC susceptibility[24,26]. These data demonstrate not only that there are extraordinary associations between PBC and the usual heritable aberrance at HLA class II, IL12A, and IL12RB2 loci, but also that the IL-12 immunoregulatory signaling axis plays an outstanding role in the physiopathology of PBC. Several recent GWAS have shown that some non-HLA genes, such as STAT4 SNPs (rs10168266, rs11889341, rs7574865, rs8179673, rs10181656)[27], ESR2 rs1256030 T allele[28], CLEC16A, SOCS1, SPIB and SIAE genes[29], may also be significant risk factors for the progression of PBC. One study demonstrated that the downregulated expression of IL-12A in lymphoblastoid cell lines obtained from Han Chinese were markedly associated with the risk alleles of rs4679868 and rs6441286 (P = 0.0031 and 0.0073, respectively)[30]. Furthermore, the risk alleles of the 2 SNPs were observably related to a decreased expression of SCHIP1 gene that is 91.5 kb, located upstream of IL-12A and associated with susceptibility to celiac disease[30]. These data have disclosed the IL-12/JAK-STAT signaling pathway as a pivotal etiologic factor for PBC. In addition, the allele of rs79267778 was observably relevant to PBC[31]. The amino acid at position 1904 (NM_001037335) from threonine (ACG) had been changed to methionine (ATG)[31]. This gene locus was exceedingly conservative in mammals and estimated to have the potential risk score of 0.469 by PolyPhen-2 (bioinformatics tools)[31]. PBC and gene expression were related to allele-specific transcription factor binding to usual and infrequent geno-variation[32]. DNA methylation analysis of the X chromosome exposes abnormal demethylation on CXCR3 promoter in PBC[33]. Furthermore, other associated risk factors include concurrent autoimmune disease, lifestyle factors (e.g., cigarette smoking), urinary tract infection, vaginal infection, and environmental influences (e.g., toxic and chemical exposure to such substances as nail polish and hair dye)[34-36]. In general, the close link between environment factors and genetic susceptibility may play a vital part in the epigenetic mechanisms of PBC.
Common clinical symptoms of PBC include fatigue, pruritus, weakness, daytime sleepiness, loss of weight, xanthelasma palpebrarum, jaundice, skin hyperpigmentation, upper abdominal discomfort, hepatosplenomegaly, osteodystrophy, osteoporosis, cholelithiasis, malabsorption syndrome, and extrahepatic manifestations of an autoimmune nature, although roughly 50% of PBC patients are asymptomatic at diagnosis[9-12]. Patients with PBC normally suffer from itching and fatigue, regardless of disease severity[14,37,38]. Serum fat-soluble vitamin D deficiency may be detected, particularly in advanced PBC patients[39,40]. Metabolic bone disease includes osteoporosis and, more rarely, osteomalacia, which have been considered important complications of PBC[41,42]. The extremely infrequent PBC complication of tubulointerstitial nephritis with Fanconi syndrome should be highly suspected in adult PBC patients in the presence of agnogenic halisteresis, even without the presence of abnormal liver function[41]. The serum levels of sclerostin were found to be observably increased in PBC patients in comparison with controls (P < 0.001), while the hepatic mRNA overexpression of sclerostin and elevated serum levels of sclerostin were inversely related to osteogenesis and reabsorption biological markers[42]. In addition, liver sclerostin was mainly distributed in the bile ducts, was relevant to the seriousness of cholangitis (P = 0.02), and was indirectly related to the extent of inflammation in the hepatic lobule (P = 0.03)[42]. These results indicated that sclerostin overexpression in the bile duct of patients with PBC in the presence of chronic intrahepatic cholestasis may affect metabolic osteopathia in PBC[42]. In general, although the main target organ is the liver, multiple systems may also be involved, such as interstitial lung disease (ILD)-related pulmonary hypertension (PH) and esophageal dysfunction. PBC is also often accompanied by nephritis, connective tissue diseases (CTDs), hepatocellular carcinoma, and other rare diseases. The concurrence of these rare diseases often augments the difficulty in establishing an exact diagnosis. Specific clinical features are as follows.
Fatigue is not an uncommon complaint of PBC patients, and is related to a lower quality of life[9-11]. In recent years, PBC is mainly diagnosed in the majority of patients who are asymptomatic[9-11]. However, fatigue is a significant problem in approximately 50% of PBC patients, with 20% of all PBC patients experiencing significant or life-altering fatigue[9-11]. The pathogenesis of fatigue in PBC has not been fully elucidated, although it isn’t relevant to the seriousness of the underlying illness and is unresponsive to UDCA[9-11]. As the symptom of fatigue is non-specific, multifactorial, and potentially incapacitating, conditions such as anemia, diabetes, hypothyroidism, and depression should be considered and excluded[9-11]. Fatigue is typically identified with a subset of PBC patients who are predominantly young women who have particularly active illness, a suboptimal response to UDCA therapy, and are more likely to develop hepatic cirrhosis and its complications[37]. At present, there is no special drug therapy for the management of PBC-related fatigue and no significant improvement following liver transplantation[9-11]. The clinical efficacy of modafinil in the treatment of PBC-related fatigue for 12 wk has been proved to be secure and reasonably well-tolerated in randomized, placebo-controlled, phase II clinical trials[38]. Nevertheless, it did not give rise to an advantageous impact on fatigue when compared to a placebo-treated group[38].
Pruritus is a pre-eminent symptom in PBC patients with chronic cholestasis and is variably reported in PBC characterized by cholestasis[9-11,14]. More than two-thirds of PBC patients experience pruritus during the process of the illness[9-11]. Compared to asymptomatic PBC patients without pruritus, symptomatic PBC patients with pruritus more frequently suffer from hepatic cirrhosis and its related complications (P = 0.004)[37], and are less likely to respond to UDCA treatment (P = 0.006). The pathogenesis of cholestatic pruritus remains largely elusive[9-11]; its natural history, related pathogenesis, and molecular mechanisms are under continued investigation[9-11]. The autotaxin (ATX)-lysophosphatidic acid signaling axis may play a vital part in the nosogenesis of pruritus, and has lately has been connected with pruritus in PBC[14]. Several pieces of evidence have showed that a circulating pruritogen will take responsibility for it, but identification of the small molecule has yet to be ultimately identified[14]. In comparison, plasma ATX activity is observably associated with pruritus in PBC, suggesting a new molecular targeting therapy[14].
Malabsorption, steatorrhea, and fat-soluble vitamin D deficiency are uncommon, except in cases of advanced liver disease and long-standing, severe cholestasis[9,10,39,40,43]. In addition to vitamin D deficiency, as luminal bile acid levels in severe cholestasis are below the critical concentration required for micelle formation and subsequent lipid absorption, clinically-relevant fat-soluble vitamin (vitamin A, E and K) deficiencies may also exist in PBC[43]. Deficiencies in fat-soluble vitamins A, D, E and K have been reported in 33.5%, 13.2%, 1.9% and 7.8% of PBC patients, respectively[43]. Vitamin A deficiency appears to be markedly associated with advanced PBC stage, decreased cholesterol, and increased Mayo risk score[43]. High Mayo risk score, low serum albumin level, and elevated total bilirubin have been shown to be independently related to vitamin D deficiency[43]. Baseline vitamin D deficiency was associated with severity of disease and response to UDCA treatment[39]. Serum 25(OH)D concentrations reduced with elevating histological grading of stage (P = 0.029) and were inversely associated with serum bilirubin and alkaline phosphatase (ALP) concentrations in PBC[39]. Serum 25(OH)D concentration at baseline was observably reduced in non-responders to UDCA (P = 0.005)[39]. Baseline vitamin D deficiency was related to an elevated risk of an inappropriate response with no relationship to advanced histological stages (P = 0.047)[39]. Mean serum concentrations of vitamin D were observably reduced among PBC patients compared to the control group (P = 0.029) and vitamin D deficiency (≤ 10 ng/mL) was observed in 33% of PBC patients vs 7% of the control group (P < 0.0001)[40]. Vitamin D concentrations were negatively associated with advanced hepatic injury, as well as the existence of accompanying autoimmune disorders[40]. The potential role of vitamin D in PBC may involve genetic and cell signaling mechanisms. Relatively few PBC patients have vitamin E or K deficiencies[43].
Portal-venous hypertension is not an uncommon aftermath of PBC, and may even occur before cirrhosis develops in PBC patients[9-11]; approximately 10% of PBC patients presented with characteristics of portal hypertension as an initial clinical symptom[9-11]. Gastroesophageal varices may occur in any of the different histological phases of PBC[9-11]. Signs of portal hypertension should therefore be carefully observed for in PBC patients at the moment of diagnosis, as well as during the observation period[9-11]. The pathogenesis of portal hypertension in PBC is still unclear[9,10]. A recent study suggested sinusoidal blockage as a potential physiopathology mechanism during the early phases of PBC, which was verified by the obvious intrahepatic portal vein in 3 non-cirrhotic PBC patients, with intrahepatic portal vein hepatica interflow being responsible for relieving the hepatic venous pressure gradient[44]. Another study indicates that angiogenetic and fibrotic responses are presumably induced by aquaporin-1 (AQP-1), resulting in the enhanced perfusion of arterial blood flow to the sinusoids[45]. The result demonstrates that AQP-1 is related to arterial capillary wall proliferation and hepatic sinusoidal transformation facilitating portal-venous hypertension in PBC[45]. Esophageal varicosities (EV) can be found in PBC patients with early histological stages[46,47]. A study revealed that 6% (8/127) of early histological stage PBC patients suffered from EV and 95% of PBC patients in the presence of varices were required to meet at least one of the following criteria: Male sex, hypoalbuminemia (< 3.5 g/dL), hyperbilirubinemia (≥ 1.2 mg/dL), and/or prolonged prothrombin time (PT) (≥ 12.9 s)[46]. Therefore, these parameters that include male sex, hypoalbuminemia, hyperbilirubinemia, and/or PT can be used as a tool for non-invasive prediction of EV[46]. A study demonstrated that among 256 cases of PBC with early histological stage, 22 cases suffered from EV at the time of diagnosis, with elevated serum ALP levels and decreasing platelet counts being markedly related to the presence of EV in early histological stage PBC[47]. The prominent relationship between these two factors with the development of EV was also disclosed, and PBC with early-stage and elevated ALP ratios ≥ 1.9 had an observably high risk of progressing EV[47]. In addition, another study has shown that quantitative parameters in the diagnosis of hepatic fibrosis in portal, septal and fibrillar areas may accurately predict gastroesophageal varices in PBC; the diagnostic specificity and sensitivity in PBC was 75% and 100%, respectively[48].
PH is generally complicated by heart or lung disorders, but it is also known to be related to PBC[49]. PH that suggests poor prognosis as a complication of PBC is not only common, but is closely related to portal-venous hypertension and immunological dysregulation[49]. PH is significantly more frequent than was previously assessed in PBC patients with portal hypertension[49]. The risk of progressing PH could be enhanced with the persistent time of portal hypertension without any explicit relationship with the degree of portal hypertension, liver failure, or amount of blood shunted[49]. The prevalence rate of PH in PBC patients with portal hypertension has been reported by McDonnell et al[50] as 0.61% in a clinical series of 2459 PBC patients with biopsy-proved cirrhosis of the liver. PH associated with PBC without portal hypertension is very infrequent; among 178 PBC patients, 21 (11.8%) suffered from PH[49]. Four cases (19.0%) suffered from medium to severe PH and one died of right ventricular dysfunction rather than hepatic dysfunction[49].
Esophageal dysmotility can exist in some PBC patients[51], particularly in those with scleroderma or Sjögren’s syndrome in the absence of scleroderma[51]. As a result, some esophageal motor disturbances could be considered associated with Sjögren’s syndrome[51]. Esophageal motor dysfunction is by no means uncommon in Sjögren’s syndrome or scleroderma; however, whether any esophageal dysmotility also exists in PBC without Sjögren’s syndrome or scleroderma is still controversial. A recent study showed that, among 37 PBC patients, 17 (45.9%) had esophageal dysmotility (10 cases of non-specific esophageal motor disorder, 5 cases of esophageal hypomotility, 1 case of nutcracker esophagus, and 1 case of hypertensive lower esophageal sphincter)[51]. These results demonstrate that sub-clinical esophageal motor dysfunction is common in PBC patients[51].
ILD is a frequent and major complication of PBC[52,53]. PBC patients who suffer from Raynaud’s phenomenon and other CTDs were considered to have the greater possibility of developing ILD[52,53]. PBC with concomitant Sjögren’s syndrome was considered to have a higher risk of developing ILD and presenting a poor prognosis[53]. A study showed that, among 178 PBC patients, 28 (15.7%) suffered from ILD, with 53.6% said patients suffering from difficult breathing and tussis, and 88.2% demonstrating restrictive and diffusing ventilation impairment by means of pulmonary function test[52]. Patients with PBC in the presence of ILD were older in age and displayed higher serum levels of sedimentation rate of erythrocyte compared to those without ILD (P < 0.05)[52]. Raynaud’s phenomenon, as well as the coexistence of PBC and CTDs, were considered to be risk factors for PBC patients developing ILD (P = 0.04, OR = 3.12 and P = 0.01, OR = 3.18, respectively), although 42.9% of patients with PBC in the presence of ILD had not suffered from other CTDs[52]. There was much higher incidence rate of ILD in PBC patients with concomitant Sjögren’s syndrome compared to those without the syndrome (P = 0.005)[53]. In some instances, ILD can even appear to precede PBC[54].
Symptomless distal renal tubular acidosis should be considered the main feature of PBC-related kidney damage, and can appear in approximately 1/3 of PBC patients[55]. However, various rarer methods of PBC-associated kidney damage have also been described in the literature, including: Fanconi syndrome, microscopic polyangiitis, membranous nephropathy, membranous glomerulonephritis, tubulointerstitial nephritis, fibrillary glomerulonephritis, interstitial nephritis, Goodpasture syndrome, anti-neutrophil cytoplasmic autoantibody (ANCA)-associated rapidly progressive glomerulonephritis, and focal segmental glomerulosclerosis[41,56-64].
PBC can be complicated by CTDs, more specifically systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA), Sjögren’s syndrome (SS), polymyositis (PM), and dermatomyositis[65]. Moreover, combined PBC and CTDs often enhance the difficulty in making an exact diagnosis and treatment of PBC[65]. One study showed that, among 322 patients with PBC, 150 cases (46.6%) suffered from CTDs, of which 11 cases (3.4%) suffered from two or more CTDs[65]. SS should be considered the most common CTD (122 cases, 36.2%)[65]. Other CTDs in this group of patients, in order of rarity from high to low, were as follow: 12 cases of SLE (3.7%), 10 cases of PM (3.1%), 9 cases of SSc (2.8%), and 9 cases of RA (2.8%)[65].
It is by no means uncommon for PBC patients to suffer from hepatocellular carcinoma (HCC)[66-69]. Of two retrospective studies in China, one demonstrated an incidence of HCC in PBC patients of 4.13% (52/1255)[66], while the other found it to be 3.75% (70/1865)[67]; this incidence was observably higher in men (9.52%) than in women (3.31%)[66]. Risk factors for PBC-related HCC in China for the two studies were found to be body mass index (BMI) ≥ 25, male sex and a history of drinking alcohol for the first study[66] and age > 54 years, male sex, co-existence of diabetes, and previous hepatitis B virus (HBV) infection for the second study[67]. A retrospective Japanese study found the incidence of HCC in PBC patients to be 5.2% (11/210), with the only risk factor for PBC-associated HCC being associated with advanced histological stage[68]. Recently, a multicenter international study demonstrated that incidence rates of HCC in PBC patients were 2.69% (123/4565), with markedly higher rates in male PBC patients compared to female patients (P < 0.0001). Univariate analysis of potential risk factors in establishing diagnosis of PBC related to HCC progression were: Male sex (P < 0.0001), increased aspartate aminotransferase (AST) (P < 0.0001), progressing liver illness (P = 0.022), platelet decline (P < 0.0001), and decompensated hepatic function (P < 0.0001)[69]. According to the Paris-I criteria, one year stratification by inappropriate biochemical response with UDCA therapy was markedly related to risk factors of progressing HCC (P < 0.0001)[69]. Biochemical non-response to UDCA therapy predicted future trends of HCC in early stage PBC (stages I-II) (P = 0.005) and advanced stage PBC (stages III-IV) (P = 0.02)[69]. The international multicenter study clearly demonstrates that one year biochemical non-response to UDCA is related to incremental future risk factors of progressing HCC in PBC[69]. In addition, another study showed that repeat liver resection for recurrent HCC complicating PBC is an option and may provide and improved outcome[70]. In general, PBC with hepatic cirrhosis or non-response to one year of UDCA therapy are at incremental risk of HCC.
The difficulty in identifying hepatitides C virus (HCV) and/or HBV infections in PBC patients is such that an accurate diagnosis of PBC is usually observably delayed in this particular patient cohort[71]. In PBC patients with accompanying HCV infection, impact therapy might be approved in consideration of the relevant and more serious cirrhosis[72]. A retrospective Greek study showed that, among 1493 HBV and 526 HCV patients, 17 were confirmed as having a coexistence of viral hepatitides and PBC (8 cases of HCV and 9 cases of HBV)[71]. It is very difficult to make an exact diagnosis of PBC in HBV or HCV-infected patients, meaning that a precise diagnosis is usually delayed[71]. Cholestasis should therefore be an important indication of PBC for physicians[71]. A study in Taiwan showed that, among 76 patients with PBC, 9 cases were confirmed as having a coexistence of HCV infection and PBC, and suffered from more serious hepatic cirrhosis on the basis of Child-Pugh (P = 0.019) and the Model for End-Stage Liver Disease (MELD) (P = 0.01) scores[72]. One case report showed that a patient with chronic HBV infection was later found to have active, asymptomatic PBC due to a persistently elevated ALP level after optimal HBV DNA suppression on antiviral therapy[73]. This report emphasizes the significance of keeping a high clinical index of suspicion for potential PBC, even after a patient with HBV has been successfully treated for a primary liver condition[73]. Clinical vigilance, particularly in atypical clinical manifestations, can result in earlier accurate diagnosis and prompt treatment of PBC[73].
Although uncommon, the coexistence of PBC and some rare diseases are frequently believed to enhance the difficulty in making an exact diagnosis of PBC, as well as its treatment, due to the very complicated clinical manifestation of diseases with coexistence conditions. PBC is occasionally associated with some rare diseases, including Guillain-Barré syndrome, warm autoimmune hemolytic anemia, primary hepatic mucosa-associated lymphoid tissue lymphoma, ANCA-associated vasculitis, pseudolymphoma, hereditary hemorrhagic telangiectasia, generalized morphea, myasthenia gravis, hepatic inflammatory pseudotumor, idiopathic retroperitoneal fibrosis, celiac disease, Wilson’s disease, bullous pemphigoid, idiopathic granulomatous hepatitis, CREST syndrome, Crohn’s disease, hepatic sarcoidosis, Evans syndrome, and Hürthle cell adenoma[74-92].
AMAs: AMAs, including AMAs-M2, are a specific and sensitive marker for the diagnosis of PBC[9,10,93,94]. The existing evidence shows that AMAs and AMAs-M2 have excellent diagnostic value, with high specificity and sensitivity for PBC[93]. Compared with AMAs-M2, AMAs is a faster and more comprehensive diagnostic marker[93]. AMAs consist of nine subtypes, four of which are associated with PBC: AMA-M2, AMA-M4, AMA-M8, and AMA-M9[9-11]. Although these four AMA subtypes have comparatively specific diagnostic value for PBC, AMA-M2 remains the foremost subtype applied as a routine diagnostic marker for PBC[9-11]. AMAs are present in 95% of PBC patients; however, 5% of patients with PBC are still AMA-negative[94]. AMA-negative PBC patients had an observably worse prognosis in comparison with AMA-positive PBC patients[94]; however, an obvious distinction between positive and negative PBC AMAs should not have been found on the basis of clinical manifestation, serum biochemical features, histopathological characteristics, disease process, or response to UDCA treatment[94]. Notably, AMA-negative PBC patients had an observably decreased free survival of liver-associated complications covering liver transplant and death in comparison with AMA-positive PBC patients (P = 0.0182)[94].
Anti-nuclear antibodies: Besides AMAs, PBC patient serum is able to demonstrate other PBC-related autoantibodies, especially anti-nuclear antibodies (ANAs) covering anti-multiple nuclear dot autoantibodies (anti-sp100, PML, NDP52, anti-sp140), anti-nuclear envelope protein autoantibodies (lamin, lamin B receptor), and anti-rim-like/membranous anti-nuclear autoantibodies (anti-gp210, anti-p62)[95-103]. Determination of AMAs and PBC-specific ANAs identified them as being associated with PBC severity[9,10]. Elevated serum concentrations of ANAs should be found in approximately 50% of PBC patients and 85% of AMA-negative PBC patients[96]. In short, 44% of PBC patients had anti-sp100, 15.1% had PML, 25% had anti-gp210 and 25% had ACAs[97-100]. AMAs and ANAs (anti-gp210, anti-sp100, ACAs) are particularly prevalent in PBC[101]. Although changes in most autoantibodies that occur naturally with the passage of time do not appear to associate with clinical results in PBC, changes in serum anti-sp100 antibody levels can be used as an evaluation of prognostic factors with regard to the progress of liver fibrosis diagnosed via hepatic biopsy[102]. Sp140L is the phylogenetically nearest family member to anti-sp100 protein, and serves as an autologous antigen in PBC patients[103]. The polymerization of anti-p62 is significantly augmented in the impaired biliary ducts of PBC and may reflect the inappropriate autophagy and subsequent senescence of biliary ducts cells in the etiopathogenesis of biliary duct injury in PBC[104]. In clinical practice, it is vital to detect these autoantibodies in order to establish PBC diagnosis, assess disease severity, determine the PBC clinical phenotype, and calculate the long-term outcome[101]. Positive anti-gp210 antibody and elevated vanishing biliary duct score were observable risk factors for elevated ALP predicted worsened response[105]. Positive anti-gp210 antibody and elevated hepatitides score were observable risk factors for elevated IgM predicted worsened response[105]. Elevation of ALP and IgM worsened response were observable risk factors for development to end-stage liver illness in the absence of jaundice[105]. Therefore, in the classical or typical form of PBC, characterized by the chronic progressive disappearance of small intrahepatic biliary ducts with a simultaneous augment in hepatic fibrosis, anti-gp210 autoantibodies are a powerful risk factor for development to icterus and liver failure[101,105]. Age, positive anti-gp210 antibody, and positive ACAs were observable risk factors for elevated of alanine aminotransferase (ALT) worsened response[105]. Elevation of ALT worsened response was an observable risk factor for development to end-stage hepatic illness with persistent icterus[105]. Of PBC patients with ACAs positivity, 30% had serious bile duct damage and portal hypertension[106]. Therefore, the presence of ACAs is a risk factor for development to hepatic cirrhosis and portal-venous hypertension[101,105,106]. Biochemical response to UDCA therapy at two years, which is affected by the serum autoantibody status of ACAs, anti-gp210, and histological and morphometric variables at baseline, may predict long-term clinical results in PBC patients[105]. By contrast, another study showed that continuous variations of anti-sp100 titers, rather than anti-gp210 titers, might be effective for the surveillance of disease procession and UDCA treatment outcome[107]. The study revealed a reduced rate of eGFR, an elevated possibility of chronic kidney disease (CKD), and an elevated rate of annual eGFR decline in PBC patients with ACAs compared to those without ACAs (P < 0.05, separately)[108]. ACAs may serve as an independent predictor for CKD in patients with PBC[108]; therefore, it is important to assess ACAs and renal function in order to deter CKD evolution in PBC[108].
New autoantibodies: The recognition of novel autoantibodies as a non-invasive serum hallmark is still an important area of PBC research[109]. Hu et al[109] created a PBC-focused microarray with 21 of these recently affirmed alternatives, as well as 9 supererogatory familiar PBC autoantigens[109]. By screening the PBC-focused microarrays with PBC patients, 6 proteins were identified as new PBC autoantigens in the presence of high specificities and sensitivities, covering hexokinase-1 (HK 1, and isoforms I and II), Kelch-like protein 7 (KLHL7), KLHL12, zinc finger, BTB domain-containing protein 2, and eukaryotic translation initiation factor 2C, subunit 1[109]. In addition, both anti-KLHL12 and anti-HK1 antibodies with higher specificity and sensitivity were more likely to be detected in PBC in comparison with controls without PBC (P < 0.001)[110]. Anti-HK1 in combination with anti-KLHL12 in the presence of usable signs (i.e., MIT3, gp210 and sp100), improved the sensitivity of PBC diagnosis[110]. Importantly, both anti-KLHL12 and anti-HK1 autoantibodies had been detected in 10% to 35% of AMA-negative patients with PBC, and increasing both biomarkers in routine PBC tests significantly increased the sensitivity in AMA-negative patients with PBC from 55% to 75% by means of immunoblot and from 48.3% to 68.5% with the ELISA method[110]. Supplementing both anti-KLHL12 and anti-HK1 autoantibodies with highly specific assays for AMAs and ANA serological tests observably enhanced the serological surveillance effect and PBC diagnosis, particularly for AMA-negative patients[110].
Serum biochemical sign in PBC: The enhanced serum activity of ALP, gamma-glutamyltransferase (γ-GT), ALT, AST, total bilirubin (TBIL), and bile acids can be detected in most patients with PBC[9,10,47,111-113]. Evidence from several studies has shown that the presence of elevated serum activity of ALP is not only an obvious guidepost of intrahepatic cholestasis, but also a pronounced succedaneous hallmark PBC severity[111-113]. A Japanese study showed that elevated serum ALP levels were not only markedly related to the presence of esophageal varicosities in PBC patients with early histological stage, but also associated with the progression of esophageal varicosities during the follow-up period[47]. In addition, a meta-analysis of individual patient information from 4845 PBC cases covering 15 European and North American countries demonstrated that serum levels of ALP and TBIL detected at research enrollment and every year for 5 years were significantly related to clinical results[111]. The study result showed that serum levels of ALP and TBIL may predict clinical results (i.e., hepatic transplant or dying) of PBC patients, and could serve as alternative terminal points in treatment tests[111]. In addition, a study in China showed that, among serum biolabeling in PBC patients, the serum concentrations of bile acids were augmented with the development of PBC, while the concentrations of carnitines were reduced with the development of PBC[113]; these factors, high serum levels of ALP, TBIL, and bile acids, are markedly associated with progressive PBC and worsened outcomes.
Serum immunoglobulins in PBC: PBC patients characteristically show elevated serum levels of IgM[9,10]. Environmental factors, but not genetic ones, are considered to play an important role in the pathogenesis of high serum IgM in PBC[114]. In addition, serum IgG2 and IgG3 levels were most prominently increased in PBC[115]. However, evidence of decreased serum levels of IgA, IgM, and IgG in a PBC patient seems to demonstrate that immunoglobulin-mediated etiopathogenesis may be unessential for the development of PBC[116].
Serum markers of infection in PBC: As a screening test, serum from 69 PBC patients were detected for IgG-antibodies against Toxoplasma gondii (anti-T. gondii), Helicobacter pylori (anti-H. pylori), Epstein-Barr virus (anti-EBV), cytomegalovirus (anti-CMV), anti-HBV and anti-HCV[117]. The results demonstrated that the prevalence rates of 4 anti-infectious agent antibodies: Scilicet anti-T. gondii (P < 0.0001), anti-H. pylori (P < 0.01), EBV early antigen (P < 0.0001), and anti-CMV (P < 0.05) in PBC patients was observably higher than in the controls[117]. The coexistence of the 4 anti-infectious agent antibodies was comparatively ordinary in PBC, but the infection burden was infrequent in normal controls (P < 0.0001)[117]. In addition, peculiar contagion reciprocities that potentially accelerate PBC patient risk were also pointed out. Seropositivity of ammodytoxin A was negatively related to hepatic cirrhosis among patients with PBC (P < 0.05)[117].
Serum biomarkers in PBC: Serum microRNAs (miRNAs), which are sufficiently steady and control RNase-mediated degeneration in body fluids, have been used as novel potential biomarkers for many illnesses. However, the expression spectrum of serum miRNAs in patients with PBC is poorly understood. Recently, a miRNA panel (hsa-miR-122-5p, hsa-miR-141-3p, and hsa-miR-26b-5p) was confirmed to have prominent diagnostic accuracy for PBC (sensitivity = 80.5%, specificity = 88.3%)[118]. There was a remarkable difference between expression profiles of the miRNA panel, those of serum ALP (P < 0.001), and those of serum ANAs (P = 0.0282)[118]. Seventeen miRNAs were confirmed to be distinctively expressed in peripheral blood mononuclear cells from PBC patients[119]. In addition, the downregulated expression of hsa-miR-505-3p and miR-197-3p can be used as biological markers of PBC[120]. Functional bioinformatics analysis showed prediction of microRNA target genes involved in multiple signaling passageways and biological processes[119]. In general, serum biological markers for inchoate diagnosis of PBC are a new subject of ongoing research.
Serum predictive marker in PBC: Non-invasive predictive markers of hepatic fibrosis in PBC patients should be used for predicting illness development. The Wisteria floribunda agglutinin-positive Mac-2-binding protein [WFA (+)-M2BP] could serve as an effortless and dependable non-invasive succedaneous serum glycol-biomarker for the diagnosis of hepatic fibrosis in PBC[121]. Serum WFA (+)-M2BP was not only considered to be better than the other non-invasive markers in determining the important and serious fibrosis stages of PBC, but was also forcefully and separately related to clinical result[121]. Serum FGF19 is related to hepatic illness severity, and can also be used as a potential predictive marker of chronic cholestatic hepatic lesion in PBC[122]. Serum ANAs, total cholesterol, and bile acids are predictors of liver failure in PBC[123]. Elevated serum levels of fractalkine in patients with PBC could serve as predictive markers of cholangitis activity at early stages[124]. Comparative proteomics analysis demonstrated not only obvious elevated serum levels of vitronectin in AMA-negative PBC patients compared to those of AMA-positive PBC (P < 0.01), but also a potential association with the more serious bile duct destruction found in this group[125]. Serum hyaluronan is considered a hopeful hallmark for the estimation of hepatic fibrosis in PBC[126]. In addition, serum cartilage oligomeric matrix protein might be a novel non-invasive biomarker for estimating PBC and the risk of HCC[127].
A retrospective analysis showed that PBC predictive scores, covering the European and Yale model, MELD score, and Child-Pugh score, should be interpreted prudently, with the Mayo Risk Score being deemed beneficial in predicting a helpful result[128]. As current approaches for risk stratification of PBC patients are limited and single-center-based, as well as often dichotomous, the novel prognostic tool of GLOBE score was recently proposed by the Global PBC Study Group on the basis of an international meta-analysis of 4119 PBC patients receiving UDCA[129]. A GLOBE score to forecast transplant-free survival of PBC patients receiving UDCA therapy within 1 year was formulated and confirmed by means of clinical and biochemical variables, and the prognostic capacity of the GLOBE score was assessed along with those of the Paris-1, Barcelona, Toronto, Rotterdam, and Paris-2 criteria[129]. Serum levels of ALP, albumin, and hematoidin, as well as blood platelet counts and age, were all independently related to patient mortality or hepatic transplant[129]. There were significantly reduced times of transplant-free survival in patients with risk scores > 0.30 compared to matched normal subjects (P < 0.0001)[129]. The 5-year and 10-year survival rates for patients with positive predictive values verified by the GLOBE score were 98% and 88%, separately[129]. The GLOBE score can therefore not only be considered predictive of the transplant-free survival of PBC patients treated with UDCA, but may also be used to choose the therapy and nursing scheme[129].
At present, there are three proposed important radiologic prediction approaches for assessing hepatic fibrosis: Acoustic radiation force impulse (ARFI), vibration controlled transient elastography (VCTE), and magnetic resonance elastography (MRE)[130-132]. The diagnostic value of the degree of liver fibrosis by means of ARFI together with the 4 serum prediction markers of hepatic fibrosis covering laminin, hyaluronan (HA), type III collagen, and type IV collagen is of a satisfying effect and has significant practical value[130]. ARFI elastography correlated observably with hepatic histological stage (r = 0.74, P < 0.001) in PBC patients[131]. The area under the receiver operating curve of ARFI elastography for predicting histological stage equal to or higher than II or III and equal to IV were 0.83, 0.93 and 0.91, respectively[131]. The optimal cut-off values of ARFI elastography were 1.51 m/s, 1.79 m/s, and 2.01 m/s for PBC stage equal to or higher than II or III and equal to IV, respectively[131]. ARFI elastography is therefore an acceptable and powerful technique for the quantitative assessment of PBC stage[131]. Dependable VCTE con–sequences can exclude advanced hepatic fibrosis and avoid the need for biopsy in the lowest 45% of patients[132]. A recent prospective study in the United States has demonstrated that three-dimensional (3D)-MRE at 40 Hz has supreme diagnostic precision in diagnosing advanced hepatic fibrosis[133]. Both 2D-MRE and 3D-MRE at 60 Hz, the standard shear-wave frequency, are also reasonably precise in diagnosing advanced hepatic fibrosis[133]. MRE has obvious diagnostic precision in advanced hepatic fibrosis and cirrhosis in hepatic transplantation receivers, independent of the extent of inflammation and BMI[134]. Magnetic resonance imaging (MRI) has potential diagnostic value for PBC, and the periportal halo sign and signal strength contribute to assessing the extent of hepatic fibrosis[135]. In addition, Gd-EOB-DTPA-enhanced MRI might offer beneficial detection approaches for hepatopathy in PBC patients[136]. In general, none of the radiologic approaches have perfect accuracy in any published study to date; however, VCTE outperformed all other non-invasive current surrogate markers of hepatic fibrosis in PBC. Due to its high acceptability and ability to predict hepatic decompensation, VCTE could be a useful tool in allocating PBC patients into different categories of risk.
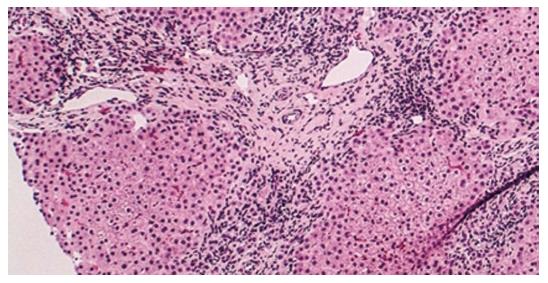
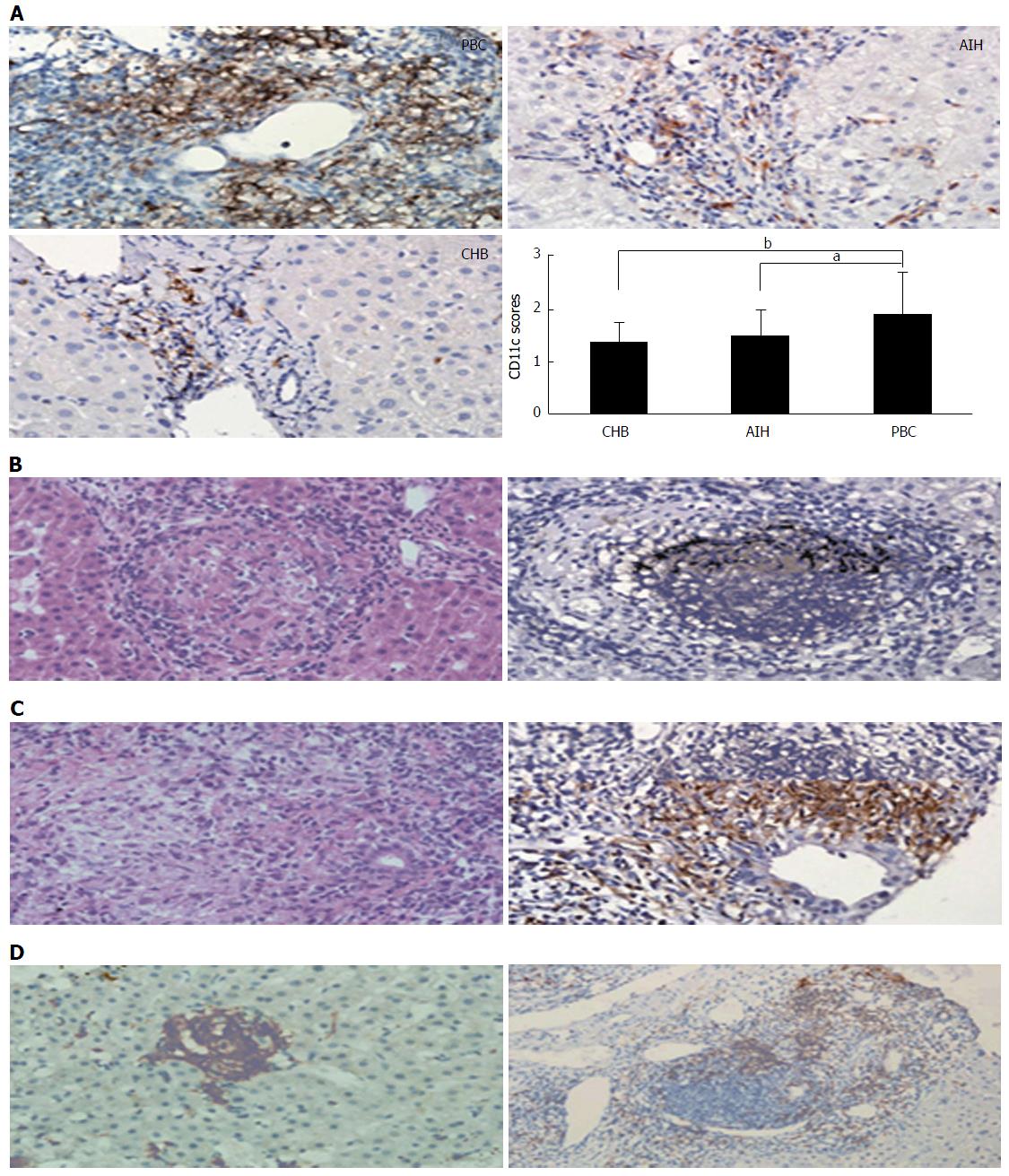
Histopathologically, PBC is not only characterized by predominantly different stages of hepatic fibrosis that eventually result in cirrhosis of the liver or hepatic failure, but also as a granulomatous lymphocytic cholangitis that consequently leads to such small bile duct loss such as vanishing bile duct syndrome and cholestasis (Figures 1 and 2)[9,137]. The typical dendritic-cellular CD11c marker has markedly effective expression and significant sensitivity compared with classical hematoxylin and eosin (H and E) staining in discovering hepatic granulomatous lesions related to PBC and other illnesses[137]. There are significantly elevated serum concentrations of IgM and earlier stages of illness in PBC patients in the presence of CD11c-positive expression hepatic granulomas[137]. There are hallmarks of immature dendritic cells, namely CD11b, decreased expression of MHC II, IL23, CD83 and CCR7, and increased expression of C1q in granulomatous lesions from PBC and other illnesses[137]. PBC-related granulomatous lesions largely represented by B lymphocytes and IgM-positive plasmocytes together with macrophagocytes[137]. Put simply, dendritic cells play a pivotal role in the etiopathogenesis of granulomas, regardless of their origin[137]. More specifically, hepatic granulomas may be caused by the reciprocities between IgM and immature dendritic cells in PBC[137]. In addition, spleen tissue samples from PBC demonstrated accumulation of IgM-positive cells, along with CXCL13-positive cells, in CD21-positive lymph follicles[138]. CXCL13-positive follicular dendritic cells might be conducive to the production of excess IgM from the spleen[138]. The deviant expression of mitochondrial autoantigens and subsequent autoimmune mechanism in PBC may be closely associated with deregulated autophagy and the following cellular senescence in biliary epithelial cells (BECs)[139]. Activated NKT cells may prompt BEC death, leading to the development of PBC[140]. A novel hepatic histological grading system for PBC, proposed by Japanese scholars, includes the degree of chronic cholangitis activity (CA 0-3), which is associated with clinic-laboratory characteristics of cholangitis, and hepatitides activity (HA 0-3), which is related to the progression of cirrhosis-related conditions[141]. French scholars proposed another novel histological scoring system for PBC, which covers assessment of hepatic fibrosis, leukomonocyte interface hepatitides, and absence of biliary ducts[142]. Abnormal expression of K-7 in hepatic cells may serve as an accessional hallmark for predicting rapid development to hepatic failure in diagnosed asymptomatic patients with PBC[143]. In other words, the histological features of PBC, in addition to typical non-suppurative destructive cholangitis and hepatic granulomatous lesions, include portal inflammation, chronic cholestasis, hepatic changes (interface hepatitides or lobular hepatitides), and bile duct loss. The two histological classifications by Ludwig’s and Scheuer’s stages have been used globally for PBC staging since the 1960s, and are based on the histopathological findings of PBC. In addition, two novel histological scoring systems for PBC have been proposed by Japanese and French scholars, respectively.
Cholestasis, which is a general clinical manifestation in hepatic illnesses that gives rise to reactive hyperplasia of the bile ducts, is the main complication in PBC patients. PBC diagnosis can be made in a patient via high serum AMAs in the presence of significantly elevated serum ALP, after ruling out other common or rare causes of cholestasis, such as viral hepatitides, drug-induced hepatic damage, alcoholic liver disease, intrahepatic cholestasis of pregnancy, progressive familial intrahepatic cholestasis, autoimmune hepatitides (AIH), primary sclerosing cholangitis (PSC), immunoglobulin G4-associated sclerosing cholangitis (IgG4-SC), and autoimmune hepatic illnesses overlap syndrome (PBC/AIH, PSC/AIH, PBC/PSC, PBC/IgG4-SC), as well as biliary obstructions such as biliary calculi, biliary ascariasis, biliary tract inflammation, postoperative bile duct benign stricture, pancreatic pseudocyst, cholangiocarcinoma, and pancreatic head carcinoma. PBC diagnosis requires two of the three following objective criteria: (1) biochemical proof of intrahepatic cholestasis based primarily on elevated levels of serum ALP greater than or equal to 1.5 times the upper limit of normal (ULN) for more than 24 wk; (2) presence of serum titers of AMAs greater than or equal to 1:40; and (3) liver histology characterized by non-suppurative cholangitis and granulomatous destruction of interlobular bile ducts[9,10,144]. Furthermore, patients with PBC frequently have elevated serum levels of ALT, AST and IgM[144].
Autoimmune liver diseases (AILDs) cover PBC, PSC, IgG4-SC and AIH; PBC should therefore be distinguished from AMA-positive AIH, PSC, or IgG4-SC. In addition, some AILDs patients present with features of PBC or PSC and AIH or IgG4-SC, either simultaneously or consecutively. They are traditionally deemed as obvious entities, although shared modes in so-called “overlap syndrome” have been recognized across the spectrum. The diagnosis of such overlap syndromes as PBC/AIH, PSC/AIH, PBC/PSC, and PBC/IgG4-SC is still challenging, but it is indispensable to diagnosis due to its rapid progression to cirrhosis and liver failure. Overlap syndromes should be considered in AIH patients with cholestatic findings, concurrent inflammatory bowel disease (IBD), or steroid-refractory disease. Clinical, biochemical, immunological, histological, and bile duct imaging characteristics contribute to the diagnosis of AILD overlap syndrome.
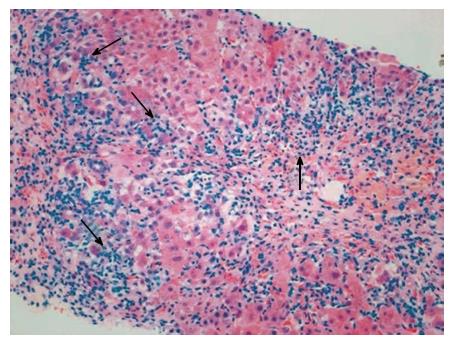
AIH is an immune-mediated severe hepatopathy characterized by elevated serum levels of ALT, AST and IgG, a high percentage of circulating non-organ-specific autoantibodies, and histologically with interface hepatitis (Figure 3)[145]. The incidence rates of AIH display a positive correlation with the national HDI (r = 0.638, P = 0.014) and the income index (r = 0.649, P = 0.012)[13]. AIH is divided into type 1, which is defined according to the seropositivity of smooth muscle autoantibody (SMA) and/or ANAs, and type 2, which is defined according to the seropositivity of liver-kidney microsome type 1 and/or liver cytosol type 1[145]. The non-classical clinical phenotypes of AIH, particularly AMA-positive AIH, should be distinguished from PBC. In general, AMAs-M2 antibody is specific to PBC patients, but may also be occasionally discovered in certain AIH patients[146]. Efficient means of discriminating between AIH and PBC are required, due to the fact that their clinical process and treatment are disparate[146]. One recent study has shown that antibodies to filamentous-actin (anti-F-actin) protein can not only be considered the serological marker of type 1 AIH, but may also predict AIH recurrence[147]. Furthermore, the application of repetition hepatic biopsy is an efficient method for AIH diagnosing comorbid liver conditions[148]. Although certain AIH patients were detected to be AMAs-M2 (+), the titers were markedly reduced compared to PBC patients[146]. During the follow-up period, the serum titers of AMAs-M2 were reduced in AIH patients[146].
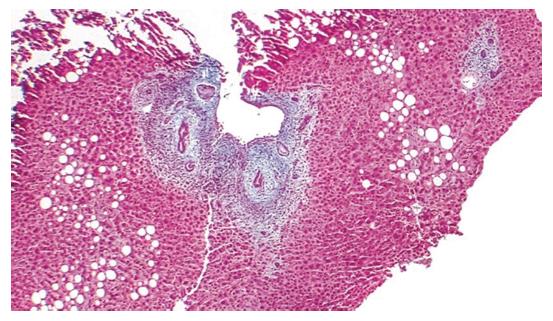
PSC is an immune-mediated chronic idiopathic cholestatic hepatobiliary illness characterized by progressive fibrosis and the stricturing of medium and large-sized extrahepatic and/or intrahepatic bile ducts (Figure 4)[9]. PSC is related to an elevated risk of cholangiocarcinoma and when IBD is present for colorectal carcinoma. Approximately 75% of PSC patients suffer from IBD; primarily ulcerative colitis (UC)[9]. Although no statistical correlation between PSC incidence and HDI was discovered (r = 0.116, P = 0.706), the income index was positively related to PSC incidence (r = 0.599, P = 0.031)[13]. PSC classically evolves slowly over 10 to 15 years, eventually leading to biliary cirrhosis and premature death due to decompensated hepatic illness in the majority of patients[149]. Additional complications of PSC include hepatic osteodystrophy, dominant bile duct stenosis, recurrent cholangitis, and such disease-related malignancies as hepatobiliary (especially cholangiocarcinoma), pancreatic, and colorectal (especially with IBD) carcinoma[149]. In one recent study, 65% of patients with long-term IBD had subclinical PSC related to progressive IBD, with no biochemical anomalousness and mild illness, based on magnetic resonance cholangiography findings[150]. There is currently no specific biomarker for PSC, although the prevalence of p-ANCA has been reported to range from 33% to 85% in patients with PSC[149]. Other non-specific autoantibodies in PSC cover ANAs and SMA[149].
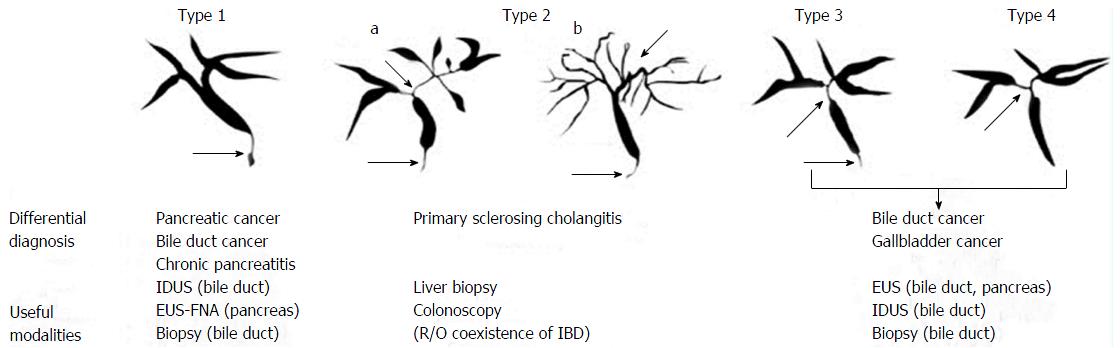
IgG4-SC is an immune-mediated peculiar sclerosing cholangitis of unknown etiopathogenesis that is frequently related to autoimmune pancreatitis (AIP)[151]. The diagnosis of IgG4-SC is performed on the basis of a combination of the following four standards: (1) distinctive cholangiography features; (2) elevated serum levels of IgG4; (3) concurrence of IgG4-associated illnesses, excluding those of the bile duct; and (4) typical histopathological characteristics[151,152]. Moreover, the efficiency of corticosteroid treatment is a selectable additional diagnostic standard to affirm a precise diagnosis of IgG4-SC[151,152]. Typical characteristics of IgG4-SC may be divided into four types on the basis of the stenosis regions disclosed by endoscopic retrograde cholangiography and anti-diastole (Figure 5)[152].
PBC/AIH overlap syndrome patients, including both characteristics of PBC and AIH, were diagnosed based on the Paris diagnostic criteria proposed by Chazouillères et al[153]. Characteristics of PBC were the following: (1) serum ALP levels more than twice the ULN value and/or γ-GT five or more times the ULN value; (2) serum AMA positivity; and (3) histopathological evidence of bile duct damage[153]. Characteristics of AIH were: (1) ALT elevation at a minimum of five times the ULN value; (2) levels of IgG at a minimum of twice the ULN valve and/or SMA positivity; and (3) hepatic biopsy revealed interface hepatitides in the presence of moderate to serious periportal lymphocyte infiltration[153]. Diagnosis of PBC/AIH overlap syndrome was considered with the presence of 2/3 of the criteria[153]. According to the Paris diagnostic criteria: (1) PBC/AIH overlap syndromes are uncommon; (2) flares of AIH can appear either voluntarily or under UDCA; and (3) a combination of corticosteroids and UDCA is requested in the majority of patients in order to achieve the best efficient biochemical response[153]. In addition, the revised and simplified diagnostic criteria for AIH were established by the International Autoimmune Hepatitis Group (IAIHG) in 1999[154] and 2008, respectively[155]. The latter is constructed on the basis of four clinical components that appear to be more peculiar in PBC/AIH patients[156]. The simplified diagnostic criteria seem to be more effective in comparison with the Paris diagnostic criteria and revised diagnostic criteria for patients with PBC/AIH overlap syndrome[157]. However, the IAIHG’s position statement on this controversial issue suggests that patients with AILDs should be classified on the basis of their dominant characteristics as PBC, AIH or PSC/small duct PSC, and that those with overlapping characteristics should not be referred to as unique diagnostic entities[158]. Combination treatment with budesonide and UDCA was more efficacious than UDCA monotherapy for PBC/AIH overlap syndrome[159]. Furthermore, combination treatment with immunosuppression and UDCA offered better short-term responses in PBC/AIH overlap syndromes[160].
PSC/AIH overlap syndrome is a comparatively infrequent variant of PSC[161]. There were remarkable distinctions in the below listed arguments, such as mean age (P < 0.01), serum levels of AST (P < 0.005), ALT (P < 0.005), and IgG (P < 0.0001) in PSC/AIH overlap syndromes compared with “typical” PSC patients[161]; the former seemingly profits from combination treatment with UDCA and immunosuppression, while survival is distinctly superior in the latter[161]. In addition, the clinical course of PSC/AIH overlap syndrome appears to be superior to typical PSC, suggesting that immunosuppression likely has an active efficacy on the development of PSC composition[162].
PBC/PSC overlap syndromes demonstrating the clinical manifestations of both PBC and PSC are an exceedingly uncommon condition that has been reported in a mere eight published cases, including the previously mentioned two cases[163].
IgG4-SC and PBC are two distinct autoimmune liver diseases. Approximately 90% of IgG4-SC patients have AIP, so therefore the presence of AIP may contribute to the diagnosis of IgG4-SC[151,152]. Nevertheless, PBC/IgG4-SC overlap syndrome is an extremely rare condition that has been reported in very few published cases to date, with the diagnosis of PBC/IgG4-SC overlap syndromes without the coexistence of AIP being particularly difficult[164]. Serum IgG4 concentrations may be worthwhile detecting in patients with PBC intractable to routine therapy[164].
The natural history pattern of PBC has observably changed over the past 20 years due to earlier diagnosis and the introduction of UDCA treatment. However, little is known about the natural history of PBC patients without efficient therapy. Hence, an epidemiological survey of the natural history of PBC patients in the absence of treatment might contribute to a greater understanding of the natural history of patients with UDCA-resistant PBC and in developing criteria for estimating UDCA response. A recent study demonstrated greatly reduced serum levels of ALP and very slight fluctuations in the other biochemical parameters of PBC patients treated with placebo at the 2 year follow-up period[165]. There was histological development in 39.4% of patients treated with placebo and a mild worsening of histological grade after 2 years of research[165]. In the meantime, histological progression was observed in 39.4% of the placebo-treated patients, with a moderate deterioration in histological scores noted after 2 years. Furthermore, the pooled 2 year rates of death, transplant, and progression of varicosities were 11.4%, 8.7% and 10.6%, respectively, in patients treated with placebo[165]. The natural history of PBC patients with AIH characteristics significantly differs from those without AIH characteristics[160]. In addition, although considered to possess a higher prevalence rate of AMAs, first-degree relatives of PBC patients have a lower risk of developing PBC over time, especially in those without baseline biochemical test evidence of intrahepatic cholestasis[166].
The optimal dosage for UDCA of 13-15 mg/kg per day is the standardized treatment for PBC[9,10], as it can postpone its development, improve long-term clinical outcomes, and is extremely safe and well-tolerated. Therefore, reliable identification of so-called treatment non-response to UDCA is very important, not only for selecting PBC patients who could benefit from new therapeutic approaches, but also for discerning those who are at low risk of developing end-stage PBC. The biochemical response to UDCA after 1 year of treatment in PBC has been deemed to be a powerful predictive indicator of long-term clinical outcomes and thus facilitate the rapid recognition of patients requiring novel treatment methods. However, another study demonstrated that, in comparison with biochemical responses assessed after 12 mo of UDCA treatment, biochemical responses at the 6 mo mark showed higher positive predictive value and negative predictive value, as well as lower negative likelihood ratio according to all criteria used in the Paris, Toronto, Barcelona, and Ehime definitions[167]. Therefore, the biochemical responses at the 6th month may be served as a new standard of prediction substitute for those assessed after 12 mo of UDCA treatment[167]. In addition, the UK-PBC risk scores (composed of baseline albumin, bilirubin, platelet count, ALT, AST and ALP) after 1 year of UDCA treatment might not only be available for identification in higher risks patients for rigorous surveillance and 2nd-line treatments, as well as lower risks patients who could possibly be tracked after observation during initial treatment, but the 5-, 12- and 15-year risk scores might also be considered extremely precise[168].
Budesonide is a corticosteroids receptor/pregnane X receptor (PXR) agonist[9,10]. Treble treatment with budesonide (6 mg/d), UDCA (13-15 mg/kg per day), and mycophenolate mofetil (1.5 g/d) may afford an advantage in non-cirrhotic PBC patients with characteristics of serious illness without biochemical response to UDCA[169]. Combination therapy of budesonide (6 mg/d) and UDCA (15 mg/kg per day) was able to ameliorate the plasma biochemical index of hepatic function and hepatic histology, particularly in PBC patients with hepatic fibrosis (grade I-III), whereas the treatment effectiveness of UDCA alone was principally on lab results[170]. Although larger studies are still required, the preparatory results of agents targeting PXR, such as budesonide, have been encouraging, particularly in subsets of patients with PBC, and may mark a new therapeutic era[169,170].
The immunosuppressive agent methotrexate (MTX) has a long history in the treatment of PBC, however little is known about its action mechanisms and roles, if any[9,10]. MTX was assessed for PBC treatment, which is currently recommended only in patients for whom PBC failed to respond adequately to UDCA and in AIH/PBC overlap syndromes[171,172]. PBC patients with characteristics of AIH should be considered for immunosuppressive therapy[158], with the therapeutic goal being to attain normal serum aminotransferase levels and histological improvement[158]. In patients who responded improperly to UDCA, MTX observably improved hepatic enzyme tests and hepatic histology[171]. Combination therapy of UDCA and MTX brought about continuous clinical anesis in a subgroup of PBC patients, and the response to a combination of MTX and UDCA appears to be more time-proof[172].
FXR is the receptor for primary bile acids expressed in enterohepatic tissues, where it regulates bile acid uptake, metabolism, and disposal, and has been considered a significant target for intrahepatic cholestatic illness therapy[173-176]. Obeticholic acid (OCA) is a semi-synthetic bile acid analogue for 6α-ethyl-chenodeoxycholic acid that is nearly 100-fold more potent than chenodeoxycholic acid (CDCA) and is a powerful, first class alternative FXR agonist derived from primary human bile acid CDCA, the natural endogenous FXR agonist[173]. OCA is being developed by Intercept Pharmaceuticals for the treatment of a variety of intrahepatic cholestatic illnesses, and has lately been permitted expedited approval in the United States for the treatment of PBC in combination with UDCA in adults with inappropriate response to UDCA or as monotherapy in adults unable to tolerate UDCA[174]; OCA (OcalivaTM) is in preregistration for this function in the European Union[174]. A randomized controlled clinical trial showed that treatment with OCA (10-50 mg/d) observably decreased the serum concentrations of γ-GT, ALP and ALT in PBC patients with inappropriate response to UDCA, in comparison with placebo[175]. Furthermore, PBC patients treated with OCA (10 mg/d) had the lowest incidence rates and seriousness of itching[175]. Clinical trials demonstrated the treatment effectiveness of OCA in PBC without biochemical response to UDCA, as evidenced by changes in laboratory parameters substituted for long-term clinical outcomes[176]. Dose-dependent itching is a usual side-effect of OCA, but can be overcome via dose-titration[176]. Furthermore, INT-767, which is another steroidal semi-synthetic bile acid analogue, has been testified to be able to modulate the activity of monocytes and macrophages, decrease inflammation though the inactivation of NF-κB via a protein kinase A dependent pattern, and decrease hepatic damage by promoting biliary bicarbonate excretion as a dual FXR and TGR5 agonist[173].
Recurrence of PBC after hepatic transplant has been proven to adversely influence transplant and patient survival. Protective potencies of cyclosporine A (CyA) against PBC recurrence after hepatic transplant have been reported[177]. Changing from tacrolimus to CyA was possible without sequelae, with no patients demonstrating recurrence of PBC[177]. Therefore, CyA might be serviceable for the prevention of PBC recurrence after living-donor hepatic transplant[177]. However, a retrospective multicenter study in Japan showed that although there was no influence on patient survival, original immunosuppression with CyA was considered to be major risk for PBC recurrence after hepatic transplant[178]. However, in subset analysis, switching from tacrolimus to CyA within 12 mo reduced recurrence[178].
Fibrates, including bezafibrate and fenofibrate, may be useful for treating asymptomatic patients with PBC who exhibit inappropriate response to UDCA[179-183]. A nationwide retrospective survey in Japan demonstrated that normalizing serum ALT concentrations with accessional bezafibrate therapy observably reduced the occurrence of hepatic illness-associated clinical signs in symptomless patients, with PBC responding incompletely to UDCA[179]. Moreover, long-term combined bezafibrate and UDCA treatment in PBC not only observably ameliorated the Mayo risk score and serum concentrations of ALP, but also observably elevated the serum concentrations of creatinine[180]. Hence, it is very important to consider adverse drug reaction associated with long-term combination treatment[180]. Although the treatment effectiveness of fenofibrate has been confirmed to be related to obvious improvement in ALP, decompensation amelioration, and hepatic transplantation-free survival in patients with PBC who reveal inappropriate responses to UDCA, fenofibrate should be more prudently applied in PBC, with frequent supervision for biochemical/clinical maladjustment[181]. Long-term fenofibrate therapy as a second-line auxiliary drug in PBC patients without appropriate response to UDCA was considered to be safe and efficient in ameliorating ALP, but did not markedly decrease the evaluated possibility of hepatic-associated death or demand for hepatic transplant[182]. In addition, the optimal dosage for fenofibrate (100-200 mg/d) seems to be efficient for assistant treatment in PBC patients without optimal biochemical response to UDCA[183].
Rituximab, an anti-CD20 monoclonal antibody that selectively depletes B cells, which are precursors of the autoantibody-producing plasmocytes, may be successfully used in autoimmune-mediated hepatic illnesses[184]. Selective depletion of B-cells with rituximab was safe and related to an obvious reduction in autoantibody product, but had limited biochemical effect in PBC patients without optimal biochemical response to UDCA[184]. The efficacy of B-cell depletion with rituximab therapy and the significant improvement in both biochemical and immunologic markers that it provides has been found in PBC patients with inappropriate biochemical response to UDCA[185]. The results of these studies demonstrate that depletion of B-lymphocyte affects the inductiveness, maintenance, and activation of both B- and T-lymphocytes, and offers an underlying principle for the treatment of PBC patients with incomplete response to UDCA[184,185].
Mesenchymal stem cell (MSC) transplantation is considered to be safe, and has been diffusely tested in autoimmune hepatic disease clinical trials with encouraging results[186,187]. MSC transplantation could modulate the systemic immune response and promote recovery in hepatic inflammation of PBC[186,187]. One single-arm clinical trial has shown that umbilical cord-derived MSC (UC-MSC) transplantation is viable and well-tolerance in patients with PBC, who response only partly to UDCA therapy, hence the need for a new treatment method for PBC patients in this subset[186]. However, the exact effect of UC-MSC transplantation in patients with PBC still requires confirmation by a larger placebo-controlled randomized clinical trial[186]. In addition, allogeneic bone marrow MSC transplantation has been confirmed to safely improve histologic fibrosis and hepatic function in UDCA-resistant PBC patients[187].
At present, liver transplantation (LT) is still a lifesaving approach with outstanding results for end-stage PBC patients[177,178]. Although the 15 year survival of PBC patients was confirmed as 52.6% after LT, regrettably, the recurrent rates of PBC were 21%-37% and 43% at 10 and 15 years after LT, respectively[178,188]. Though there is still no specific treatment for recurrent PBC (rPBC), cyclosporine A and UDCA may be useful for the prevention of rPBC after LT[177,188,189]. Furthermore, the expression of mitochondrial proteins in small biliary ducts may be a beneficial diagnostic hallmark for both end-stage PBC and rPBC after LT[190].
In the past decade, recent advances in PBC have attempted to improve the accuracy of the disease’s diagnosis and prognosis, as well as affording the chance to refine therapeutic methods. Promising novel therapies, including budesonide, fibrates, and rituximab, are being tested in PBC patients on the basis of understanding thoroughly the cellular and molecular mechanisms touched upon in all histological stages of PBC, from the early autoimmune-mediated bile duct epithelial cell damage to the destructive and illness-persistent influences of intrahepatic cholestasis, and finally giving rise to hepatic fibrosis and hepatic cirrhosis progression. Although much progress has been seen in the last 5 to 10 years, including in the diagnosis and treatment of PBC, the ultimate challenge for physicians is reducing UDCA non-responders and recurrent PBC after liver transplantation. Some novel therapeutic agents, including FXR agonists like OCA, FXR/TGR5 agonists like as INT-767, and PPAR-alpha have been identified as novel targets for drug development, with further investigation in PBC-related clinical trials still being implemented. Results of ongoing clinical trials and burgeoning therapeutic paradigms for PBC patients will likely further improve medical management and stride toward accurate treatment in the near foreseeable future.
| 1. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Gastroenterology. 2015;149:1627-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing Nomenclature for PBC: From ‘Cirrhosis’ to ‘Cholangitis’. Am J Gastroenterol. 2015;110:1536-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Gut. 2015;64:1671-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Hepatology. 2015;62:1620-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 5. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. J Hepatol. 2015;63:1285-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Dig Liver Dis. 2015;47:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Clin Res Hepatol Gastroenterol. 2015;39:e57-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing Nomenclature for PBC: From ‘Cirrhosis’ to ‘Cholangitis’. Clin Gastroenterol Hepatol. 2015;13:1867-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 908] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1227] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 11. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Floreani A, Franceschet I, Perini L, Cazzagon N, Gershwin ME, Bowlus CL. New therapies for primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Pan HY, Dai YN, Zheng JN, Shi KQ, Van Poucke S, Zou H, Zheng MH. National incidence of autoimmune liver diseases and its relationship with the human development index. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sun Y, Zhang W, Evans JF, Floreani A, Zou Z, Nishio Y, Qi R, Leung PS, Bowlus CL, Gershwin ME. Autotaxin, Pruritus and Primary Biliary Cholangitis (PBC). Autoimmun Rev. 2016;15:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kim WR, Lindor KD, Locke GR, Therneau TM, Homburger HA, Batts KP, Yawn BP, Petz JL, Melton LJ, Dickson ER. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631-1636. [PubMed] |
| 16. | Myers RP, Shaheen AA, Fong A, Burak KW, Wan A, Swain MG, Hilsden RJ, Sutherland L, Quan H. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology. 2009;50:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lleo A, Jepsen P, Morenghi E, Carbone M, Moroni L, Battezzati PM, Podda M, Mackay IR, Gershwin ME, Invernizzi P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci Rep. 2016;6:25906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Koulentaki M, Mantaka A, Sifaki-Pistolla D, Thalassinos E, Tzanakis N, Kouroumalis E. Geoepidemiology and space-time analysis of Primary biliary cirrhosis in Crete, Greece. Liver Int. 2014;34:e200-e207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Boonstra K, Kunst AE, Stadhouders PH, Tuynman HA, Poen AC, van Nieuwkerk KM, Witteman EM, Hamann D, Witteman BJ, Beuers U. Rising incidence and prevalence of primary biliary cirrhosis: a large population-based study. Liver Int. 2014;34:e31-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Baldursdottir TR, Bergmann OM, Jonasson JG, Ludviksson BR, Axelsson TA, Björnsson ES. The epidemiology and natural history of primary biliary cirrhosis: a nationwide population-based study. Eur J Gastroenterol Hepatol. 2012;24:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470-475. [PubMed] |
| 22. | Kim KA, Ki M, Choi HY, Kim BH, Jang ES, Jeong SH. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment Pharmacol Ther. 2016;43:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Liu H, Liu Y, Wang L, Xu D, Lin B, Zhong R, Gong S, Podda M, Invernizzi P. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544-2555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 476] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Invernizzi P, Ransom M, Raychaudhuri S, Kosoy R, Lleo A, Shigeta R, Franke A, Bossa F, Amos CI, Gregersen PK. Classical HLA-DRB1 and DPB1 alleles account for HLA associations with primary biliary cirrhosis. Genes Immun. 2012;13:461-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Joshita S, Umemura T, Nakamura M, Katsuyama Y, Shibata S, Kimura T, Morita S, Komatsu M, Matsumoto A, Yoshizawa K. STAT4 gene polymorphisms are associated with susceptibility and ANA status in primary biliary cirrhosis. Dis Markers. 2014;2014:727393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Yang L, Zhang H, Jiang YF, Jin QL, Zhang P, Li X, Gao PJ, Niu JQ. Association of Estrogen Receptor Gene Polymorphisms and Primary Biliary Cirrhosis in a Chinese Population: A Case-Control Study. Chin Med J (Engl). 2015;128:3008-3014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Hirschfield GM, Xie G, Lu E, Sun Y, Juran BD, Chellappa V, Coltescu C, Mason AL, Milkiewicz P, Myers RP. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun. 2012;13:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Li P, Lu G, Cui Y, Wu Z, Chen S, Li J, Wen X, Zhang H, Mu S, Zhang F. Association of IL12A Expression Quantitative Trait Loci (eQTL) With Primary Biliary Cirrhosis in a Chinese Han Population. Medicine (Baltimore). 2016;95:e3665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Li P, Lu G, Wang L, Cui Y, Wu Z, Chen S, Li J, Wen X, Zhang H, Mu S. A rare nonsynonymous variant in the lipid metabolic gene HELZ2 related to primary biliary cirrhosis in Chinese Han. Allergy Asthma Clin Immunol. 2016;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Cavalli M, Pan G, Nord H, Wallerman O, Wallén Arzt E, Berggren O, Elvers I, Eloranta ML, Rönnblom L, Lindblad Toh K. Allele-specific transcription factor binding to common and rare variants associated with disease and gene expression. Hum Genet. 2016;135:485-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Lleo A, Zhang W, Zhao M, Tan Y, Bernuzzi F, Zhu B, Liu Q, Tan Q, Malinverno F, Valenti L. DNA methylation profiling of the X chromosome reveals an aberrant demethylation on CXCR3 promoter in primary biliary cirrhosis. Clin Epigenetics. 2015;7:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Lammert C, Nguyen DL, Juran BD, Schlicht E, Larson JJ, Atkinson EJ, Lazaridis KN. Questionnaire based assessment of risk factors for primary biliary cirrhosis. Dig Liver Dis. 2013;45:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Smyk D, Rigopoulou EI, Bizzaro N, Bogdanos DP. Hair dyes as a risk for autoimmunity: from systemic lupus erythematosus to primary biliary cirrhosis. Auto Immun Highlights. 2013;4:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 443] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 37. | Quarneti C, Muratori P, Lalanne C, Fabbri A, Menichella R, Granito A, Masi C, Lenzi M, Cassani F, Pappas G. Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver Int. 2015;35:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Silveira MG, Gossard AA, Stahler AC, Jorgensen RA, Petz JL, Ali AH, Lindor KD. A Randomized, Placebo-Controlled Clinical Trial of Efficacy and Safety: Modafinil in the Treatment of Fatigue in Patients With Primary Biliary Cirrhosis. Am J Ther. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Guo GY, Shi YQ, Wang L, Ren X, Han ZY, Guo CC, Cui LN, Wang JB, Zhu J, Wang N. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment Pharmacol Ther. 2015;42:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Agmon-Levin N, Kopilov R, Selmi C, Nussinovitch U, Sánchez-Castañón M, López-Hoyos M, Amital H, Kivity S, Gershwin EM, Shoenfeld Y. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol Res. 2015;61:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Yamaguchi S, Maruyama T, Wakino S, Tokuyama H, Hashiguchi A, Tada S, Homma K, Monkawa T, Thomas J, Miyashita K. A case of severe osteomalacia caused by Tubulointerstitial nephritis with Fanconi syndrome in asymptomotic primary biliary cirrhosis. BMC Nephrol. 2015;16:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Guañabens N, Ruiz-Gaspà S, Gifre L, Miquel R, Peris P, Monegal A, Dubrueil M, Arias A, Parés A. Sclerostin Expression in Bile Ducts of Patients with Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J Bone Miner Res. 2016;31:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Phillips JR, Angulo P, Petterson T, Lindor KD. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol. 2001;96:2745-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Maruyama H, Kondo T, Sekimoto T, Takahashi M, Fujiwara K, Imazeki F, Yokosuka O. Retrograde detection of the intrahepatic portal vein in primary biliary cirrhosis: is sinusoidal blockage the underlying pathophysiology? Eur J Gastroenterol Hepatol. 2015;27:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Iguchi H, Oda M, Yamazaki H, Yoshimura K, Ando W, Yokomori H. Aquaporin-1 is associated with arterial capillary proliferation and hepatic sinusoidal transformation contributing to portal hypertension in primary biliary cirrhosis. Med Mol Morphol. 2014;47:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Ali AH, Sinakos E, Silveira MG, Jorgensen RA, Angulo P, Lindor KD. Varices in early histological stage primary biliary cirrhosis. J Clin Gastroenterol. 2011;45:e66-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Ikeda F, Okamoto R, Baba N, Fujioka S, Shoji B, Yabushita K, Ando M, Matsumura S, Kubota J, Yasunaka T. Prevalence and associated factors with esophageal varices in early primary biliary cirrhosis. J Gastroenterol Hepatol. 2012;27:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Wu QM, Zhao XY, You H. Quantitative fibrosis parameters highly predict esophageal-gastro varices in primary biliary cirrhosis. Eur Rev Med Pharmacol Sci. 2016;20:1037-1043. [PubMed] |
| 49. | Shen M, Zhang F, Zhang X. Pulmonary hypertension in primary biliary cirrhosis: a prospective study in 178 patients. Scand J Gastroenterol. 2009;44:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441. [PubMed] |
| 51. | Bektas M, Seven G, Idilman R, Yakut M, Doğanay B, Kabacam G, Ustun Y, Korkut E, Kalkan Ç, Sahin G, Cetinkaya H, Bozkaya H, Yurdaydin C, Bahar K, Cinar K, Soykan I. Manometric assessment of esophageal motor function in patients with primary biliary cirrhosis. Eur J Intern Med. 2014;25:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Shen M, Zhang F, Zhang X. Primary biliary cirrhosis complicated with interstitial lung disease: a prospective study in 178 patients. J Clin Gastroenterol. 2009;43:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Chen CT, Tseng YC, Yang CW, Lin HH, Chen PJ, Huang TY, Shih YL, Chang WK, Hsieh TY, Chu HC. Increased Risks of Spontaneous Bacterial Peritonitis and Interstitial Lung Disease in Primary Biliary Cirrhosis Patients With Concomitant Sjögren Syndrome. Medicine (Baltimore). 2016;95:e2537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Franco I, Dubini A, Piciucchi S, Casoni G, Poletti V. Interstitial lung disease preceding primary biliary cirrhosis in a male patient. Rev Port Pneumol (2006). 2015;21:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Parés A, Rimola A, Bruguera M, Mas E, Rodés J. Renal tubular acidosis in primary biliary cirrhosis. Gastroenterology. 1981;80:681-686. [PubMed] |
| 56. | Iannone F, Falappone P, Pannarale G, Gentile A, Grattagliano V, Covelli M, Lapadula G. Microscopic polyangiitis associated with primary biliary cirrhosis. J Rheumatol. 2003;30:2710-2712. [PubMed] |
| 57. | Sakamaki Y, Hayashi M, Wakino S, Fukuda S, Konishi K, Hashiguchi A, Hayashi K, Itoh H. A case of membranous nephropathy with primary biliary cirrhosis and cyclosporine-induced remission. Intern Med. 2011;50:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Goto T, Komatsu M, Fujii T, Ohshima S, Nakane K, Yoneyama K, Shibuya T, Meng XW, Masamune O, Imai H. Primary biliary cirrhosis associated with membranous glomerulonephritis. Intern Med. 1999;38:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Iwakura T, Fujigaki Y, Matsuyama T, Fujikura T, Ohashi N, Yasuda H, Kato A, Baba S. Tubulointerstitial nephritis and primary biliary cirrhosis with a T cell-dominant profile of infiltrating cells and granulomas in both organs. Intern Med. 2013;52:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Kornblihtt LI, Vassalllu PS, Heller PG, Lago NR, Alvarez CL, Molinas FC. Primary myelofibrosis in a patient who developed primary biliary cirrhosis, autoimmune hemolytic anemia and fibrillary glomerulonephritis. Ann Hematol. 2008;87:1019-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Macdougall IC, Isles CG, Whitworth JA, More IA, MacSween RN. Interstitial nephritis and primary biliary cirrhosis: a new association? Clin Nephrol. 1987;27:36-40. [PubMed] |
| 62. | Komatsu T, Utsunomiya K, Oyaizu T. Goodpasture’s syndrome associated with primary biliary cirrhosis. Intern Med. 1998;37:611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Nakamura T, Kawagoe Y, Ueda Y, Koide H. Antineutrophil cytoplasmic autoantibody-associated rapidly progressive glomerulonephritis in a patient with primary biliary cirrhosis. Am J Med Sci. 2004;328:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Azak A, Koçak G, Huddam B, Koçak E, Ergül B, Duranay M. Focal segmental glomerulosclerosis associated with primary biliary cirrhosis. Ren Fail. 2011;33:1052-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Wang L, Zhang FC, Chen H, Zhang X, Xu D, Li YZ, Wang Q, Gao LX, Yang YJ, Kong F. Connective tissue diseases in primary biliary cirrhosis: a population-based cohort study. World J Gastroenterol. 2013;19:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 66. | Zhang XX, Wang LF, Jin L, Li YY, Hao SL, Shi YC, Zeng QL, Li ZW, Zhang Z, Lau GK. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol. 2015;21:3554-3563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Rong G, Wang H, Bowlus CL, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Chen Y, An L. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Tomiyama Y, Takenaka K, Kodama T, Kawanaka M, Sasaki K, Nishina S, Yoshioka N, Hara Y, Hino K. Risk factors for survival and the development of hepatocellular carcinoma in patients with primary biliary cirrhosis. Intern Med. 2013;52:1553-1559. [PubMed] |
| 69. | Trivedi PJ, Lammers WJ, van Buuren HR, Parés A, Floreani A, Janssen HL, Invernizzi P, Battezzati PM, Ponsioen CY, Corpechot C. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 70. | Mochizuki S, Nakayama H, Higaki T, Okubo T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S, Sugitani M, Takayama T. Repeat liver resection for hepatocellular carcinoma complicating primary biliary cirrhosis. Int Surg. 2013;98:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Rigopoulou EI, Zachou K, Gatselis NK, Papadamou G, Koukoulis GK, Dalekos GN. Primary biliary cirrhosis in HBV and HCV patients: Clinical characteristics and outcome. World J Hepatol. 2013;5:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Chen HW, Huang HH, Lai CH, Chang WE, Shih YL, Chang WK, Hsieh TY, Chu HC. Hepatitis C virus infection in patients with primary biliary cirrhosis. Ann Hepatol. 2013;12:78-84. [PubMed] |
| 73. | Javaid A, Poongkunran M, Allard FD, Kyaw W, Maung HH, Lau D. Subtle presentation of active primary biliary cirrhosis in chronic hepatitis B: a case report. Gastroenterol Rep (Oxf). 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Munday WR, DiCapua D, Vortmeyer A, Gomez JL. Guillain-Barré syndrome mimics primary biliary cirrhosis-related myopathy. Oxf Med Case Reports. 2015;2015:272-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Gonzalez-Moreno EI, Martinez-Cabriales SA, Cruz-Moreno MA, Borjas-Almaguer OD, Cortez-Hernandez CA, Bosques-Padilla FJ, Garza AA, Gonzalez-Gonzalez JA, Garcia-Compean D, Ocampo-Candiani J. Primary biliary cholangitis associated with warm autoimmune hemolytic anemia. J Dig Dis. 2016;17:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Nakayama S, Yokote T, Kobayashi K, Hirata Y, Akioka T, Miyoshi T, Oka S, Hiraoka N, Iwaki K, Takayama A. Primary hepatic MALT lymphoma associated with primary biliary cirrhosis. Leuk Res. 2010;34:e17-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Yamashita H, Suzuki A, Takahashi Y, Kaneko H, Kano T, Mimori A. Anti-neutrophil Cytoplasmic Antibody (ANCA)-associated Vasculitis Associated with Primary Biliary Cirrhosis: A Case Report and Literature Review. Intern Med. 2015;54:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Calvo J, Carbonell N, Scatton O, Marzac C, Ganne-Carrie N, Wendum D. Hepatic nodular lymphoid lesion with increased IgG4-positive plasma cells associated with primary biliary cirrhosis: a report of two cases. Virchows Arch. 2015;467:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Macaluso FS, Maida M, Alessi N, Cabibbo G, Cabibi D. Primary biliary cirrhosis and hereditary hemorrhagic telangiectasia: When two rare diseases coexist. World J Hepatol. 2013;5:288-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Iga N, Otsuka A, Iwata M, Ueda Y, Kabashima K. Generalized morphea with preceding severe pain and coexistent early primary biliary cirrhosis. Eur J Dermatol. 2015;25:365-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Taddy H, Yoshida EM, Gibson G, Chatur N. Acetylcholine receptor antibody positive generalized myasthenia gravis in association with primary biliary cirrhosis. Ann Hepatol. 2010;9:471-472. [PubMed] |
| 82. | Koide H, Sato K, Fukusato T, Kashiwabara K, Sunaga N, Tsuchiya T, Morino S, Sohara N, Kakizaki S, Takagi H. Spontaneous regression of hepatic inflammatory pseudotumor with primary biliary cirrhosis: case report and literature review. World J Gastroenterol. 2006;12:1645-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Tang KH, Schofield JB, Powell-Jackson PR. Primary biliary cirrhosis and idiopathic retroperitoneal fibrosis: a rare association. Eur J Gastroenterol Hepatol. 2002;14:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Volta U, Caio G, Tovoli F, De Giorgio R. Gut-liver axis: an immune link between celiac disease and primary biliary cirrhosis. Expert Rev Gastroenterol Hepatol. 2013;7:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Zhao SX, Zhang YG, Wang RQ, Li WC, Kong LB, Kong L, Nan YM. A Patient With Primary Biliary Cirrhosis Accompanied by Wilson’s Disease. Hepat Mon. 2016;16:e29077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Guerra-Uribe NB, González-Huezo MS. Bullous pemphigoid and primary biliary cirrhosis, an infrequent association: A case report. Rev Gastroenterol Mex. 2016;81:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Paul S, Sepehr GJ, Weinstein B, Roper J. Co-occurrence of idiopathic granulomatous hepatitis and primary biliary cirrhosis. Dig Dis Sci. 2014;59:2831-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Riviere E, Vergniol J, Reffet A, Lippa N, Le Bail B, de Ledinghen V. Gastric variceal bleeding uncovering a rare association of CREST syndrome, primary biliary cirrhosis, nodular regenerative hyperplasia and pulmonary hypertension. Eur J Gastroenterol Hepatol. 2010;22:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Triantafillidis JK, Durakis S, Merikas E. Crohn’s disease of the small bowel, complicated by primary biliary cirrhosis, Hashimoto thyroiditis, and Raynaud’s phenomenon: favorable response of all disorders to adalimumab treatment. Gastroenterol Hepatol Bed Bench. 2013;6:101-105. [PubMed] |
| 90. | Alempijević T, Sokić-Milutinović A, Toncev L, Pavlović-Marković A, Djuranović S, Tomanović N, Drulović J. Primary biliary cirrhosis and hepatic sarcoidosis--a case report. Vojnosanit Pregl. 2014;71:83-86. [PubMed] |
| 91. | Korkmaz H, Bugdaci MS, Temel T, Dagli M, Karabagli P. Autoimmune hepatitis-primary biliary cirrhosis overlap syndrome concomitant with immune hemolytic anemia and immune thrombocytopenic purpura (Evans syndrome). Clin Res Hepatol Gastroenterol. 2013;37:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Yaşar DG, Ozenirler S, Doğan M. A patient with primary biliary cirrhosis accompanied by Graves disease and Hurthle cell adenoma. Turk J Gastroenterol. 2007;18:198-200. [PubMed] |
| 93. | Hu S, Zhao F, Wang Q, Chen WX. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: a meta-analysis. Clin Chem Lab Med. 2014;52:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Juliusson G, Imam M, Björnsson ES, Talwalkar JA, Lindor KD. Long-term outcomes in antimitochondrial antibody negative primary biliary cirrhosis. Scand J Gastroenterol. 2016;51:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Cancado EL, Harriz M. The Importance of Autoantibody Detection in Primary Biliary Cirrhosis. Front Immunol. 2015;6:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Yamagiwa S, Kamimura H, Takamura M, Aoyagi Y. Autoantibodies in primary biliary cirrhosis: recent progress in research on the pathogenetic and clinical significance. World J Gastroenterol. 2014;20:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Bauer A, Habior A, Kraszewska E. Detection of anti-SP100 antibodies in primary biliary cirrhosis. Comparison of ELISA and immunofluorescence. J Immunoassay Immunochem. 2013;34:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Villalta D, Sorrentino MC, Girolami E, Tampoia M, Alessio MG, Brusca I, Daves M, Porcelli B, Barberio G, Bizzaro N. Autoantibody profiling of patients with primary biliary cirrhosis using a multiplexed line-blot assay. Clin Chim Acta. 2015;438:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Valour F, Durupt S, Khenifer S, Durieu I. Diagnostic value of anti-gp210 antibodies in primary biliary cirrhosis: a case-based review. BMJ Case Rep. 2013;2013:pii: bcr2013009803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Himoto T, Tanaka N, Saito A, Muro Y, Sugiura K, Tani J, Miyoshi H, Morishita A, Yoneyama H, Haba R. Diversity of humoral responses to the centromere proteins among HCV-related chronic liver disease, PBC and AIH patients. Clin Res Hepatol Gastroenterol. 2015;39:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Nakamura M. Clinical significance of autoantibodies in primary biliary cirrhosis. Semin Liver Dis. 2014;34:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Tana MM, Shums Z, Milo J, Norman GL, Leung PS, Gershwin ME, Noureddin M, Kleiner DE, Zhao X, Heller T. The Significance of Autoantibody Changes Over Time in Primary Biliary Cirrhosis. Am J Clin Pathol. 2015;144:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Saare M, Hämarik U, Venta R, Panarina M, Zucchelli C, Pihlap M, Remm A, Kisand K, Toots U, Möll K. SP140L, an Evolutionarily Recent Member of the SP100 Family, Is an Autoantigen in Primary Biliary Cirrhosis. J Immunol Res. 2015;2015:526518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. A possible involvement of p62/sequestosome-1 in the process of biliary epithelial autophagy and senescence in primary biliary cirrhosis. Liver Int. 2012;32:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Nakamura M, Kondo H, Tanaka A, Komori A, Ito M, Yamamoto K, Ohira H, Zeniya M, Hashimoto E, Honda M. Autoantibody status and histological variables influence biochemical response to treatment and long-term outcomes in Japanese patients with primary biliary cirrhosis. Hepatol Res. 2015;45:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Liberal R, Grant CR, Sakkas L, Bizzaro N, Bogdanos DP. Diagnostic and clinical significance of anti-centromere antibodies in primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:572-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Gatselis NK, Zachou K, Norman GL, Gabeta S, Papamichalis P, Koukoulis GK, Dalekos GN. Clinical significance of the fluctuation of primary biliary cirrhosis-related autoantibodies during the course of the disease. Autoimmunity. 2013;46:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Mandai S, Kanda E, Arai Y, Hirasawa S, Hirai T, Aki S, Inaba N, Aoyagi M, Tanaka H, Ikeda T. Anti-centromere antibody is an independent risk factor for chronic kidney disease in patients with primary biliary cirrhosis. Clin Exp Nephrol. 2013;17:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Hu CJ, Song G, Huang W, Liu GZ, Deng CW, Zeng HP, Wang L, Zhang FC, Zhang X, Jeong JS. Identification of new autoantigens for primary biliary cirrhosis using human proteome microarrays. Mol Cell Proteomics. 2012;11:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Norman GL, Yang CY, Ostendorff HP, Shums Z, Lim MJ, Wang J, Awad A, Hirschfield GM, Milkiewicz P, Bloch DB. Anti-kelch-like 12 and anti-hexokinase 1: novel autoantibodies in primary biliary cirrhosis. Liver Int. 2015;35:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.e5; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 112. | Giljaca V, Stimac D, Gluud C. Are levels of alkaline phosphatases and bilirubin surrogate markers of outcomes of patients with primary biliary cirrhosis? Gastroenterology. 2015;148:860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Tang YM, Wang JP, Bao WM, Yang JH, Ma LK, Yang J, Chen H, Xu Y, Yang LH, Li W. Urine and serum metabolomic profiling reveals that bile acids and carnitine may be potential biomarkers of primary biliary cirrhosis. Int J Mol Med. 2015;36:377-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 114. | Lleo A, Liao J, Invernizzi P, Zhao M, Bernuzzi F, Ma L, Lanzi G, Ansari AA, Coppel RL, Zhang P. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 115. | Zhang H, Li P, Wu D, Xu D, Hou Y, Wang Q, Li M, Li Y, Zeng X, Zhang F. Serum IgG subclasses in autoimmune diseases. Medicine (Baltimore). 2015;94:e387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Kawaguchi T, Tanaka T, Hashiguchi M, Miyoshi H, Akiba J, Kage M, Yano H, Ohshima K, Okamura T, Sata M. Decreased serum levels of immunoglobulin A, immunoglobulin M and immunoglobulin G in a patient with primary biliary cirrhosis: A case report. Hepatol Res. 2014;44:E261-E266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Shapira Y, Agmon-Levin N, Renaudineau Y, Porat-Katz BS, Barzilai O, Ram M, Youinou P, Shoenfeld Y. Serum markers of infections in patients with primary biliary cirrhosis: evidence of infection burden. Exp Mol Pathol. 2012;93:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Tan Y, Pan T, Ye Y, Ge G, Chen L, Wen D, Zou S. Serum microRNAs as potential biomarkers of primary biliary cirrhosis. PLoS One. 2014;9:e111424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 119. | Qin B, Huang F, Liang Y, Yang Z, Zhong R. Analysis of altered microRNA expression profiles in peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Ninomiya M, Kondo Y, Funayama R, Nagashima T, Kogure T, Kakazu E, Kimura O, Ueno Y, Nakayama K, Shimosegawa T. Distinct microRNAs expression profile in primary biliary cirrhosis and evaluation of miR 505-3p and miR197-3p as novel biomarkers. PLoS One. 2013;8:e66086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 121. | Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, Komatsu M, Matsumoto A, Ota M, Tanaka E. Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis. Am J Gastroenterol. 2015;110:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 122. | Wunsch E, Milkiewicz M, Wasik U, Trottier J, Kempińska-Podhorodecka A, Elias E, Barbier O, Milkiewicz P. Expression of hepatic Fibroblast Growth Factor 19 is enhanced in Primary Biliary Cirrhosis and correlates with severity of the disease. Sci Rep. 2015;5:13462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 123. | Zhao P, Liu WW, Li JF, Wang CY, Wang H, Xu J, Wang RF, Yang HZ, Jin C, Wei ZM. Predictors of liver failure in primary biliary cirrhosis. Ups J Med Sci. 2015;120:47-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 124. | Harada K, Kakuda Y, Nakamura M, Shimoda S, Nakanuma Y. Clinicopathological significance of serum fractalkine in primary biliary cirrhosis. Dig Dis Sci. 2013;58:3037-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 125. | Deng C, Hu C, Wang L, Zhang S, Li P, Wu Z, Chen S, Zhang F, Li Y. Serological comparative proteomics analysis of mitochondrial autoantibody-negative and -positive primary biliary cirrhosis. Electrophoresis. 2015;36:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Voumvouraki A, Koulentaki M, Notas G, Sfakianaki O, Kouroumalis E. Serum surrogate markers of liver fibrosis in primary biliary cirrhosis. Eur J Intern Med. 2011;22:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 127. | Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. 2015;7:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Weinmann A, Sattler T, Unold HP, Grambihler A, Teufel A, Koch S, Schuchmann M, Biesterfeld S, Wörns MA, Galle PR. Predictive scores in primary biliary cirrhosis: a retrospective single center analysis of 204 patients. J Clin Gastroenterol. 2015;49:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015;149:1804-1812.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 130. | Zhang HC, Hu RF, Zhu T, Tong L, Zhang QQ. Primary biliary cirrhosis degree assessment by acoustic radiation force impulse imaging and hepatic fibrosis indicators. World J Gastroenterol. 2016;22:5276-5284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Zhang DK, Chen M, Liu Y, Wang RF, Liu LP, Li M. Acoustic radiation force impulse elastography for non-invasive assessment of disease stage in patients with primary biliary cirrhosis: A preliminary study. Clin Radiol. 2014;69:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 133. | Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, Ang B, Bhatt A, Wang K, Aryafar H. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016;111:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 134. | Singh S, Venkatesh SK, Keaveny A, Adam S, Miller FH, Asbach P, Asbach P, Godfrey EM, Silva AC, Wang Z. Diagnostic accuracy of magnetic resonance elastography in liver transplant recipients: A pooled analysis. Ann Hepatol. 2016;15:363-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 135. | Meng Y, Liang Y, Liu M. The value of MRI in the diagnosis of primary biliary cirrhosis and assessment of liver fibrosis. PLoS One. 2015;10:e0120110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Takeyama Y, Tsuchiya N, Kunimoto H, Fukunaga A, Sakurai K, Hirano G, Yokoyama K, Morihara D, Anan A, Irie M. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging as a useful detection method for advanced primary biliary cirrhosis. Hepatol Res. 2015;45:E108-E114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 137. | You Z, Wang Q, Bian Z, Liu Y, Han X, Peng Y, Shen L, Chen X, Qiu D, Selmi C. The immunopathology of liver granulomas in primary biliary cirrhosis. J Autoimmun. 2012;39:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Kikuchi K, Tsuneyama K, Yamada H, Kajiyama Y, Matsumoto K, Tsunashima H, Yamashita R, Takai A, Negishi M, Hara M. Splenic lymph follicles generate immunoglobulin M-producing B cells in primary biliary cirrhosis. Hepatol Res. 2014;44:E253-E256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Sasaki M, Yoshimura-Miyakoshi M, Sato Y, Nakanuma Y. A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis. J Gastroenterol. 2015;50:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 140. | Aso-Ishimoto Y, Yamagiwa S, Ichida T, Miyakawa R, Tomiyama C, Sato Y, Watanabe H, Aoyagi Y. Increased activated natural killer T cells in the liver of patients with advanced stage primary biliary cirrhosis. Biomed Res. 2014;35:161-169. [PubMed] |
| 141. | Harada K, Hsu M, Ikeda H, Zeniya M, Nakanuma Y. Application and validation of a new histologic staging and grading system for primary biliary cirrhosis. J Clin Gastroenterol. 2013;47:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 142. | Wendum D, Boëlle PY, Bedossa P, Zafrani ES, Charlotte F, Saint-Paul MC, Michalak S, Chazouillères O, Corpechot C. Primary biliary cirrhosis: proposal for a new simple histological scoring system. Liver Int. 2015;35:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 143. | Seki H, Ikeda F, Nanba S, Moritou Y, Takeuchi Y, Yasunaka T, Onishi H, Miyake Y, Takaki A, Nouso K. Aberrant Expression of Keratin 7 in Hepatocytes as a Predictive Marker of Rapid Progression to Hepatic Failure in Asymptomatic Primary Biliary Cirrhosis. Acta Med Okayama. 2015;69:137-144. [PubMed] |
| 144. | Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev. 2014;13:441-444. [PubMed] |
| 145. | Liberal R, Vergani D, Mieli-Vergani G. Update on Autoimmune Hepatitis. J Clin Transl Hepatol. 2015;3:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 146. | Tomizawa M, Shinozaki F, Fugo K, Motoyoshi Y, Sugiyama T, Yamamoto S, Kishimoto T, Ishige N. Anti-mitochondrial M2 antibody-positive autoimmune hepatitis. Exp Ther Med. 2015;10:1419-1422. [PubMed] |
| 147. | Himoto T, Fujita K, Nomura T, Tani J, Miyoshi H, Morishita A, Yoneyama H, Haba R, Masaki T. Diagnostic Dilemma in the Detection of Antibodies to Filamentous Actin. Clin Lab. 2016;62:839-847. [PubMed] |
| 148. | Putra J, Toor A, Suriawinata AA. The utility of repeat liver biopsy in autoimmune hepatitis: a series of 20 consecutive cases. Pathology. 2016;48:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 149. | Schulze K, Weismüller TJ, Bubenheim M, Huebener P, Zenouzi R, Lenzen H, Rupp C, Gotthardt D, de Leuw P, Teufel A. Criteria Used in Clinical Practice to Guide Immunosuppressive Treatment in Patients with Primary Sclerosing Cholangitis. PLoS One. 2015;10:e0140525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 150. | Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, Viktil E, Henriksen M, Hovde Ø, Huppertz-Hauss G, Høie O, Lie Høivik M, Monstad I, Solberg IC, Jahnsen J, Karlsen TH, Moum B, Vatn M, Negård A. Prevalence of Sclerosing Cholangitis, Detected by Magnetic Resonance Cholangiography, in Patients with Long-term Inflammatory Bowel Disease. Gastroenterology. 2016;151:660-669.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 151. | Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 152. | Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 153. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 485] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 154. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [PubMed] |
| 155. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1308] [Article Influence: 72.7] [Reference Citation Analysis (1)] |
| 156. | Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 157. | Liu F, Pan ZG, Ye J, Xu D, Guo H, Li GP, Xu KS, Hou XH, Song YH. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: simplified criteria may be effective in the diagnosis in Chinese patients. J Dig Dis. 2014;15:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 159. | Zhang H, Yang J, Zhu R, Zheng Y, Zhou Y, Dai W, Wang F, Chen K, Li J, Wang C. Combination therapy of ursodeoxycholic acid and budesonide for PBC-AIH overlap syndrome: a meta-analysis. Drug Des Devel Ther. 2015;9:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 160. | Yang F, Wang Q, Wang Z, Miao Q, Xiao X, Tang R, Chen X, Bian Z, Zhang H, Yang Y. The Natural History and Prognosis of Primary Biliary Cirrhosis with Clinical Features of Autoimmune Hepatitis. Clin Rev Allergy Immunol. 2016;50:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 161. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. [PubMed] |
| 162. | Zenouzi R, Lohse AW. Long-term outcome in PSC/AIH “overlap syndrome”: does immunosuppression also treat the PSC component? J Hepatol. 2014;61:1189-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Floreani A, Motta R, Cazzagon N, Franceschet I, Roncalli M, Del Ross T, Rosina F, Lleo A, Mescoli C, Colloredo G. The overlap syndrome between primary biliary cirrhosis and primary sclerosing cholangitis. Dig Liver Dis. 2015;47:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 164. | Takemoto R, Miyake Y, Harada K, Nakanuma Y, Moriya A, Ando M, Hirohata M, Yamamoto K. Overlap of IgG4-related sclerosing cholangitis and primary biliary cirrhosis. Intern Med. 2014;53:1429-1433. [PubMed] |
| 165. | Xu P, Li L, Li G, Yu C, Li Y. Insight into the natural history of primary biliary cirrhosis: A systemic review of data from placebo-controlled clinical trials. Turk J Gastroenterol. 2016;27:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 166. | Gulamhusein AF, Juran BD, Atkinson EJ, McCauley B, Schlicht E, Lazaridis KN. Low incidence of primary biliary cirrhosis (PBC) in the first-degree relatives of PBC probands after 8 years of follow-up. Liver Int. 2016;36:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Zhang LN, Shi TY, Shi XH, Wang L, Yang YJ, Liu B, Gao LX, Shuai ZW, Kong F, Chen H. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: results of a 14-year cohort study. Hepatology. 2013;58:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 168. | Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, Griffiths L, Lim R, Trembling P, Williamson K. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 169. | Rabahi N, Chrétien Y, Gaouar F, Wendum D, Serfaty L, Chazouillères O, Corpechot C, Poupon R. Triple therapy with ursodeoxycholic acid, budesonide and mycophenolate mofetil in patients with features of severe primary biliary cirrhosis not responding to ursodeoxycholic acid alone. Gastroenterol Clin Biol. 2010;34:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 170. | Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 171. | Kaplan MM, Bonder A, Ruthazer R, Bonis PA. Methotrexate in patients with primary biliary cirrhosis who respond incompletely to treatment with ursodeoxycholic acid. Dig Dis Sci. 2010;55:3207-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 172. | Leung J, Bonis PA, Kaplan MM. Colchicine or methotrexate, with ursodiol, are effective after 20 years in a subset of patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2011;9:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 173. | Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 174. | Markham A, Keam SJ. Obeticholic Acid: First Global Approval. Drugs. 2016;76:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 175. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC, Parés A. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-761.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 448] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 176. | Trivedi PJ, Hirschfield GM, Gershwin ME. Obeticholic acid for the treatment of primary biliary cirrhosis. Expert Rev Clin Pharmacol. 2016;9:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 177. | Shiba H, Wakiyama S, Futagawa Y, Gocho T, Ito R, Furukawa K, Ishida Y, Misawa T, Yanaga K. Switching from tacrolimus to cyclosporine A to prevent primary biliary cirrhosis recurrence after living-donor liver transplantation. Int Surg. 2013;98:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 178. | Egawa H, Sakisaka S, Teramukai S, Sakabayashi S, Yamamoto M, Umeshita K, Uemoto S. Long-Term Outcomes of Living-Donor Liver Transplantation for Primary Biliary Cirrhosis: A Japanese Multicenter Study. Am J Transplant. 2016;16:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 179. | Tanaka A, Hirohara J, Nakanuma Y, Tsubouchi H, Takikawa H. Biochemical responses to bezafibrate improve long-term outcome in asymptomatic patients with primary biliary cirrhosis refractory to UDCA. J Gastroenterol. 2015;50:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 180. | Hosonuma K, Sato K, Yamazaki Y, Yanagisawa M, Hashizume H, Horiguchi N, Kakizaki S, Kusano M, Yamada M. A prospective randomized controlled study of long-term combination therapy using ursodeoxycholic acid and bezafibrate in patients with primary biliary cirrhosis and dyslipidemia. Am J Gastroenterol. 2015;110:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 181. | Cheung AC, Lapointe-Shaw L, Kowgier M, Meza-Cardona J, Hirschfield GM, Janssen HL, Feld JJ. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 182. | Hegade VS, Khanna A, Walker LJ, Wong LL, Dyson JK, Jones DE. Long-Term Fenofibrate Treatment in Primary Biliary Cholangitis Improves Biochemistry but Not the UK-PBC Risk Score. Dig Dis Sci. 2016;61:3037-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 183. | Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 184. | Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 185. | Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, Yang GX, Nakatani T, Vierling J, Lindor K. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 186. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 187. | Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, Yang YJ, Li YZ, Zhang X, Dong F. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 188. | Raczyńska J, Habior A, Pączek L, Foroncewicz B, Pawełas A, Mucha K. Primary biliary cirrhosis in the era of liver transplantation. Ann Transplant. 2014;19:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 189. | Bosch A, Dumortier J, Maucort-Boulch D, Scoazec JY, Wendum D, Conti F, Morard I, Rubbia-Brandt L, Terris B, Radenne S. Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence. J Hepatol. 2015;63:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 190. | Sasaki M, Hsu M, Yeh MM, Nakanuma Y. In recurrent primary biliary cirrhosis after liver transplantation, biliary epithelial cells show increased expression of mitochondrial proteins. Virchows Arch. 2015;467:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Invernizzi P, Lalor P, Licinio R S- Editor: Qiu S L- Editor: Rutherford A E- Editor: Li D