Published online Nov 8, 2016. doi: 10.4254/wjh.v8.i31.1327
Peer-review started: May 14, 2016
First decision: June 14, 2016
Revised: June 28, 2016
Accepted: August 15, 2016
Article in press: August 16, 2016
Published online: November 8, 2016
Processing time: 175 Days and 0.8 Hours
To compare patients who underwent resection of early stage hepatocellular cancer (HCC) in three different countries.
This retrospective study characterizes 573 stage I/II HCC patients treated with liver resection in 3 tertiary-referral centers: Tokyo (n = 250), Honolulu (n = 146) and Shanghai (n = 177).
Shanghai patients were younger, predominantly male, hepatitis-B seropositive (94%) and cirrhotic (93%). Tokyo patients were older and more likely to have hepatitis-C (67%), smaller tumors, low albumin, and normal alpha-fetoprotein. The Honolulu cohort had the largest tumors and 30% had no viral hepatitis. Age-adjusted mortality at 1 and 5-years were lower in the Tokyo cohort compared to Honolulu and there was no difference in mortality between Shanghai and Honolulu cohorts. Elevated alpha-fetoprotein, low albumin and tumor > 5 cm were associated with increased 1-year mortality. These factors and cirrhosis were independently associated with increased 5-year mortality. Independent risk factors of survival varied when examined separately by center.
The profile of early-stage HCC patients is strikingly different across countries and likely contributes to survival differences. Underlying differences in patient populations including risk factors/comorbidities influencing disease progression may also account for variation in outcomes.
Core tip: Treatment for hepatocellular cancer (HCC) depends on stage and liver function. Single-institution studies have characterized resection for HCC but this unique study combines the experience of three large hepatobiliary centers in different countries with 573 resections for stage I/II HCC in Tokyo (n = 250), Honolulu (n = 146) and Shanghai (n = 177). Groups differed in viral hepatitis, tumor size, alpha fetal protein (AFP) and cirrhosis. One and 5-year mortality was lowest in the Tokyo cohort. Elevated AFP, low albumin, tumor > 5 cm and cirrhosis were independently-associated with increased 5-year mortality. The profile of early-stage HCC patients is strikingly different across countries and likely contributes to survival differences.
- Citation: Wong LL, Hernandez BY, Shvetsov YB, Kawano Y, Tang ZY, Ji JF. Liver resection for early hepatocellular cancer: Comparison of centers in 3 different countries. World J Hepatol 2016; 8(31): 1327-1335
- URL: https://www.wjgnet.com/1948-5182/full/v8/i31/1327.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i31.1327
Hepatocellular cancer (HCC) is the fifth most common cancer in males and the ninth in females worldwide and is the second most deadly cancer. In 2012, there were 782000 HCC cases and 745000 deaths. HCC is more prominent in less-developed countries and more than 50% of cases were diagnosed in Asia[1]. In the United States, there were 35000 new cases of liver and intrahepatic bile duct cancer in 2015 and HCC is one of the few cancers that is increasing in both incidence and mortality[2]. The best treatments for early stage HCC include liver resection for those with adequate liver function and liver transplant for those with decompensated cirrhosis or tumor that is not amenable to resection. Multiple single center studies have demonstrated success with liver resection and transplant, but patient populations largely differ in underlying risk factors (viral hepatitis, diabetes, obesity, alcohol and smoking) and may differ by technique of resection, indications for resection, patient management, use of adjuvant therapy, and follow-up[3-8]. The use of liver resection may also vary depending on the availability of liver transplantation. Countries with relatively limited donor liver availability or new transplant programs may depend more on resection for curative therapy. Because of limited donor livers, some countries, such as Japan, have made great efforts at developing successful surveillance and diagnosis programs that detect more than 60% of HCC at a very early stage[9]. Early detection allows more patients to undergo resection or liver-directed therapy such as local ablation with curative intent.
Because of potential differences in surveillance, tumor size, and available therapies, it is difficult to directly compare a particular therapy for HCC in different countries. The aim of the present study is to compare patient and clinical characteristics and survival of early (stage I, II) HCC patients treated by resection in three different countries. These centers include large tertiary referral centers for HCC in Shanghai (China), Nippon (Japan) and Hawaii, the United States with the highest incidence of HCC.
This is a retrospective analysis of 573 liver resections performed in 3 tertiary referral centers for liver disease performed in 3 different countries.
The Honolulu cohort consisted of 936 HCC cases referred between1993 and 2014 to the only liver transplant program in Hawaii and the only referral center for liver disease/surgery for the American territories of the Pacific Basin (including Samoa, Guam, Saipan, and the Marshall Islands). Patients were primarily United States citizen of diverse racial/ethnic backgrounds including Whites, Asians, and Pacific Islanders but also included foreign nationals from Asian countries who sought medical care in the United States Race/ethnicity and birthplace were assessed as risk factors for HCC were previously shown to vary by these demographic characteristics in this study population[10]. This clinic and the transplant center were initially affiliated with Hawaii Medical Center-East (formerly St. Francis Medical Center) and after 2012, the Queens Medical Center. This center sees about 60%-70% of the HCC cases in Hawaii. Liver resections were performed by a single group of hepatobiliary/transplant surgeons, with about 80% of these cases done by a single surgeon (LW).
HCC was confirmed histologically by percutaneous biopsy or at surgery. In the first decade, HCC consistent with the previous United Network for Organ Sharing policy regarded transplant for HCC patients without biopsy. More recently, the diagnosis of HCC was made with only imaging if a dynamic contrast-enhanced study showed typical arterial enhancement with venous “washout” as described by the American Association for the Study of Liver Disease guidelines[11,12].
Data collected included demographic data (age, sex, birthplace, self-reported ethnicity) and the presence of diabetes mellitus, hyperlipidemia, smoking, viral hepatitis, alcohol abuse, obesity and other chronic liver diseases. Laboratory data collected included bilirubin, albumin, prothrombin time, creatinine, alanine aminotransferase, aspartate aminotranferase, platelet count, Model for End-stage Liver Disease score and alpha fetal protein (AFP). The size, number, and location of the tumor(s) were used to determine the Tumor Node Metastases stage according to the American Joint Commission on Cancer (AJCC) staging manual[13].
After excluding patients who presented with ruptured HCC and underwent embolization prior to resection, 146 HCC cases were included in the study. During this time period, 84 patients underwent liver transplant for HCC. This study was approved by the University of Hawaii Institutional Review Board.
The Shanghai cohort was comprised of 241 HCC cases diagnosed between 2002-2003 and followed for up to 70 mo. Patients were diagnosed and treated at Zhongshan Hospital (Fudan University) in Shanghai, China. Zhongshan Hospital is a major teaching hospital affiliated with the Ministry of Health of China. This is a 1700 bed medical facility that serves approximately 80000 inpatients and 3 million outpatients/emergency visits annually.
All patients were of Chinese ethnicity and were initially seen by medical organizations in the surrounding areas but the final diagnosis was made in this facility. Patients were diagnosed based on imaging criteria, as well as with a history of chronic viral hepatitis and elevated AFP. Three surgeons including Dr. Zhao-You Tang (author) performed all of the liver resections in this cohort. The diagnosis of HCC was confirmed by two independent pathologists.
The patient enrollment criteria included those with detailed information on clinical presentation and pathological characteristics; and detailed follow-up data for at least 3 years, which included recurrence-free survival, overall survival, as well as the cause of death. The detailed clinical presentation characteristics included but were not limited to sex, age, OKUDA staging, CLIP staging, BCLC staging, Child-Pugh score, TNM staging, multiple nodules, satellite nodule, tumor size, tumor capsule, cirrhosis, tumor thrombosis, lymph node, alanine transaminase, Albumin, international normalized ratio, hepatitis B surface (HBV) Ag, hepatitis C antibody, HBV viral status, pre-treatment AFP, preoperative therapy, and postoperative other therapies. A majority of patients were long-term carriers of HBV (94%). The updated TNM classification was used in this cohort, and 177 early stage HCC patients (TNM stage I and II) with survival information were therefore chosen to perform comparison analysis in our study. Data for this cohort are publically available and have been used in many HCC translational research studies[14,15].
The Tokyo cohort consisted of 504 HCC cases diagnosed between1986 and 2014 in the Department of Surgery at Nippon Medical School, which has a primary medical center (1000 beds) and 3 smaller branch hospitals. Decisions on therapy were made by hepatologists and surgeons and all liver resections were performed at the primary medical center by members of a dedicated liver surgery team (10 hepatobiliary surgeons, surgical residents and medical students). Living-donor liver transplantation is done in this medical center, but only 15 cases have been done and no deceased-donor liver transplants were performed during this time period.
Although the treatment strategy has been changing in Japan, decisions on therapy were based on an algorithm for treatment of HCC reported by Makuuchi et al[16,17]. This algorithm was based on three factors: Degree of liver damage (Childs A, B or C), number of tumors (single, 2-3 or 4 or more), and tumor diameter (≤ 3 cm or > 3 cm). Indications for surgery were according to modified-Makuuchi criteria incorporating the indocyanine green test[18]. The final diagnosis of HCC was histologically confirmed at surgery by a group of expert pathologists. Use of transplantation for HCC was extremely limited because of scarcity of organs from deceased donors. Hepatectomy is generally the first choice for Child-Pugh class A and selected class B cirrhotic patients.
In this cohort of 504 patients with HCC, the vast majority of patients were Japanese. The pre-operative diagnosis of HCC was made primarily with imaging and confirmed at resection. Liver biopsy prior to surgery was rarely performed. Data collected in this cohort included: Age, gender, HBV, hepatitis C virus (HCV), presence of coma, ascites, bilirubin, albumin, protime, AFP, Childs-Pugh class, presence of cirrhosis, stage, tumor size, recurrence and survival. Additional data that were collected but not used in this analysis included ICG (indocyanine green), AFP-LC, PIVKA, tumor differentiation, vascular invasion and details on the segments of liver that were removed. Of the 504 HCC patients in this cohort, 250 diagnosed at TNM stage I and II were included in the present analysis.
All analyses were conducted with SAS version 9.3 (SAS Institute, Inc., Cary NC). All P-values were two-sided, and P < 0.05 was defined as significant. Characteristics of Honolulu, Tokyo and Shanghai HCC patients were compared using generalized linear models (continuous variables) and χ2 tests (categorical variables). Differences in HCC mortality among patients treated in Honolulu, Tokyo and Shanghai were examined using Kaplan-Meier estimates and Cox proportional hazards regression. Survival period was computed from the date of HCC diagnosis to the date of death from any cause. Patients alive at the end of the follow-up period were considered censored. The proportional hazard assumption for Cox models was checked by plotting scaled Schoenfeld residuals against time to event[19]. There was evidence of non-proportionality of hazards with respect to time. For this reason and due to the uneven follow-up period between the three centers, survival was partitioned into two time periods: Survival at 1 year after diagnosis and at 5 years following diagnosis were modeled separately. Analyses were adjusted for patients’ age at time of diagnosis. Predictors of overall survival were also evaluated in 1-year and 5-year models. Univariate analyses were used to model age (< 50 year; ≥ 50 year), sex (male; female); stage (I; II); Child-Pugh Score (A; B); tumor size (< 5 cm; ≥ 5 cm) presence/absence of cirrhosis; AFP (< 20 ng/mL; ≥ 20 ng/mL); albumin levels (< 3.5 g/dL; ≥ 3.5 g/dL), HBV (positive; negative); and HCV (positive; negative). The three center locations were modeled as indicator variables with Honolulu as the reference. Along with age, factors found to be significant at the P≤ 0.10 level in univariate analyses were included in the full multivariate models. A statistical review of the study was performed by a biomedical statistician.
A total of 573 HCC patients diagnosed at AJCC stage I or II who underwent resection were included in the present analyses. Patients included 146 from Honolulu, 250 from Tokyo, and 177 from Shanghai. Patient and clinical characteristics varied widely across countries (Table 1). Patients were youngest in Shanghai and oldest in Tokyo (P < 0.0001). Males comprised 82% of Shanghai patients, 72% of Tokyo cases, and 69% of Honolulu cases (P = 0.01). HBV seropositivity was highest among Shanghai HCC cases (94%), followed by Honolulu (43%) and Tokyo (14%) cases (P < 0.0001). Conversely, HCV seropositivity was highest among Tokyo cases (67%), followed by Honolulu (27%) and Shanghai (3%) patients (P < 0.0001). Stage I cases were predominant in Honolulu (89%), compared to Shanghai (51%) and Tokyo (27%) cases (P < 0.0001). Cirrhosis was present in most Shanghai cases (93%), compared to 57% of Tokyo and 41% of Honolulu cases (P < 0.0001). Mean tumor size was largest in Honolulu cases (6.4 cm), compared to Shanghai and Tokyo cases (3.9 cm and 3.0 cm, respectively) (P < 0.0001). Elevated AFP levels were present in 60% of Shanghai patients, 51% of Honolulu cases, and 42% of Tokyo patients (P = 0.001). Abnormal albumin levels (< 3.5 g/dL) were present in 34% of Tokyo patients compared to 16% and 12% in the Honolulu and Shanghai cohorts, respectively (P < 0.0001).
| Characteristic | Honolulu (n = 146) n (%) | Tokyo (n = 250) n (%) | Shanghai (n = 177) n (%) | P value |
| Mean age in years | 62.7 (SD 11.4) | 67.0 (SD 8.7) | 50.6 (SD11.1) | < 0.0001 |
| Age < 50 yr | 18 (12.3) | 8 (3.2) | 83 (46.9) | < 0.0001 |
| Males | 100 (68.5) | 180 (72.0) | 145 (81.9) | 0.01 |
| Hepatitis B positive | 62 (42.5) | 35 (14.3) | 167 (94.4) | < 0.0001 |
| Hepatitis C positive | 39 (26.7) | 163 (66.8) | 5 (3.3) | < 0.0001 |
| Hepatitis B and C positive | 5 (3.4) | 4 (1.6) | 3 (2.0) | 0.50 |
| Stage 1 | 129 (88.4) | 68 (27.2) | 91 (51.4) | < 0.0001 |
| Childs A | 143 (99.3) | 224 (89.6) | 172 (97.2) | < 0.0001 |
| Cirrhosis | 60 (41.1) | 133 (56.8) | 163 (92.6) | < 0.0001 |
| Mean tumor size (cm) | 6.4 (SD 4.6) | 3.0 (SD 2.1) | 3.9 (SD 2.6) | < 0.0001 |
| Tumor size < 5.0 cm | 75 (51.4) | 213 (85.2) | 137 (77.4) | < 0.0001 |
| AFP < 20 ng/mL | 72 (49.3) | 144 (57.6) | 70 (39.6) | 0.0011 |
| Albumin < 3.5 g/dL | 23 (15.8) | 85 (34) | 21 (11.9) | < 0.0001 |
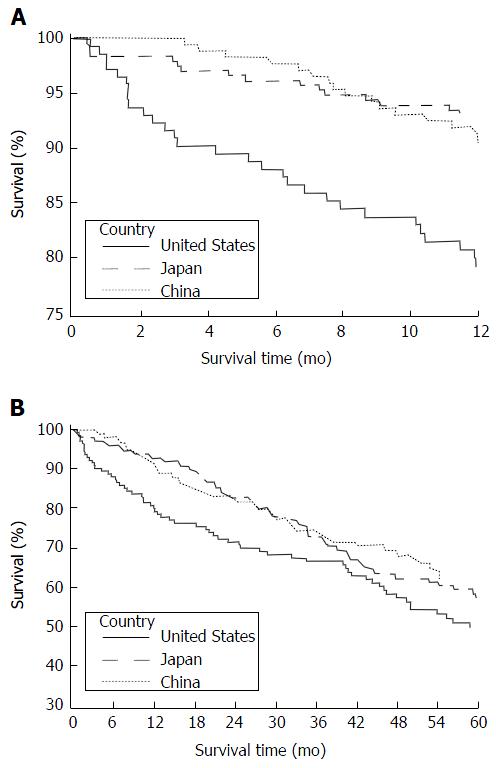
Overall, 1-year and 5-year mortality varied across the three centers (Figure 1). Thirty-day mortality was 2.8%, 1.6% and 0% for Honolulu, Tokyo and Shanghai groups, respectively. Mortality was compared across the three centers with Honolulu as the reference (Table 2). (Estimates adjusted for age at diagnosis only and additionally adjusted for the year of surgery were comparable. Therefore, estimates adjusted for age at diagnosis only are reported). During the 1-year survival and 5-year periods, Tokyo patients had lower mortality than those in Honolulu (age-adjusted HR = 0.28; 95%CI: 0.15-0.51 and age-adjusted HR = 0.70; 95%CI: 0.50-0.98, respectively). One-year and 5-year survival did not differ between the Shanghai and Honolulu cohorts.
| Center | Honolulu | Tokyo | Shanghai | |
| No. patients | 146 | 250 | 177 | |
| No. deaths | 29 | 17 | 6 | |
| Mean follow-up (yr) | 4.04 | 3.75 | 3.55 | |
| Median follow-up (yr) | 3.33 | 2.83 | 4.36 | |
| 1-yr survival | Hazard ratio1 | 1 | 0.28 | 0.63 |
| 95%CI | Reference | 0.15-0.51 | 0.32-1.21 | |
| P value | < 0.0001 | 0.17 | ||
| 5-yr survival | Hazard ratio | 1 | 0.7 | 0.74 |
| 95%CI | Reference | 0.50-0.98 | 0.51-1.09 | |
| P value | 0.04 | 0.13 |
Predictors of overall 1-year and 5-year survival were examined (Table 3). For 1-year survival, the multivariate model included age, AFP, tumor size, albumin and center as covariates. In the final multivariate model, the following were positively associated with increased risk of mortality at 1-year: AFP levels ≥ 20 (adjusted HR = 2.27; 95%CI: 1.32-3.90), tumor size ≥ 5 cm (adjusted HR = 2.00; 95%CI: 1.17-3.43) and albumin < 3.5 g/dL (adjusted HR = 2.10; 95%CI: 1.20-3.68). The Tokyo cohort had lower 1-year mortality than Honolulu (adjusted HR = 0.33; 95%CI: 0.17-0.65). For 5-year survival, the multivariate model included age, stage, Child-Pugh Score, AFP, albumin, cirrhosis, tumor size, and center as covariates. In the final multivariate model, predictors of 5-year survival were AFP ≥ 20 (adjusted HR = 1.57; 95%CI: 1.17-2.10), cirrhosis (adjusted HR = 1.59; 95%CI: 1.12-2.26), tumor size ≥ 5 cm (adjusted HR = 1.84; 95%CI: 1.34-2.54), and albumin < 3.5 g/dL (adjusted HR = 1.72; 95%CI: 1.21-2.46,). Both Tokyo and Shanghai centers had better 5-year survival than the Honolulu cohort (adjusted HR = 0.51; 95%CI: 0.33-0.78 and adjusted HR = 0.47; 95%CI: 0.31-0.72). Predictors of overall 1-year and 5-year survival were examined separately by center. In Honolulu, predictors of both 1-year and 5-year survival were AFP ≥ 20 (1-year: adjusted HR = 3.21; 95%CI: 1.36-7.56; 5-year: adjusted HR = 3.18; 95%CI: 1.35-7.51) and albumin < 3.5 g/dL (1-year: adjusted HR = 4.17; 95%CI: 1.92-9.04; 5-year: adjusted HR = 4.13; 95%CI: 1.90-8.98). In the Tokyo cohort, there were no significant predictors of 1-year survival. Five-year survival was associated with cirrhosis (adjusted HR = 1.90; 95%CI: 1.17-3.09) and tumor size ≥ 5 cm (adjusted HR = 2.29; 95%CI: 1.33-3.94). For the Shanghai cohort, 1-year mortality risk was associated with tumor size ≥ 5 cm (adjusted HR = 2.99; 95%CI: 1.14-7.80) and 5-year predictors included AJCC stage 2 (vs 1) (adjusted HR = 2.30; 95%CI: 1.35-3.92) and Child-Pugh Score (B vs A) (adjusted HR = 4.51; 95%CI: 1.59-12.81).
| Covariates | Univariate | Multivariate | ||||
| Hazard ratio | Confidence interval | P value | Hazard ratio1 | Confidence interval | P value | |
| 1-yr | ||||||
| Age (yr, ≥ 50 vs < 50) | 1.29 | 0.66-2.55 | 0.46 | 1.41 | 0.68-2.92 | 0.36 |
| Sex (male vs female) | 1.39 | 0.82-2.38 | 0.22 | |||
| AFP (ng/mL, ≥ 20 vs < 20) | 2.28 | 1.33-3.91 | 0.003 | 2.27 | 1.32-3.90 | 0.003 |
| Cirrhosis (yes vs no) | 1.13 | 0.65-1.95 | 0.66 | |||
| Tumor size (cm, ≥ 5 vs < 5) | 2.64 | 1.60-4.34 | 0.0001 | 2.00 | 1.17-3.43 | 0.01 |
| Albumin (g/dL, < 3.5 vs≥ 3.5) | 1.72 | 1.01-2.94 | 0.045 | 2.10 | 1.20-3.68 | 0.01 |
| AJCC stage (2 vs 1) | 0.90 | 0.55-1.49 | 0.16 | |||
| Childs Pugh (B vs A) | 1.27 | 0.46-3.49 | 0.64 | |||
| Hepatitis B (+ vs -) | 0.77 | 0.47-1.28 | 0.33 | |||
| Hepatitis C (+ vs -) | 0.73 | 0.42-1.25 | 0.25 | |||
| Tokyo vs Honolulu | 0.32 | 0.17-0.58 | 0.0002 | 0.33 | 0.17-0.65 | 0.001 |
| Shanghai vs Honolulu | 0.43 | 0.23-0.78 | 0.005 | 0.54 | 0.28-1.03 | 0.06 |
| 5-yr | ||||||
| Age (yr, ≥ 50 vs < 50) | 1.12 | 0.79-1.60 | 0.52 | 1.14 | 0.77-1.69 | 0.53 |
| Sex (male vs female) | 1.14 | 0.84-1.56 | 0.40 | |||
| AFP (ng/mL, ≥ 20 vs < 20) | 1.56 | 1.17-2.06 | 0.002 | 1.57 | 1.17-2.10 | 0.002 |
| Cirrhosis (yes vs no) | 1.32 | 0.97-1.80 | 0.08 | 1.59 | 1.12-2.26 | 0.009 |
| Tumor size (cm, ≥ 5 vs < 5) | 1.66 | 1.24-2.23 | 0.0007 | 1.84 | 1.34-2.54 | 0.0002 |
| Albumin (g/dL, < 3.5 vs≥ 3.5) | 1.91 | 1.41-2.58 | < 0.0001 | 1.72 | 1.21-2.46 | 0.003 |
| AJCC stage (2 vs 1) | 1.30 | 0.98-1.72 | 0.07 | |||
| Childs Pugh (B vs A) | 1.78 | 1.05-3.02 | 0.03 | 1.33 | 0.73-2.43 | 0.35 |
| Hepatitis B (+ vs -) | 0.87 | 0.65-1.15 | 0.31 | |||
| Hepatitis C (+ vs -) | 1.11 | 0.83-1.49 | 0.48 | |||
| Tokyo vs Honolulu | 0.74 | 0.53-1.03 | 0.08 | 0.51 | 0.33-0.78 | 0.002 |
| Shanghai vs Honolulu | 0.66 | 0.46-0.94 | 0.02 | 0.47 | 0.31-0.72 | 0.0006 |
Therapy for hepatocellular cancer has evolved and there are current practice guidelines based on Barcelona Clinic Liver Cancer (BCLC) staging[11,12]. These guidelines provide a general framework, but what occurs in the real world is likely center and country specific. In developed countries with resources to perform liver transplant, multiple studies have compared outcome between resection and transplant and those treated with transplant have better survival and less recurrence[7,20-30]. Nonetheless, widespread use of transplant is constrained by the availability of donor livers. Other reports compared liver resection and local ablation. Although ablation was effective especially for tumors less than 2.0 cm and can be performed with fewer complications, liver resections had better long-term, recurrence free-survival in some series[3,31-34]. Good short-term outcomes occur in small tumors, whether resected, ablated or transplanted, but recurrence rates, cost and donor livers are major factors in the decision-making. Worldwide, strategies to treat liver cancer have evolved based on the burden of liver cancer and the available resources in that particular area.
This is the first study that attempts to assess one surgical modality in 3 different countries, each of which has a high burden of disease, but different resources and treatment strategies. We chose liver resection because this was uniformly available and not dependent on technology or donor livers. Rather than comparing data in the form of a meta-analysis or systematic review, we developed a working relationship between the surgeons and scientists in these three centers. In this study, we selected only stage I/II HCC who underwent liver resection, in an attempt to make a comparison in as homogeneous a group as possible. We showed that although the survival outcomes are different in various centers, overall survival is mostly dependent on tumor factors and underlying liver function. Although the patients in each center differ in many respects (mean age, gender, viral risk factor, tumor size, mean tumor size and AFP), all centers had excellent 30-d mortality. Our study showed that tumor size, AFP, and albumin were factors associated with early mortality. By 5 years post-resection, these same factors in addition to the presence of cirrhosis were predictors of mortality. Both the Tokyo and Shanghai cohorts had better 1- and 5-year survival compared to the Honolulu cohort even after adjustment for clinical factors. Differences in patient populations across the centers may account for these differences. Compared to the generally homogeneous Tokyo and Shanghai patients, the Honolulu cohort was comprised of racially and ethnic diverse individuals born within and outside the United States. Many Honolulu patients had comorbidities including those that may contribute to disease progression (obesity, type-2 diabetes, excess alcohol consumption and past intravenous drug use)[10]. We were unable to account for these differences in comorbidities and risk factors as this information was not available for the Tokyo and Shanghai cohorts. Differences in the patient populations are further supported by our observation that independent risk factors of survival differed across centers. In Honolulu, elevated AFP and albumin were associated with both 1-year and 5-year survival. In the Tokyo cohort, cirrhosis large and tumor size were associated with 1-year survival. For the Shanghai cohort, tumor size was a predictor of 1-year survival while AJCC stage and Child-Pugh Score were associated with 5-year mortality risk.
Single-center studies have similarly demonstrated that tumor characteristics (size, vascular invasion) and underlying liver function are predictors of survival[3,5,21,35,36]. Kao et al[3] examining 1265 liver resections for early stage HCC, showed that low albumin, AFP > 20 ng/mL, and tumor size > 3 cm affected mortality. Kang et al[34] studying 353 South Korean patients, found that vascular invasion and thrombocytopenia were risk factors for poor disease-free survival. Many of these studies were large series of liver resections in centers outside the United States. Large United States studies of liver resection for HCC have been primarily based on cancer databases with limited information on underlying liver function[30,33,37], or were conducted in single centers that focused on the comparison between liver transplant and resection[29-38].
A few studies have also compared the outcome of liver resections in patients with HBV vs HCV. Chen et al[38] studying 2920 patients in Taiwan, showed that patients with HBV were younger, had higher AFP and larger tumor size and lower mean survival (11.1 mo vs 23.9 mo with HCV). Dohmen et al[39] demonstrated that among 692 patients in Japan, HBV patients were younger, presented with more advanced stage and had poorer overall survival. Wu et al[40] reported that among 110 Taiwanese patients who underwent hepatic resection for HCC, neither underlying cirrhosis nor viral status affected operative morbidity or mortality, but the poorer liver reserve in HCV cirrhotic patients resulted in worse survival compared to the HBV patients. Franssen et al[37] reported that among 567 United States patients who underwent liver resection, HBV rather than HCV-related HCC had better survival and less recurrence. Our study allowed comparison of a primarily HBV-related HCC group of patients (China), a primarily HCV-related HCC group (Japan) and a mixed group (Hawaii), and when considered together viral hepatitis status had little bearing on overall 1- and 5-year survival as the cirrhosis, tumor size, AFP and underlying liver function had the greatest effect on outcome.
This study is limited in that the time frame was different in the three groups. In the Tokyo and Honolulu cohorts, this study represented a 20+ year experience, whereas the Shanghai cohort underwent liver resection over a 2-year period. Because this study was done retrospectively, each group collected different parameters, so there was limited data collected by all groups that could be directly compared. There are also likely differences in the quality of long-term follow up between the centers. This study also has variable data on recurrence of HCC and treatment of these recurrences, which may affect long-term survival. Survival was also expressed as all-cause survival so it is difficult to determine the contribution of HCC to overall patient outcome. Finally, differences in survival after liver resection may be due to availability of liver transplant and other locoregional therapies in a particular country. The increased availability of liver transplant in the Honolulu group may have prompted fewer resections in those with smaller tumors, leaving liver resections for larger tumors with reasonable liver function. Unfortunately, we would not be able to determine this without information on all HCC referred to each of these centers.
In spite of these differences and limitations, this study represents a large experience of liver resections by expert hepatobiliary surgeons in their respective countries. In the final analysis, the very early outcome after liver resection for HCC is similar in specialized centers in different countries but later survival is better in the Tokyo and Shanghai groups. Tumor factors, underlying liver function, comorbidities and availability of other therapies may be playing a role patient selection for resection and the ultimate outcome. Nevertheless, this study demonstrates that collaborations at an international level will be important for understanding how to better manage and treat HCC.
Treatment for hepatocellular cancer depends on stage and liver function. How liver cancer is treated in different country may also depend on available therapy. Liver transplant has the best long-term disease free survival for early liver cancer, however the availability of liver transplant differs in various countries and may limit this therapy.
Single-institution studies have characterized resection for hepatocellular but this unique study combines the experience of three large hepatobiliary centers in different countries with 573 resections for stage I/II hepatocellular cancer in Tokyo (n = 250), Honolulu (n = 146) and Shanghai (n = 177).
Groups differed in viral hepatitis, tumor size, alpha fetal protein (AFP) and cirrhosis. One and 5-year mortality was lowest in the Tokyo cohort. Elevated AFP, low albumin, tumor > 5 cm and cirrhosis were independently-associated with increased 5-year mortality. The profile of early-stage hepatocellular patients is strikingly different across countries and likely contributes to survival differences.
This study is important as it demonstrates the importance of collaboration between centers in different countries so that we can better diagnose and manage hepatocellular cancer.
Liver resection is a surgical procedure involving removal of a portion of liver that has a malignant cancer. Liver transplantation is performed for those patients with hepatocellular cancer and poor underlying liver function.
The authors of this paper observed excellent early outcomes after liver resection for early stage hepatocellular cancer. Differences in longer term survival were likely related to tumor size, albumin, AFP and the presence of cirrhosis.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20723] [Article Influence: 1883.9] [Reference Citation Analysis (23)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9982] [Article Influence: 907.5] [Reference Citation Analysis (15)] |
| 3. | Kao WY, Chao Y, Chang CC, Li CP, Su CW, Huo TI, Huang YH, Chang YJ, Lin HC, Wu JC. Prognosis of Early-Stage Hepatocellular Carcinoma: The Clinical Implications of Substages of Barcelona Clinic Liver Cancer System Based on a Cohort of 1265 Patients. Medicine (Baltimore). 2015;94:e1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Yim SY, Seo YS, Jung CH, Kim TH, Lee JM, Kim ES, Keum B, Jong YK, An H, Kim JH. The management and prognosis of patients with hepatocellular carcinoma: what has changed in 20 years? Liver Int. 2016;36:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Lee WC, Lee CF, Cheng CH, Wu TJ, Chou HS, Wu TH, Soong RS, Chan KM, Yu MC, Chen MF. Outcomes of liver resection for hepatocellular carcinoma in liver transplantation era. Eur J Surg Oncol. 2015;41:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Zhu Q, Li N, Zeng X, Han Q, Li F, Yang C, Lv Y, Zhou Z, Liu Z. Hepatocellular carcinoma in a large medical center of China over a 10-year period: evolving therapeutic option and improving survival. Oncotarget. 2015;6:4440-4450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Yamashita Y, Tsuijita E, Takeishi K, Ishida T, Ikegami T, Ezaki T, Maeda T, Utsunomiya T, Nagasue N, Shirabe K. Trends in surgical results of hepatic resection for hepatocellular carcinoma: 1,000 consecutive cases over 20 years in a single institution. Am J Surg. 2014;207:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hsueh KC, Lee TY, Kor CT, Chen TM, Chang TM, Yang SF, Hsieh CB. The role of liver transplantation or resection for patients with early hepatocellular carcinoma. Tumour Biol. 2016;37:4193-4201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Wong LL, Hernandez B, Kwee S, Albright CL, Okimoto G, Tsai N. Healthcare disparities in Asians and Pacific Islanders with hepatocellular cancer. Am J Surg. 2012;203:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6630] [Article Influence: 442.0] [Reference Citation Analysis (1)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4520] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 13. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th Edition. New York: Springer, 2009: 191-199. . |
| 14. | Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 665] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 15. | Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 569] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 16. | Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol. 2006;12:828-829. [PubMed] |
| 17. | Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. Second English edition. Kanehara & Co., Ltd., Tokyo: 2003. . |
| 18. | Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22. [PubMed] |
| 19. | Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51:1469-1482. [PubMed] |
| 20. | Scatton O, Goumard C, Cauchy F, Fartoux L, Perdigao F, Conti F, Calmus Y, Boelle PY, Belghiti J, Rosmorduc O. Early and resectable HCC: Definition and validation of a subgroup of patients who could avoid liver transplantation. J Surg Oncol. 2015;118:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Wong RJ, Wantuck J, Valenzuela A, Ahmed A, Bonham C, Gallo A, Melcher ML, Lutchman G, Concepcion W, Esquivel C. Primary surgical resection versus liver transplantation for transplant-eligible hepatocellular carcinoma patients. Dig Dis Sci. 2014;59:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Chirica M, Tranchart H, Tan V, Faron M, Balladur P, Paye F. Infection with hepatitis C virus is an adverse prognostic factor after liver resection for early-stage hepatocellular carcinoma: implications for the management of hepatocellular carcinoma eligible for liver transplantation. Ann Surg Oncol. 2013;20:2405-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol. 2012;19:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, Tang J, Anderson M, Misra S, Solomon NL. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527-537; discussion 537-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Huang J, Hernandez-Alejandro R, Croome KP, Yan L, Wu H, Chen Z, Prasoon P, Zeng Y. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in Childs A cirrhotics-a retrospective study of 1,061 cases. J Gastrointest Surg. 2011;15:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 27. | Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, Majno P, Mira P, Rubbia-Brandt L, Ferrero A. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointest Surg. 2008;12:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Chapman WC, Klintmalm G, Hemming A, Vachharajani N, Majella Doyle MB, DeMatteo R, Zaydfudim V, Chung H, Cavaness K, Goldstein R. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. 2015;220:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Seshadri RM, Besur S, Niemeyer DJ, Templin M, McKillop IH, Swan RZ, Martinie JB, Russo MW, Iannitti DA. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford). 2014;16:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Liu PH, Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Chiou YY, Lin HC, Huo TI. When to Perform Surgical Resection or Radiofrequency Ablation for Early Hepatocellular Carcinoma?: A Nomogram-guided Treatment Strategy. Medicine (Baltimore). 2015;94:e1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg. 2016;263:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Li GZ, Speicher PJ, Lidsky ME, Darrabie MD, Scarborough JE, White RR, Turley RS, Clary BM. Hepatic resection for hepatocellular carcinoma: do contemporary morbidity and mortality rates demand a transition to ablation as first-line treatment? J Am Coll Surg. 2014;218:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zhou Z, Lei J, Li B, Yan L, Wang W, Wei Y, Cheng K. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule & lt; 2 cm): a single-center study. Eur J Gastroenterol Hepatol. 2014;26:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Kang CM, Choi GH, Kim DH, Choi SB, Kim KS, Choi JS, Lee WJ. Revisiting the role of nonanatomic resection of small (& lt; or = 4 cm) and single hepatocellular carcinoma in patients with well-preserved liver function. J Surg Res. 2010;160:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Ulahannan SV, Duffy AG, McNeel TS, Kish JK, Dickie LA, Rahma OE, McGlynn KA, Greten TF, Altekruse SF. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 2014;60:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, Roayaie S, Florman S, Schwartz ME. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg. 2014;260:650-656; discussion 656-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, Wang JD, Sheu JC. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524-2529. [PubMed] |
| 39. | Dohmen K, Shigematsu H, Irie K, Ishibashi H. Comparison of the clinical characteristics among hepatocellular carcinoma of hepatitis B, hepatitis C and non-B non-C patients. Hepatogastroenterology. 2003;50:2022-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Wu CC, Tang JS, Lin MC, Yeh DC, Liu TJ, P’eng FK. Comparison of liver resection for hepatocellular carcinoma in hepatitis B and hepatitis C-related cirrhotic patients. Hepatogastroenterology. 1999;46:651-655. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bramhall S, He ST, Sipos F S- Editor: Qiu S L- Editor: A E- Editor: Li D