Published online Oct 28, 2016. doi: 10.4254/wjh.v8.i30.1269
Peer-review started: May 9, 2016
First decision: June 13, 2016
Revised: July 28, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: October 28, 2016
Processing time: 171 Days and 8.3 Hours
To evaluate the bidirectional association between metabolic syndrome (MS) components and antiviral treatment response for chronic hepatitis C virus (HCV) infection.
This retrospective cohort study included 119 HCV + patients treated with pegylated-interferon-α and ribavirin. Metabolic characteristics and laboratory data were collected from medical records. Differences in baseline clinical and demographic risk factors between responders and non-responders were assessed using independent samples t-tests or χ2 tests. The effects of sustained viral response (SVR) to antiviral treatment on de novo impairments in MS components, including impaired fasting glucose (IFG) and type 2 diabetes mellitus (T2DM), were assessed using univariable and multivariable logistic regression analysis, while the effect of MS components on SVR was assessed using univariable logistic regression analysis.
Of the 119 patients, 80 (67%) developed SVR over the average 54 ± 13 mo follow-up. The cumulative risks for de novo T2DM and IFG were 5.07- (95%CI: 1.261-20.4, P = 0.022) and 3.87-fold higher (95%CI: 1.484-10.15, P = 0.006), respectively for non-responders than responders, when adjusted for the baseline risk factors age, sex, HCV genotype, high viral load, and steatosis. Post-treatment triglyceride levels were significantly lower in non-responders than in responders (OR = 0.27; 95%CI: 0.069-0.962, P = 0.044). Age and HCV genotype 3 were significantly different between responders and non-responders, and MS components were not significantly associated with SVR. Steatosis tended to attenuate SVR (OR = 0.596; 95%CI: 0.331-1.073, P = 0.08).
SVR was associated with lower de novo T2DM and IFG incidence and higher triglyceride levels. Patients infected with HCV should undergo T2DM screening and antidiabetic treatment.
Core tip: Hepatitis C virus (HCV) is associated with a unique metabolic syndrome (MS) type: Insulin resistance with type 2 diabetes mellitus (T2DM), hypocholesterolemia, and liver steatosis. We retrospectively investigated the association between MS components and HCV infection, including antiviral therapy response, for 119 patients infected with HCV treated with interferon alpha and ribavirin. After long-term follow-up, de novo T2DM incidence significantly decreased, and triglyceride levels significantly increased in treatment responders. Only steatosis tended to affect treatment response. The association between HCV and lipid metabolic pathways may be important even with new direct antiviral agents. Patients infected with HCV should be screened for T2DM.
- Citation: Yair-Sabag S, Nussinson E, Ben-Assuli O, Shibli F, Shahbari A, Zelber-Sagi S. Retrospective study of the associations between hepatitis C virus infection and metabolic factors. World J Hepatol 2016; 8(30): 1269-1278
- URL: https://www.wjgnet.com/1948-5182/full/v8/i30/1269.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i30.1269
A mutual association between hepatitis C virus (HCV) infection and host metabolism has been demonstrated in several studies. HCV depends on host lipids for entry into the hepatocytes and for its replication; in return, HCV also affects the metabolism of host lipids[1-3].
HCV causes insulin resistance, hepatic steatosis, type 2 diabetes mellitus (T2DM), and low serum cholesterol and triglyceride (TG) levels. Insulin resistance contributes to HCV-related disruption of glucose and lipid metabolism[4], and it is a key factor in metabolic syndrome (MS). In addition, HCV infection might lead to hepatic steatosis via several pathways.
Hepatic steatosis might aggravate MS directly by causing further insulin resistance[5] or indirectly because of resultant hepatic fibrosis[6] or cirrhosis[4,7,8]. After HCV infection, cholesterol and TG levels decrease, creating a different lipid profile from that for MS[9]. However, T2DM might be twice as prevalent in patients infected with HCV compared to the general population[5]. HCV has been associated with a unique type of MS called hepatitis C-associated dysmetabolic syndrome (HCADS), which includes liver steatosis, insulin resistance, and hypocholesterolemia[5,10]. Reversal of hypocholesterolemia and steatosis after achieving sustained viral response (SVR) with antiviral therapy has been observed in several studies[11-13].
Therefore, although MS is not clearly associated with HCV, there is an association between HCV and some MS components. HCV-induced fatty liver and insulin resistance leads to T2DM; with the additional presence of MS, HCV replication is accelerated by activation of hepatocyte transcription factors, leading to increased lipogenesis and the provision of lipids for HCV replication[5,9,10]. Furthermore, in patients with MS, immune responses to HCV can be attenuated by leptin resistance or other changes in adipokine secretion[5]. Thus, MS might interfere with SVR after treatment[11,14-17].
Previous studies showed that HCV eradication decreases the risk of de novo glucose abnormalities and insulin resistance. On the other hand, some studies reported neither an association between metabolic syndrome and HCV infection[18] nor reduced incidence of de novo glucose abnormalities in responders to treatment with interferon alpha and ribavirin[19].
Our study aimed to assess the association between MS components and HCV infection based on the response to the therapy as well as to evaluate the influence of MS components on the response to antiviral therapy in a younger cohort of HCV-infected patients with a long term follow-up.
During 2004-2008, 119 patients diagnosed with chronic HCV infection, based on positive HCV RNA findings on polymerase chain reaction (PCR), were treated with combination pegylated-interferon α (Peg-IFNα) and ribavirin in the department of gastroenterology at Emek Medical Center in Afula, Israel. All patients were eligible for the Peg-IFNα and ribavirin treatment, which consisted of 180 μg Peg-IFNα administered subcutaneously once a week and 800-1200 mg ribavirin administered orally daily. Treatment lasted 24 wk for patients with genotypes 2/3 and 48 wk for those with genotypes 1/4.
Patients with hepatocellular carcinoma (HCC), human immunodeficiency virus infection, other serious conditions, or evidence of drug abuse or excessive alcohol consumption during the year preceding the enrollment were excluded.
Patients were evaluated before treatment, 6 mo after treatment, and every year after the end of treatment until 2011. Age, body mass index (BMI), serum fasting blood glucose, TG, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, aspartate transaminase, alanine transaminase, viral load, blood pressure, liver biopsy results, and liver ultrasonography were obtained from medical records and were not available in some cases. To reduce data bias, all data were extracted from several independent sources, including patient hospital files, electronic files from the family physician, and laboratory results every year after the end of treatment. The study was performed after obtaining local ethics committee approval.
Impaired fasting glucose (IFG) was defined as a serum fasting glucose level > 100 mg/dL, and T2DM was identified based on diagnoses documented in medical records, serum fasting glucose level > 126 mg/dL, or use of antidiabetic drugs. MS was defined using the World Health Organization clinical criteria for MS [20].
Serum anti-HCV antibodies were measured using a 2nd generation immunoassay, and HCV RNA was measured using real time-PCR (RT-PCR; Amplicor HCV test, Roche Diagnostic; detection rate = 50 IU/mL). HCV genotyping was determined using RT-PCR (HCV genotyping, DNA immunoassay).
Most, but not all, patients agreed to and underwent a baseline liver biopsy to determine hepatic inflammation and steatosis. In addition, ultrasonography was performed before treatment and during the follow-up period to determine hepatic steatosis.
The statistical methods of this study were reviewed by Shira Zelber-Sagi from the School of Public Health at the University of Haifa and Ofir Ben-Assuli from Ono Academic College.
Continuous variables (MS components) are presented as means ± SD. Statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, United States), and P < 0.05 was considered statistically significant for all analyses.
The response to treatment was the independent variable, and metabolic components were initially evaluated separately as dependent variables using independent samples t-tests or Mann-Whitney U tests, when appropriate, for continuous variables (e.g., age and BMI) and Pearson’s χ2 tests and odds ratios (ORs) for categorical variables (e.g., sex and genotype).
To test differences from baseline to the average follow-up duration in continuous variables between the treatment responders and non-responders, independent samples t-tests were performed.
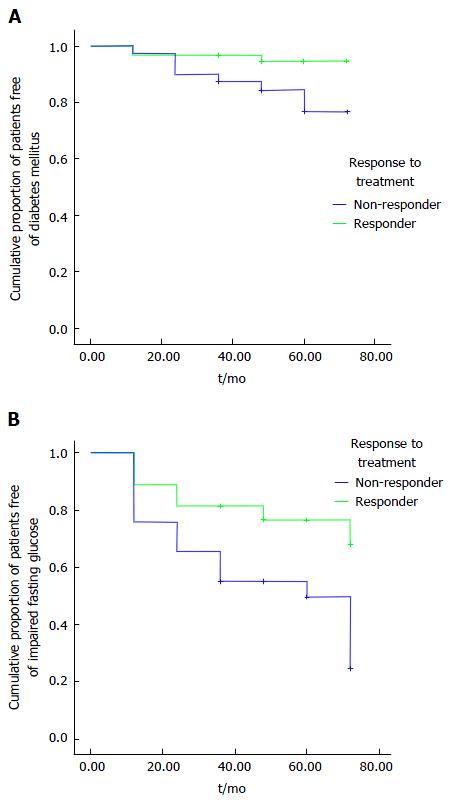
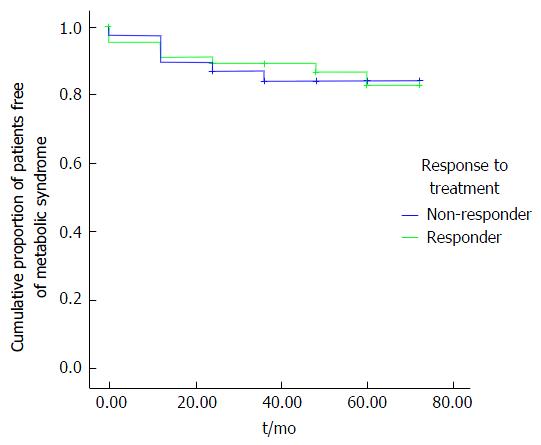
The difference in de novo occurrence of MS components (0 = normal values; 1 = abnormal values indicating presence of the component) between responders and non-responders was calculated after excluding patients with MS components prior to antiviral treatment. Then, logistic regression analysis of de novo occurrence of T2DM and other MS components was conducted at different time intervals using an unadjusted (univariable) logistic regression (model 1) and an adjusted (multivariable) logistic regression (model 2, adjusted for age, sex, BMI, and genotype). Additionally, the cumulative rates of the patients without de novo occurrence of IFG, T2DM, and MS were estimated using the Kaplan-Meier method and compared between responders and non-responders using the log-rank test.
The effects of metabolic, demographic, and clinical variables on treatment response were determined using univariable logistic regression analysis.
The mean age of the 119 HCV-positive patients treated with Peg-IFNα and ribavirin (57% men, 43% women) was 41 ± 11.3 years (Table 1). The sample population primarily included immigrants from the Union of Soviet Socialist Republics (77%). The proportions of patients with HCV genotypes 1, 2, 3 and 4 were 66%, 9.2%, 22% and 1.7%, respectively (Table 1). The mean follow-up duration for MS components after treatment was 47.5 ± 13.3 mo.
| Variable (normal values) | n1 | |
| Age (yr) | 119 | 41 ± 11.3 |
| Sex (men %) | 57.1 | |
| Birth place (%) | ||
| Israel | 12.6 | |
| Union of Soviet Socialist Republics | 77.3 | |
| Other (Europe, North America, South Africa, Georgia) | 10 | |
| Viral load (IU/mL) | 114 | 461.234 ± 251.445 |
| HCV genotype (%) | ||
| 1 | 66.4 | |
| 2 | 9.2 | |
| 3 | 22.7 | |
| 4 | 1.7 | |
| BMI (19-25 kg/m2) | 81 | 27.0 ± 5.4 |
| Systolic BP (15.99 kPa) | 108 | 15.59 ± 2.26 |
| Diastolic BP (10.66 kPa) | 108 | 9.06 ± 1.56 |
| Serum glucose (70-100 mg/dL) | 98 | 96.96 ± 20.5 |
| Cholesterol (100-200 mg/dL) | 117 | 176 ± 49 |
| Triglycerides (30-150 mg/dL) | 90 | 116 ± 65 |
| HDL (40-60 mg/dL) | 74 | 48.8 ± 12 |
| AST (3-32 IU) | 119 | 51 ± 32 |
| ALT (3-33 IU) | 119 | 77 ± 59 |
| T2DM (diagnosis, fasting blood glucose > 126 mg/dL, or use of anti-diabetic drugs) (%) | 9.2 | |
| IFG or T2DM (fasting blood glucose > 100 mg/dL) (%) | 27.7 | |
| Steatosis determined using liver biopsy (n = 85)2 | ||
| Without steatosis (%) | 36 | |
| Mild | 37.3 | |
| Moderate | 25.3 | |
| Severe | 1.3 | |
| Steatosis determined using abdominal ultrasound (n = 110; with steatosis) (%) | 16.5 |
Regarding MS components, hypertension, T2DM, and IFG were present in 17%, 9.2% and 27.7% of patients, respectively (Table 1). Serum HDL values were within the lower limit of the normal range, and serum TG levels were within the normal range. Mean BMI was in the overweight range (27 ± 5.4 kg/m2). Steatosis was present in 36% or 16.5% of patients, as determined with liver biopsy or abdominal ultrasound, respectively (Table 1).
SVR was obtained in 67% (n = 80) of patients. Only baseline age and HCV genotype 3 were significantly different between responders and non-responders (Table 2). Non-responders were significantly older (P = 0.017), and significantly fewer non-responders had HCV genotype 3 (P = 0.005).
| Variable | Non-responders | Responders | P value1 |
| Age (yr) | 44.62 ± 11.22 | 39.42 ± 11.14 | 0.017 |
| BMI (kg/m2) | 26.88 ± 5.11 | 27.34 ± 5.70 | 0.717 |
| Genotype 3 (%) | 2.7 | 17.4 | 0.005 |
| Male sex (%) | 20.8 | 35.6 | 0.736 |
In the unadjusted regression analysis of the effect of the baseline metabolic factors on treatment response, metabolic syndrome and metabolic components (except T2DM) negatively affected treatment response (OR = 0.448; OR = 0.597; respectively), though none of them were significantly associated with treatment response (P = 0.847 and P = 0.483; respectively) (Table 3).
| Variables | Crude OR | 95%CI (P value) |
| BMI > 30 kg/m2 | 0.825 | 0.303-2.243 (0.706) |
| IFG (> 100 mg/dL) | 0.609 | 0.266-1.393 (0.140) |
| T2DM (diagnosis, fasting blood glucose > 126 mg/dL, or use of anti-diabetic drugs) | 1.094 | 0.301-3.975 (0.892) |
| High blood pressure (> 16/10.66 kPa) | 0.713 | 0.269-1.889 (0.495) |
| High triglycerides | 1.075 | 0.338-2.978 (0.889) |
| High cholesterol and low HDL levels | 0.782 | 0.367-1.666 (0.523) |
| Presence of any metabolic syndrome components (high cholesterol levels, hyperlipidemia, high BP, or BMI > 30), without T2DM | 0.448 | 0.551-1.301 (0.847) |
| Metabolic syndrome | 0.597 | 0.141-2.520 (0.483) |
In the unadjusted regression analysis of the effect of the baseline demographic and clinical variables on treatment response, the ORs for HCV genotype 3 and age were significant (OR = 5.35; 95%CI: 1.48-19.3; P = 0.01 and OR = 0.959; 95%CI: 0.926-9.93; P = 0.019; respectively), suggesting positive effects of genotype 3 and relatively young age on response to treatment (Table 4). While the rate of hepatic steatosis as determined using abdominal ultrasound (16.5%) was not significant, the rate of hepatic steatosis as determined by liver biopsy (64%) tended to result in a better response (P = 0.085) (Table 4).
| Variables | Crude OR | 95%CI (P value) |
| Sex (n = 115) | 0.878 | 0.412-1.873 (0.737) |
| Mean age (yr) (n = 115) | 0.959 | 0.926-9.93 (0.019) |
| Birth place (Israel/Union of Soviet Socialist Republics/other) (n = 115) | 0.839 | 0.530-1.329 (0.455) |
| Current smoker (yes/no) (n = 105) | 1.487 | 0.762-2.901 (0.245) |
| Alcohol consumption (none/past) (n = 103) | 1.133 | 0.266-4.824 (0.866) |
| Drug use (none/past user) (n = 106) | 1.476 | 0.550-3.961 (0.439) |
| Genotype 3 (n = 115) (genotypes 1, 2, and 4 are grouped as the reference) | 5.35 | 1.48-19.3 (0.010) |
| Liver steatosis determined by biopsy (yes/no) (n = 74) | 0.596 | 0.331-1.079 (0.085) |
| Liver steatosis determined by ultrasound (yes/no) (n = 107) | 0.515 | 0.181-1.462 (0.213) |
Univariable and multivariable logistic regression analyses of the effect of antiviral therapy response on the de novo impaired MS components resulted in significant crude ORs of 3.87 for IFG and 5.07 for T2DM (P = 0.006 and 0.022, respectively) in the unadjusted model, and a significant OR of 4.7 for IFG in the adjusted model (P = 0.02; model 2). Because of the low incidence of T2DM in responders (n = 3), T2DM could not be evaluated in the adjusted model (model 2; multivariable analysis) (Table 5). The crude OR for hypertriglyceridemia was 0.27 (P = 0.044). Because of the low occurrence of hypertriglyceridemia in the non-responders (n = 3), hypertriglyceridemia could not be evaluated in the adjusted model.
| Variable | Model 1 | Model 2 | ||||
| n | OR | 95%CI (P value) | n | OR | 95%CI (P value) | |
| T2DM (diagnosis, fasting blood glucose > 126 mg/dL, or use of anti-diabetic drugs) | 83 | 5.07 | 1.261-20.494 (0.022) | - | ||
| IFG (fasting blood glucose > 100 mg/dL) | 83 | 3.87 | 1.484-10.154 (0.006) | 53 | 4.71 | 1.280-17.316 (0.020) |
| Hypertriglyceridemia (triglycerides > 150 mg/dL) | 96 | 0.27 | 0.069-0.967 (0.044) | - | ||
| Low HDL levels | 54 | 0.70 | 0.188-2.607 (0.595) | 39 | 1.5241 | 0.185-12.588 (0.695) |
| Men: HDL ≤ 35 mg/dL | - | |||||
| Women: HDL ≤ 39 mg/dL | ||||||
| Obesity (BMI > 30 kg/m2) | 96 | 1.12 | 0.178-7.030 (0.91) | 96 | 0.782 | 0.115-5.339 (0.80) |
| Hypertension (defined by WHO) | 95 | 1.176 | 0.379-3.626 (0.782) | 62 | 1.921 | 0.246-5.636 (0.458) |
| Hepatic steatosis determined by ultrasound | 90 | 2.66 | 0.929-7.636 (0.068) | 64 | 2.1511 | 0.555-8.33 (0.268) |
According to Kaplan-Meier analyses, there were lower rates of IFG and T2DM in responders than in the non-responders (P = 0.006 and 0.023, respectively) (Figure 1). Overall, the occurrence of MS in responders was not different from that in non-responders (Figure 2).
Our study, which aimed to examine the association between MS components and HCV infection based on treatment response and the influence of MS components on the success of antiviral therapy, did not detect any differences in most pre- or post-treatment MS component values between responders and non-responders to antiviral therapy. However, de novo IFG and T2DM occurred significantly more often in non-responders than in responders.
Our results regarding T2DM are consistent with those of other studies conducted with larger community cohorts. In a study conducted in Taiwan with a 7-year follow-up period, the cumulative incidence of T2DM was 14.3% in anti-HCV-positive patients and 8.6% in seronegative individuals (P < 0.0001)[21]. In another study conducted in Japan, 143 of 2842 HCV-positive patients treated with IFNα monotherapy or IFNα and ribavirin combination therapy developed T2DM during a mean observation period of 6.4 years, and only 26 of these patients with T2DM had SVR[22].
HCV infection causes insulin resistance in the very early stage hepatic lesions (fibrosis stage 0 or 1). The progression of fibrosis, primarily owing to insulin resistance, worsens insulin resistance[23], which may lead to T2DM in predisposed individuals. In addition to SVR, fibrosis stage is independently associated with T2DM in HCV-infected patients[24]. Furthermore, a recent meta-analysis[25] and systematic review included 11 studies, of which only five examined the influence of HCV eradication on the risk of de novo glucose abnormalities[19,24,26,27] and insulin resistance[27]. Of the 2 studies with long follow-up periods [19,26], one (8-year follow-up) failed to demonstrate reduced de novo T2DM in patients with SVR[19]. The other (follow-up of 5.7 ± 2 years) showed that SVR reduced de novo glucose abnormalities in patients with chronic HCV. However, patient ages were not reported[26]. In another study with a relatively short follow-up (24 mo), SVR in patients with chronic HCV who did not have T2DM (51.8 ± 12.2 years old) prevented the development of de novo insulin resistance[28]. A significant two-third reduction in T2DM development was reported in a large cohort of patients with HCV and SVR (51.8 ± 9 years old) after IFNα monotherapy or combination therapy with IFNαand ribavirin[22]. Thus, curing HCV infections decreases the incidence of T2DM or improves homeostatic model assessment insulin resistance (HOMA-IR) in most studies[22-33]. These effects might be specific to patients with particular genotypes, with a reduction in HOMA-IR in patients with HCV genotype 1 but not those with genotypes 2/3[23]. Antiviral therapy might improve insulin resistance independent of virological outcomes[32] although a greater reduction in HOMA was observed in the patients who achieved persistent HCV clearance[33]. Antiviral therapy might also improve hepatic steatosis and fibrosis[29,34,35]. Thus, there might be an association between HCV and MS, and patients with HCV infection and MS have higher HOMA-IR values.
Several other components of MS, including waist circumference, BMI, and arterial hypertension, have been reported more frequently in non-responders to antiviral therapy[15]. The present study failed to demonstrate a significant relationship between baseline metabolic factors and treatment responses, potentially owing to the relatively young sample population. However, some metabolic factors (apart from T2DM) showed trends for differences based on the treatment response (e.g., de novo hypertriglyceridemia was 3.7 times more frequent in the responders than in the non-responders).
A bidirectional relationship between serum lipid levels and success of antiviral therapy for HCV has been reported[36]. Successful antiviral therapy might reverse the low LDL cholesterol, HDL cholesterol, and TG levels associated with HCV infection[7,12,13]. Low serum LDL levels in HCV infection result from the utilization of geranyl-geranyl phosphate, a product of the mevalonate pathway that is an early branch point of the cholesterol synthetic pathway, for HCV replication[1,7]. Higher baseline serum LDL cholesterol and lower serum TG levels were associated with higher rates of SVR[34], and lower serum LDL cholesterol levels correlated with low rates of SVR[33,35] in non-diabetic, non-cirrhotic patients infected with genotype 1 HCV. High serum LDL cholesterol levels might improve the rates of SVR by competing with binding to hepatocyte LDL receptors and subsequently reducing the infection of hepatocytes with HCV[4,11,34]. In contrast, HDL cholesterol enhances HCV infection by facilitating its entry into hepatocytes[2]. However, high baseline serum HDL cholesterol levels reportedly interfere with the early viral response, but not with SVR[3,4], while serum HDL cholesterol inversely correlates with the rate of SVR in men but not in women, resulting in a lack of an association between overall baseline HDL levels and SVR[11].
Hepatic steatosis might also attenuate the antiviral treatment response, a trend that was demonstrated in the present study based on steatosis identified via liver biopsy. The insulin resistance and hepatic steatosis present during HCV infection are genotype-specific. Lower HOMA values are reported in patients infected with genotype 3 than in those infected with genotype 1. Insulin resistance-associated steatosis, which is present in patients with HCV genotype 3, is caused mainly by viral inhibition of the enzyme microsomal triglyceride transfer protein (viral steatosis), which might resolve with successful antiviral therapy. With the other HCV genotypes, steatosis is due to insulin resistance, stimulation of fatty acid synthesis, and inhibition of mitochondrial β-oxidation (metabolic steatosis)[37-39]. Metabolic steatosis might be associated with a high BMI and central obesity, which are not usually improved by viral eradication. HCV 1 and 4 core proteins might cause insulin resistance by functionally inhibiting insulin signaling pathways via increased levels of pro-inflammatory cytokines including tumor necrosis factor (TNF)-α and suppressors of cytokine signaling (SOCS) proteins, which impair insulin signaling and activate sterol regulatory element binding proteins, resulting in increased hepatic lipid synthesis[37-39]. The increased levels of hepatic proinflammatory cytokines have additional effect of negative regulate IFNα transduction.
This might explain the molecular link between insulin resistance and the nonresponse to antiviral therapy[4]. Furthermore, steatosis has been demonstrated to decrease SVR in HCV genotype 1 but not in HCV genotype 3, although steatosis is a predictor of HCV infection relapse with genotype 3 HCV[16]. This effect of steatosis on SVR might be the result of its association with insulin resistance[40], which is caused by excretion of TNFα and SOCS protein from the increased trunk fat in HCV infection. The lower level of PPAR-alpha mRNA also mediates genotype 3 hepatic steatogenesis[7,8,39]. However, Peg-INFα and ribavirin treatment response with HCV genotype 3 infection is better than with HCV genotype 1, despite more severe steatosis and lower cholesterol levels[10,37,40]. This might be related to the association between steatosis and higher BMI with genotypes 1 and 4[14,15].
It is noteworthy that, even in the era of direct acting antiviral (DAA; e.g., telaprevir and boceprevir)-based triple therapy, some baseline metabolic variables might affect SVR, albeit to a lesser degree than with IFN and ribavirin combination therapy[41]. However, insulin resistance does not predict SVR to telaprevir-based triple therapy or to the protease inhibitor danoprevir monotherapy[16,42], while low serum LDL levels might affect SVR in telaprevir-treated patients, and obesity impairs SVR in patients treated with boceprevir-based regimen[16,41]. An additional link between DAA and MS has been demonstrated with danoprevir monotherapy, an inhibitor of NS3/3A HCV serine protease, which might increase insulin sensitivity considerably, independent of its antiviral effect[5,38,42]. Furthermore, DAAs are less effective against genotype 3 HCV infection, partly due to steatosis[43] and relapse after IFN-free therapy with the polymerase inhibitor sofosbuvir and ribavirin is associated with a low baseline LDL level.
This study has certain limitations. First, the study was retrospective, and some data were missing for some cases. Serum glucose levels were examined for diagnosis and monitoring glycemic control of T2DM and IFG. However, due to the retrospective nature of this manuscript, HbA1c and glycated albumin (GA) values were not available in most of the patients’ charts. Nevertheless, liver cirrhosis and INF alpha treatment may falsely decrease HbA1c owing to hemolysis. On the other hand, GA as a glycemic control marker in patients with chronic liver disease may be overestimated, due to prolonged albumin half-life.
Additionally, the small sample size resulted in a small number of responders with de novo T2DM and non-responders with de novo hypertriglyceridemia, which limited the ability to assess these data in the multivariable logistic regression analysis.
However, the strengths of the study include the relatively young age of the patients and the relatively long follow-up period for all MS components after antiviral treatment, which enabled us to observe the effect of SVR on the cumulative incidences of IFG and T2DM. MS, including insulin resistance, hyperglycemia, high BMI, and liver steatosis, might complicate the disease course of patients infected with HCV, by enhancing cirrhosis, HCC, and cardiovascular disease[29,43-47]. Thus, it is worthwhile to screen these patients for T2DM[46,47]. Furthermore, with T2DM in the presence of HCV, there are specific considerations for antidiabetic treatment. Insulin and sulfonylurea administration might increase the risk of HCC[45,48], while the insulin sensitizers metformin and pioglitazone might decrease the risk of HCC and steatosis. However, these agents are harmful and might cause lactic acidosis and hepatic toxicity, respectively, in patients with liver cirrhosis[45]. The new dipeptidyl peptidase-4 inhibitors appear promising[45,47,49].
In conclusion, the results presented here suggest that MS components did not have any significant effect on the response to antiviral therapy, although hepatic steatosis tended to impair the response to antiviral treatment. There were no differences in the post-treatment changes in most MS components between responders and non-responders to antiviral therapy. However, the incidences of de novo T2DM and IFG were significantly higher in non-responders. Given the younger age of the patient population in the present study compared to previous similar studies, the findings might suggest a direct effect of HCV on the development of T2DM independent of fibrosis or cirrhosis. The higher serum TG levels after SVR exemplify the interaction between HCV infection and lipid metabolic pathways. Due to the increased risk of HCV infection with T2DM, it might be appropriate to screen HCV patients for T2DM and insulin resistance and to consider treatment of T2DM in the presence of HCV with new antidiabetic agents.
A bidirectional association exists between chronic hepatitis C virus (HCV) infection and some components of metabolic syndrome (MS).
Most, but not all, previous studies showed an association between chronic HCV infection and MS components, including type 2 diabetes mellitus (T2DM), insulin resistance, elevated body mass index, and hepatic steatosis. Several host MS components might affect the disease course and sustained virological response (SVR) of HCV-infected patients treated with pegylated-interferon α (Peg-IFNα) and ribavarin. On the other hand, SVR can affect some MS components, mainly decreased insulin resistance (IR) and decreased de novo occurrence of T2DM.
This study, which included a younger patient cohort, was designed to assess associations between MS components and HCV infection, but it did not show increased prevalence of T2DM with HCV infection. However, after long term follow-up T2DM was more frequent in non-responders to treatment with Peg-IFNα and ribavarin.
Patients with HCV infection should be frequently monitored for T2DM and treated appropriately, considering the increased risk of cirrhosis and HCC in young patients with HCV-associated diabetes mellitus. The authors think that further studies are needed to evaluate the mutual association of MS components with direct acting antiviral (DAA) drug therapy in chronic HCV infection.
MS components refer to the metabolic syndrome factors; IR refers to insulin resistance; SVR refers to sustained virological response, which means viral eradication and cure; DAA refers to direct acting antiviral agents.
This is a very interesting study which shows the association between metabolic syndrome mechanism and response to antiviral treatment for chronic HCV infection. Patients were followed up for about 4 years; univariable and multivariable logistic regression analysis were applied. Data are well presented and discussed.
| 1. | Felmlee DJ, Hafirassou ML, Lefevre M, Baumert TF, Schuster C. Hepatitis C virus, cholesterol and lipoproteins--impact for the viral life cycle and pathogenesis of liver disease. Viruses. 2013;5:1292-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Pécheur EI. Lipoprotein receptors and lipid enzymes in hepatitis C virus entry and early steps of infection. Scientifica (Cairo). 2012;2012:709853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ye J. Reliance of host cholesterol metabolic pathways for the life cycle of hepatitis C virus. PLoS Pathog. 2007;3:e108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World J Gastroenterol. 2014;20:2888-2901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011;12:2215-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Lecube A, Hernández C, Genescà J, Simó R. Glucose abnormalities in patients with hepatitis C virus infection: Epidemiology and pathogenesis. Diabetes Care. 2006;29:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Negro F. Mechanisms and significance of liver steatosis in hepatitis C virus infection. World J Gastroenterol. 2006;12:6756-6765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Negro F. HCV infection and metabolic syndrome: which is the chicken and which is the egg? Gastroenterology. 2012;142:1288-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Adinolfi LE, Restivo L, Marrone A. The predictive value of steatosis in hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2013;7:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ramcharran D, Wahed AS, Conjeevaram HS, Evans RW, Wang T, Belle SH, Yee LJ. Associations between serum lipids and hepatitis C antiviral treatment efficacy. Hepatology. 2010;52:854-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Fernández-Rodríguez CM, López-Serrano P, Alonso S, Gutiérrez ML, Lledó JL, Pérez-Calle JL, Temiño R, Cacho G, Nevado M, Casas ML. Long-term reversal of hypocholesterolaemia in patients with chronic hepatitis C is related to sustained viral response and viral genotype. Aliment Pharmacol Ther. 2006;24:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Soresi M, Tripi S, Franco V, Giannitrapani L, Alessandri A, Rappa F, Vuturo O, Montalto G. Impact of liver steatosis on the antiviral response in the hepatitis C virus-associated chronic hepatitis. Liver Int. 2006;26:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Cheng FK, Torres DM, Harrison SA. Hepatitis C and lipid metabolism, hepatic steatosis, and NAFLD: still important in the era of direct acting antiviral therapy? J Viral Hepat. 2014;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Tarantino G, Conca P, Sorrentino P, Ariello M. Metabolic factors involved in the therapeutic response of patients with hepatitis C virus-related chronic hepatitis. J Gastroenterol Hepatol. 2006;21:1266-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Cheng YL, Wang YC, Lan KH, Huo TI, Huang YH, Su CW, Lin HC, Lee FY, Wu JC, Lee SD. Anti-hepatitis C virus seropositivity is not associated with metabolic syndrome irrespective of age, gender and fibrosis. Ann Hepatol. 2015;14:181-189. [PubMed] |
| 19. | Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, Pellicano R, Cassader M, Gambino R, Bo S. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 476] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 24. | Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, Diago M, Alonso S, Planas R, Solá R, Pons JA, Salmerón J, Barcena R. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Zhang W, Rao HY, Feng B, Liu F, Wei L. Effects of interferon-alpha treatment on the incidence of hyperglycemia in chronic hepatitis C patients: a systematic review and meta-analysis. PLoS One. 2012;7:e39272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kawaguchi Y, Mizuta T, Oza N, Takahashi H, Ario K, Yoshimura T, Eguchi Y, Ozaki I, Hisatomi A, Fujimoto K. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int. 2009;29:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Aghemo A, Prati GM, Rumi MG, Soffredini R, D’Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Clearance of HCV by Combination Therapy of Pegylated Interferon alpha-2a and Ribavirin Improves Insulin Resistance. Gut Liver. 2009;3:108-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Brandman D, Bacchetti P, Ayala CE, Maher JJ, Khalili M. Impact of insulin resistance on HCV treatment response and impact of HCV treatment on insulin sensitivity using direct measurements of insulin action. Diabetes Care. 2012;35:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Petta S, Cammà C, Di Marco V, Cabibi D, Ciminnisi S, Caldarella R, Licata A, Massenti MF, Marchesini G, Craxì A. Time course of insulin resistance during antiviral therapy in non-diabetic, non-cirrhotic patients with genotype 1 HCV infection. Antivir Ther. 2009;14:631-639. [PubMed] |
| 34. | Gopal K, Johnson TC, Gopal S, Walfish A, Bang CT, Suwandhi P, Pena-Sahdala HN, Clain DJ, Bodenheimer HC, Min AD. Correlation between beta-lipoprotein levels and outcome of hepatitis C treatment. Hepatology. 2006;44:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 36. | Dai CY, Yeh ML, Huang CF, Hou CH, Hsieh MY, Huang JF, Lin IL, Lin ZY, Chen SC, Wang LY. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol. 2015;30:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Patel A, Harrison SA. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol Hepatol (N Y). 2012;8:305-312. [PubMed] |
| 39. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 40. | Basaranoglu M, Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol. 2011;17:4055-4062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Grasso A, Malfatti F, Testa R. Are metabolic factors still important in the era of direct antiviral agents in patients with chronic hepatitis C? World J Gastroenterol. 2013;19:6947-6956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Moucari R, Forestier N, Larrey D, Guyader D, Couzigou P, Benhamou Y, Voitot H, Vidaud M, Seiwert S, Bradford B. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59:1694-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 43. | Lim T. Metabolic syndrome in chronic hepatitis C infection: does it still matter in the era of directly acting antiviral therapy? Hepat Med. 2014;6:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol. 2014;61:S69-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 45. | Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and Hepatitis C: A Two-Way Association. Front Endocrinol (Lausanne). 2015;6:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | García-Compeán D, González-González JA, Lavalle-González FJ, González-Moreno EI, Villarreal-Pérez JZ, Maldonado-Garza HJ. Current Concepts in Diabetes Mellitus and Chronic Liver Disease: Clinical Outcomes, Hepatitis C Virus Association, and Therapy. Dig Dis Sci. 2016;61:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013;19:2298-2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 129] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Osna NA, Tanaka Y S- Editor: Qiu S L- Editor: A E- Editor: Li D