Published online Sep 8, 2016. doi: 10.4254/wjh.v8.i25.1047
Peer-review started: March 23, 2016
First decision: May 16, 2016
Revised: May 16, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: September 8, 2016
Processing time: 175 Days and 23.7 Hours
Advanced liver cirrhosis is usually accompanied by portal hypertension. Long-term portal hypertension results in various vascular alterations. The systemic hemodynamic state in patients with cirrhosis is termed a hyperdynamic state. This peculiar hemodynamic state is characterized by an expanded blood volume, high cardiac output, and low total peripheral resistance. Vascular alterations do not disappear even long after liver transplantation (LT), and recipients with cirrhosis exhibit a persistent systemic hyperdynamic state even after LT. Stability of optimal systemic hemodynamics is indispensable for adequate portal venous flow (PVF) and successful LT, and reliable parameters for optimal systemic hemodynamics and adequate PVF are required. Even a subtle disorder in systemic hemodynamics is precisely indicated by the balance between cardiac output and blood volume. The indocyanine green (ICG) kinetics reflect the patient’s functional hepatocytes and effective PVF, and PVF is a major determinant of the ICG elimination constant (kICG) in the well-preserved allograft. The kICG value is useful to set the optimal PVF during living-donor LT and to evaluate adequate PVF after LT. Perioperative management has a large influence on the postoperative course and outcome; therefore, key points and unexpected pitfalls for intensive management are herein summarized. Transplant physicians should fully understand the peculiar systemic hemodynamic behavior in LT recipients with cirrhosis and recognize the critical importance of PVF after LT.
Core tip: In patients with advanced cirrhosis who undergo liver transplantation (LT), perioperative management greatly influences the postoperative course and outcome. This review covers key points and unexpected pitfalls of intensive management in these patients. A peculiar systemic hemodynamic state (hyperdynamic state) persists in recipients with cirrhosis even after LT, and stability of optimal systemic hemodynamics is important for adequate portal venous flow (PVF) and successful LT. Reliable parameters for optimal systemic hemodynamics (a balance between cardiac output and blood volume) and adequate PVF (indocyanine clearance) during and after LT are herein described. Transplant physicians should fully understand these peculiar hemodynamic phenomena.
- Citation: Hori T, Ogura Y, Onishi Y, Kamei H, Kurata N, Kainuma M, Takahashi H, Suzuki S, Ichikawa T, Mizuno S, Aoyama T, Ishida Y, Hirai T, Hayashi T, Hasegawa K, Takeichi H, Ota A, Kodera Y, Sugimoto H, Iida T, Yagi S, Taniguchi K, Uemoto S. Systemic hemodynamics in advanced cirrhosis: Concerns during perioperative period of liver transplantation. World J Hepatol 2016; 8(25): 1047-1060
- URL: https://www.wjgnet.com/1948-5182/full/v8/i25/1047.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i25.1047
Advanced liver cirrhosis (LC) is usually accompanied by portal hypertension (PH). Long-term PH results in various vascular alterations, such as venous dilatation, endothelial damage, collateral pathway formation, and shunt development[1-3]. Some pathognomonic findings (e.g., varices, splanchnic congestion, intractable ascites, hepatic encephalopathy, and hepatorenal syndrome) are directly related to PH[3,4], and the pathophysiology of PH involves a complex of humoral and neural mechanisms[3]. These mechanisms determine hemodynamic changes and lead to a peculiar systemic circulation pattern[3]. The clinical implications of these peculiar systemic hemodynamics in patients with LC have been described as a hyperdynamic state (so-called “hyperdynamic syndrome”)[3]. Specific manifestations that have been described include high cardiac output (CO), a large blood volume (BV), low total peripheral resistance (TPR), hyponatremic electrolyte abnormalities, and a lower potassium level due to secondary aldosteronism[5].
Here, we reviewed the peculiar systemic hemodynamics in patients with advanced LC. We focused particularly on the systemic hemodynamic phenomena in liver transplantation (LT) recipients with LC because such LT recipients usually have long-term PH due to advanced LC. Adequate portal venous flow (PVF) to acquire satisfactory graft function is attributed to continuous optimal systemic hemodynamic stability beginning immediately after LT[1,2]. Therefore, we herein review the optimal state of the systemic hemodynamics after LT for excellent outcomes and discuss key points and unexpected pitfalls in the perioperative intensive managements of recipients with LC. We also demonstrate the usefulness of indocyanine green (ICG) during and after LT to estimate optimal systemic hemodynamics and adequate PVF.
The systemic hemodynamic state in patients with LC has been characterized as hyperdynamic[3,6,7]. Cirrhotic hemodynamics are characterized as hyperdynamic by a high CO, large BV, low TPR, mildly tachycardic heart rate (HR), and low or normal mean arterial pressure (MAP)[1-4,6,8-10]. Parameters of peripheral resistance, such as TPR, clearly reflect various vascular alterations[1-3,9,11,12].
The ICG dye dilution curve can be used to measure hemodynamic parameters[13,14]. The currently available noninvasive method for measuring systemic hemodynamic parameters is pulse dye densitometry (PDD). Its basic principles have been described in detail elsewhere[13-16]. This noninvasive method is more reliable than invasive methods[13-15] and is suitable for clinical use because of its simplicity for bedside use, real-time presentation of results, and cost-effectiveness[15-17].
The principles of BV measurement using radioactive isotopes have already been established[18-20]. However, these techniques are associated with potential biohazards due to the use of radioactive indicators and require complex management. Indeed, these invasive methods using radioactive isotopes are completely unsuitable for BV monitoring during the perioperative period[14,15]. BV measurement by noninvasive PDD is considerably correlated with BV measurement by radioactive isotope methods[21-23]; it is thus advantageous for real-time evaluation of BV[1,15].
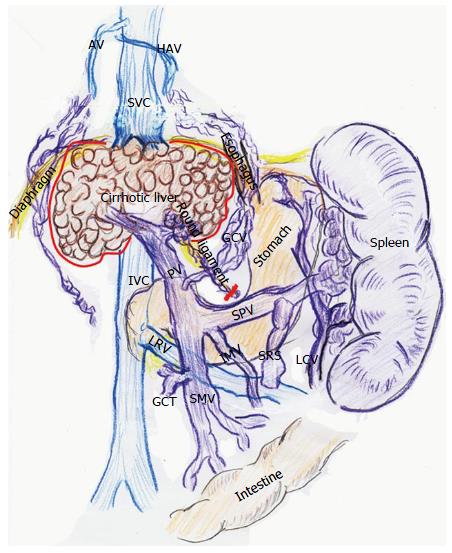
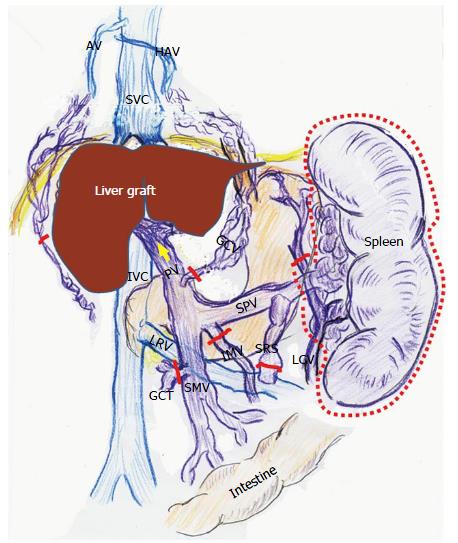
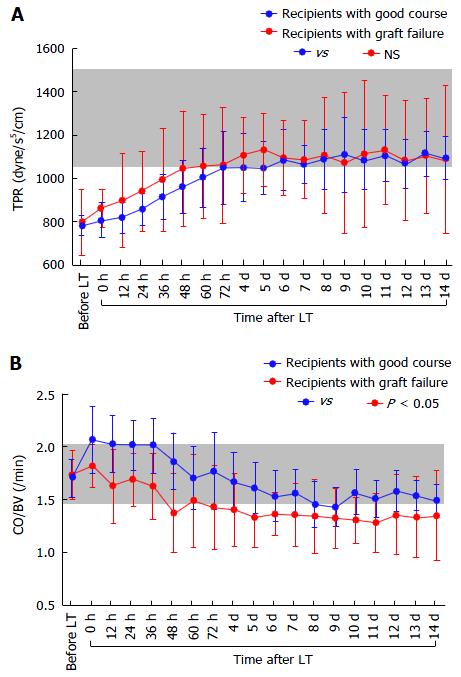
Adult LT recipients often develop peculiar hemodynamics due to advanced LC[1,2] (Figure 1). Mainly in the 1990s, various researchers focused on systemic hemodynamics after LT[8,9,11,12,24-28]. Controversial opinions exist regarding these systemic hemodynamic behaviors after LT. While several investigators found persistence of hyperdynamic state[8,11,24-26], others insisted on a decrease toward normal ranges[12,27,28]. This discrepancy is believed to be due to the peculiarity of cirrhotic hemodynamics[9,11].
According to studies of TPR in recipients with LC, vascular alterations including venous dilatation and the development of collateral vessels and shunts do not disappear within the first month after LT[1,2]. These vascular alterations remain on imaging studies even several years after LT[29,30]. Various alterations in systemic hemodynamics in recipients with LC should be maintained despite restoration of the liver function and portal venous pressure (PVP) after LT[8,11,24-26], and most systemic parameters are very slowly restored to the normal range after LT[9,11] (Figure 2).
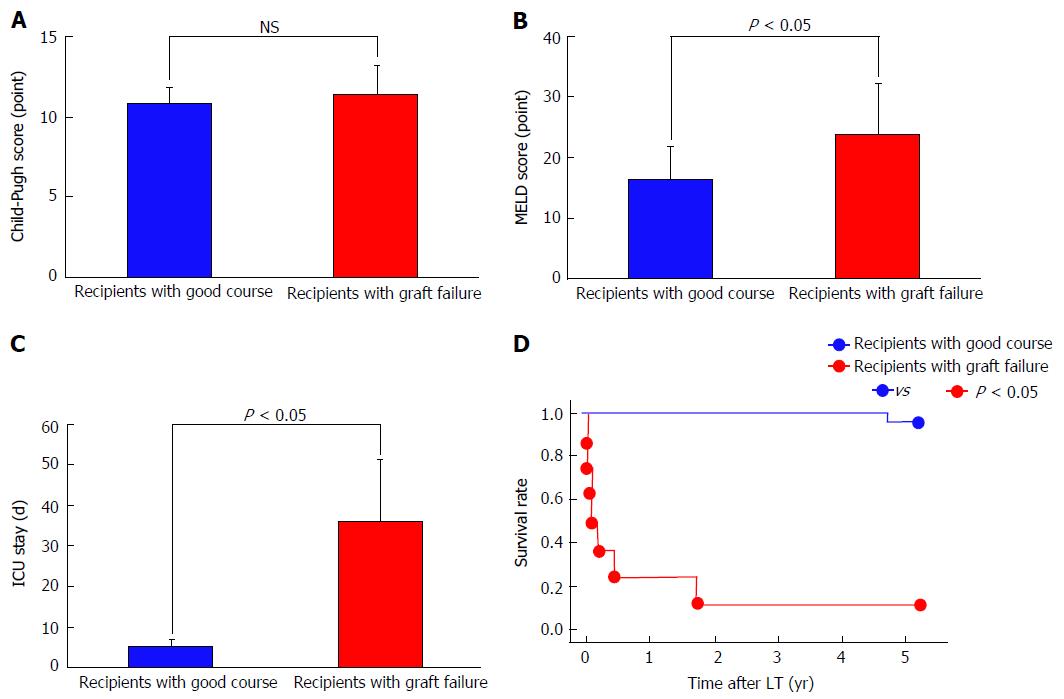
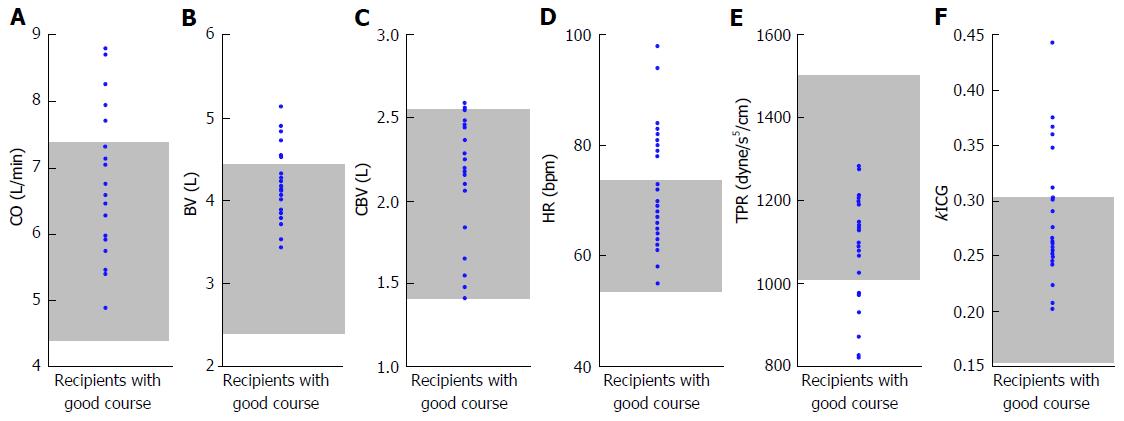
Hemodynamic and splanchnic systemic parameters were analyzed in 35 adult recipients who underwent living-donor LT (LDLT). All patients had advanced LC based on imaging studies and histopathological assessments. ABO blood groups were identical or compatible. Combinations of lymphoid cross-matches were all negative. The CO, CI, BV, central blood volume (CBV), and HR were measured with a PDD apparatus. The TPR was measured simultaneously with the PDD examination; calculation of the TPR has been described in detail elsewhere[2,9]. Splanchnic circulatory parameters were simultaneously assessed using Doppler ultrasound. Measurements of splanchnic parameters including PVF has been described in detail elsewhere[2]. Measurements were performed before LDLT and from 1 to 14 d after LDLT. Measurements were repeated every 12 h until 72 h after LDLT. To establish the normal ranges of each parameter, the variables were investigated in 16 healthy individuals (live donors before LDLT). Our 35 recipients were retrospectively classified into 2 groups based on graft functions that corresponded to outcomes[31,32]. Twenty-seven recipients had good clinical courses after LDLT, although eight recipients developed graft failure. No significant differences were found in the Child-Pugh score (Figure 3A), graft-to-recipient weight ratio (GRWR), operative time, or intraoperative blood loss between the two groups; however, significant differences were found in the Model for End-Stage Liver Disease score (Figure 3B), duration of intensive care unit al) stay (Figure 3C), and survival rate (Figure 3D). In addition, in the patients who survived, the above-mentioned parameters were measured 3 mo after LDLT. All protocols used in the present study were approved by our institutional review board (approved No. C-297) and were based on the ethical guidelines of the Helsinki Declaration. Informed consent was obtained from all patients before enrollment. For individually, temporally, and repeatedly measured data, differences in the changes over time after LDLT between the two groups were analyzed by repeated-measures analysis of variance. Differences in unpaired discontinuous data between the two groups were analyzed by the Mann-Whitney U test. Survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used for between-group comparisons of recipient survival. Values of P < 0.05 were considered statistically significant.
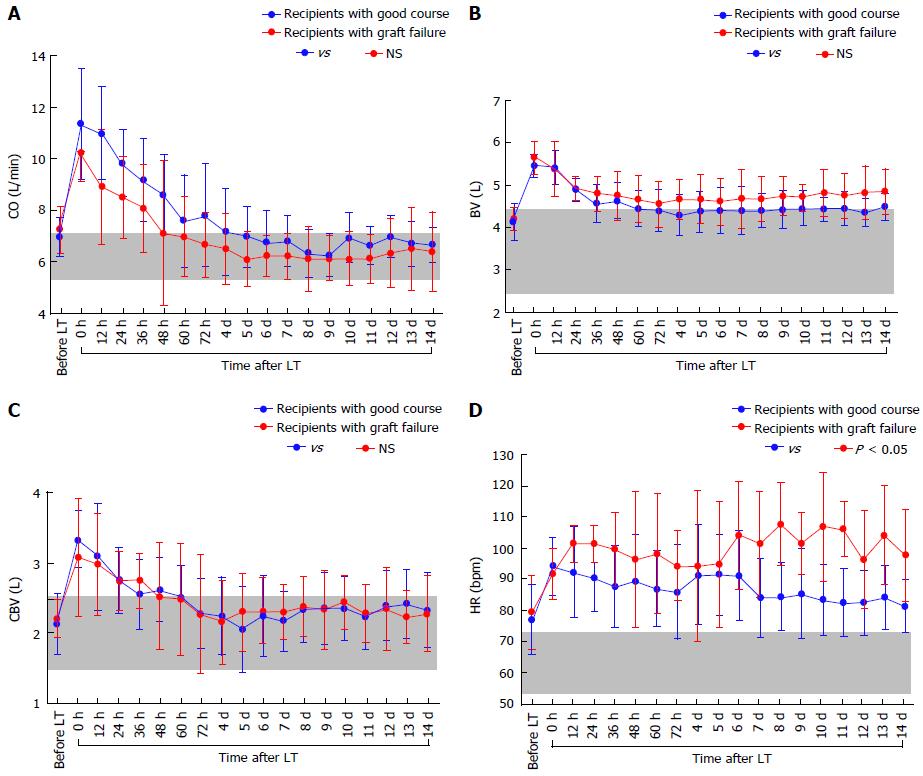
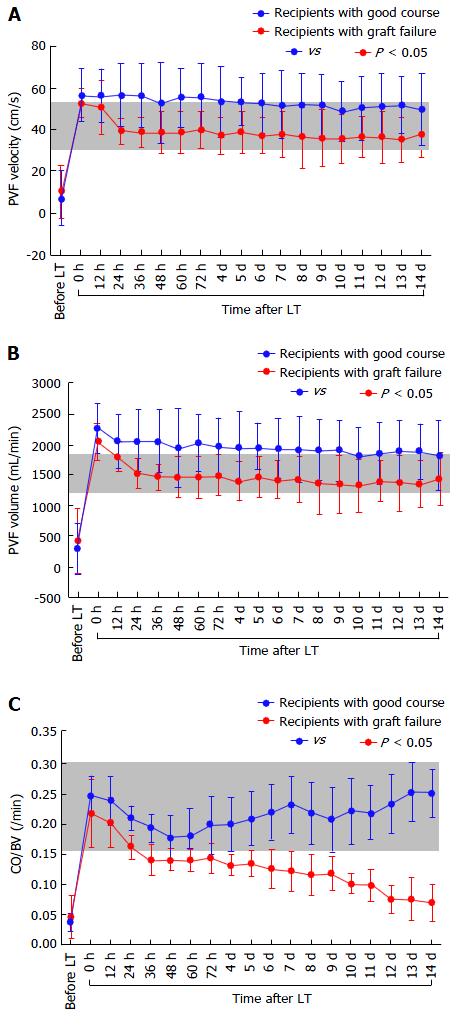
There were no significant differences in the absolute CO (Figure 4A), CI, BV (Figure 4B), CBV (Figure 4C), or MAP between the two groups, although the absolute HR showed differences (Figure 4D). There were also no significant differences in the absolute TPR, which closely reflected vascular alterations (Figure 5A). The balance between CO and BV (i.e., CO/BV) clearly showed significant differences between the groups (Figure 5B). There were significant differences in the PVF velocity (Figure 6A) and PVF volume (Figure 6B) between the groups, although the variables for hepatic arterial flow showed no differences. There were also significant differences in the ICG elimination constant (kICG), which mainly reflects PVF in the early postoperative period[1,2,32].
The CBV reflects the greater circulatory system, and some researchers have suggested that this greater circulation in patients with LC may be slightly lower than that in healthy individuals[33], although the total BV is significantly higher in patients with LC. Our data also demonstrated no remarkable differences in the greater circulation itself between patients with LC and healthy individuals.
The absolute CO, BV, CBV, HR, TPR, and kICG in LT recipients who were still alive 3 mo after LDLT are summarized in Figure 7. Our data support the previous opinion that cirrhotic vascular alterations still remain long after LT[29,30].
As described above, recipients with LC exhibit a persistent systemic hyperdynamic state even after LT[1,2,8,11,24-26]. Stability of characteristic systemic hyperdynamic parameters after LT is necessary for successful LT in recipients with LC[1,2]. Because recipients with LC exhibit these peculiar systemic hyperdynamics even after LT[8,9,11,24-26], an accurate real-time evaluation is necessary to ensure appropriate intensive management after LT[1,2,15,32]. The optimal systemic hemodynamics needed for excellent outcomes and the precise parameters for the most appropriate clinical strategy remain unclear[1,32] because the absolute values themselves, such as CO, CI, BV, CBV, and MAP, are not necessarily satisfactory for the detection of the subtle instabilities of these patients’ peculiar hyperdynamic state[1,2].
Several investigators have used CO and/or CI to assess hemodynamics after LT[8,11,12,25]. Use of the CI, an index that concisely standardizes CO against the body surface area, has been popularized as a standardized CO value for better assessment. Similar to CO, BV is also one of the most important factors affecting cardiac preload[14,34]. Intrinsically, preload is a concept that represents the blood load in the left ventricle and considers the left ventricle as the center of blood ejection[1,15]. Therefore, the left ventricular end-diastolic volume becomes a quantitative parameter[1,15]. The preload usually replaces actual clinical assessment with parameters representing pressures such as the pulmonary capillary wedge pressure and central venous pressure[14,35]. The central venous pressure can be a useful indicator of the filling status of the right ventricle; it is especially useful when followed over time and combined with a measurement of cardiac output[36]. Pressure-expressing parameters including the pulmonary capillary wedge pressure and central venous pressure are mainly provided by the CO and BV[35]. Therefore, although pressure-expressing parameters do not necessarily reflect the left ventricular end-diastolic volume[14,15], pressure-expressing parameters that reduce the precision of assessment of the systemic hemodynamics have been paradoxically used to judge distinct factors that represent the amount of BV and strength of CO clinically because BV monitoring has been impossible in the past[14,15]. It is necessary to standardize CO against BV, but not against the body surface area, for precise evaluation of preload[1]. Currently, the PDD guarantees noninvasive vigilance of the balance between CO and BV as an index for precise assessment of the systemic hemodynamic state[1,15]. The CO/BV ratio is a reliable indicator of the optimal systemic hemodynamic state after LT[1,2]. Preload focuses on the balance between CO and BV, and cirrhotic systemic hemodynamics are characterized by a high CO and large BV[6-9,12,24-26,35]. Real-time assessment of CO and BV by making the best use of noninvasive PDD may become an effective strategy for evaluating the systemic hemodynamic state in LT recipients with LC.
Postoperatively, LT recipients with LC show a clear tendency toward PVF overflow compared with healthy individuals[2]. The systemic hemodynamics impact the local graft circulation after LT[1,2], and even a subtle systemic hyperdynamic disorder strongly affects the splanchnic circulation. An imbalance between CO and BV decreases the PVF, which results in critical outcomes[1,2]. In brief, an optimal balance between CO and BV guarantees adequate PVF after LT[1,2]. Interestingly, subtle disorders in the optimal systemic hyperdynamic state more easily influence the PVF than the hepatic arterial flow[2]. Vascular alterations secondary to PH develop in the vessels that originally flow into the portal vein under normal PVP. Such alterations are one cause of a large BV[2]. The intestine and spleen become a pool for the large BV[37]. Postoperative imbalance between the greater CO and larger BV cause stagnation of the tributary blood flow in the dilated veins and collateral pathways, resulting in a decrease in PVF[2]. Transplant physicians should never forget that the systemic hyperdynamic state persists in recipients with LC even after LT[1,2,8,11,24-26] and that this peculiar systemic hemodynamic stability is indispensable for adequate PVF after LT[1,2].
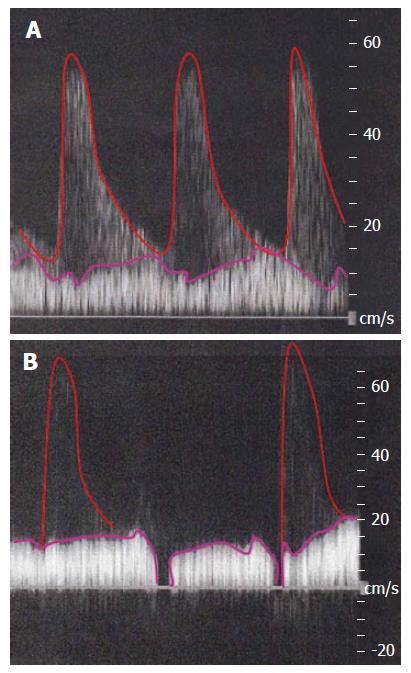
Actual images of Doppler ultrasound in cases without stability of systemic hemodynamic state (i.e., an imbalance of CO and BV in the lower TPR) are shown in Figure 8. The PVF should be detected as a stationary wave. However, in a case of unstable systemic hyperdynamic state, the waveform of PVF may seem to be undulant. Moreover, HA waveform may blend into the background of a decreased PVF.
Partial liver grafting is inevitable in the LDLT setting, and the allograft size from the live donor is therefore insufficient. Intentional modulation of the PVP to ≤ 15 mmHg is a simple and sure strategy during LDLT[38-42]. Detailed surgical procedures for intentional modulation of PVP have been described elsewhere[40,41]. Paradoxically, the acceptable minimum GRWR of < 0.7 is possible at graft selection[40] because intentional PVP modulation during LDLT will prevent small-for-size syndrome after LDLT[38-42]. Although intentional PVP control seems to overcome an GRWR of < 0.7, these grafts still cause critical problems when evaluated retrospectively[40]. Selection of a graft with an GRWR of ≥ 0.8 and establishment of a target PVP of ≤ 15 mmHg during LDLT are considered keys for successful LDLT[40]. Optimal PVF is required for successful LDLT[2,43]. Ligation of collaterals and shunts often require an advanced surgical technique because these vessels are always abnormal[41,42]. However, intentional setting of the PVF during LDLT is effective not only to trigger liver regeneration after LT, but also to prevent steal of PVF after LDLT.
ICG is widely used for analysis of liver functions because it is exclusively eliminated by the liver without involvement of the enterohepatic circulation and does not accumulate in the body[44]. Asialoglycoprotein receptors on hepatocytes are characteristic of functional liver cells[45], and liver scintigraphy using 99mTc-galactosyl human serum albumin has been used as a reliable method of assessment of the hepatic functional reserve in hepatectomy and graft parenchymal function after LT[46-48]. There is a correlation between ICG clearance and the hepatic uptake ratio assayed by liver scintigraphy[45,46].
ICG kinetics reflect the functional hepatocytes (cell volume) and effective PVF (clearance)[31,49-52], and PVF is a major determinant of kICG in the normal liver[32,34,49,51,53]. The PVF has a large influence on liver regeneration after LT[32,43], and reversible damage to hepatocytes begins immediately after graft recirculation[32,38,39,43]. Some researchers have focused on ICG kinetics as a liver function test after LT[31,32], and kICG values can predict clinical outcomes in the early postoperative period after LDLT by closely reflecting the influence of systemic dynamics on the splanchnic circulation[32].
Hepatocytes are well preserved in LDLT because the cold storage time (CIT) is shorter. The kICG reflects the optimum PVF value during LT and in the early postoperative period[41,42]. Hence, a division by graft weight is a simple resolution to ensure that the kICG reflects only the PVF based on the advantage of well-preserved hepatocytes during LDLT[41,42]. Intentional PVP modulation based on real-time PVP monitoring and the confirmation of an optimal kICG/graft weight value reflecting the PVF are useful procedures used by transplant surgeons during LDLT[41,42]. Actually, in some cases, the kICG value did not change even with intentional controls to decrease or increase the PVP[41]. In other cases, the kICG values improved with an increased PVP by ligation of portosystemic collaterals or a decrease in the PVP by splenectomy[41]. Thus, these factors seemed to show some discrepancies in some cases[41,42]. The relationship between PVP and PVF remains unclear[42]. The usefulness of ICG kinetics during LT was first described in 2012[41]. Simultaneous fulfillment of a final PVP of ≤ 15 mmHg and a final kICG of > 4 × 10-4/g × the graft weight (g) is a sure strategy for achieving the optimal PVF during LDLT[41]. Thereafter, the cut-off level of the final kICG/graft weight was demonstrated as 3.1175 × 10-4/g[42]. The final kICG/graft weight during LT has potential as an accurate parameter for the optimal PVF and as a reliable predictor of the postoperative course and outcome after LT[41,42].
Liver allografts are at risk of problems such as cold ischemia/warm reperfusion injury, acute rejection, disease recurrence and hepatic blood flow disorders[32]. Transplant physicians should consider many factors simultaneously.
Eventration of the diaphragm because of intractable ascites, or easily broken ribs, often disrupts ventilation[54]. Vascular alteration due to long-term PH causes endothelial injury and permeant breakdown and subsequently results in large amounts of ascites, pleural effusion, and gastric fluid[55]. The electrolyte composition of these third-space fluids may not be similar to that of the extracellular fluid, and the electrolyte composition of third-space fluids should be checked once if the quantity is large[56]. Replenishment for third-space loss should be performed using not Ringer’s solution but bicarbonated Ringer’s solution[57-59] if the electrolyte composition is similar to that of the extracellular fluid and if the third-space loss is quantitatively large.
Careless management techniques, such as rapid increases or decreases of transfusions and medications, are detrimental[60,61]. Effects of increases or decreases of transfusions are usually reflected on a day-to-day basis because of the peculiar cirrhotic hemodynamics[55,60,61], and a roller-coaster management technique that repeatedly changes within a single day will trigger poor clinical courses with unexpected complications[60,61]. All transfusion management plans should be handled with great caution, and transplant physicians should very carefully evaluate the effects of increases or decreases of transfusions[60-62]. A response time lag due to endothelial injury and permeant breakdown should be considered in LC recipients with long-term PH[63-65]. Adequate hydration is also required; dehydration should be avoided because of these patients’ peculiar hemodynamics. Even temporal dehydration causes unexpected thrombosis, renal failure, and impaired drug metabolism[60-62]. Plans to stay within stable systemic hemodynamics (e.g., noradrenaline to maintain CO and well-hydration with human atrial natriuretic peptide) should be considered. Tachycardia may lower the CO. A lower CO that is insufficient to circulate the larger BV decreases the PVF, and a lower PVF results in a poor outcome. As described above, vascular alterations cause the large BV in these patients[2], and the intestine and spleen become pools for the large BV[37]. Even a subtle imbalance between the greater CO and larger BV induced by roller-coaster management triggers a decrease in the PVF[2,60,61].
Long-term PH causes splanchnic congestion and intractable ascites. Splanchnic congestion results in breakdown of the enteric barrier[66], and portal venous gas and/or abdominal compartment syndrome may be temporally observed[66-68]. Induction of drugs with fibrolytic activity (not heparin, but urokinase and warfarin) should be initiated without hesitation based on the endothelial damage in patients with LC, although heparin induction may be effective from the viewpoint of thromboprophylaxis[69]. Notably, long-term biliary drainage may cause coagulopathy due to impaired absorption of vitamins[70]. Massive ascites is usually intractable due to endothelial injury and permeant breakdown, and systemic arterial pressure may be effected even by body motion[63-65]. Diuretics (e.g., furosemide and potassium-conserving diuretics) and a water-clearance mediator (e.g., tolvaptan) are available[71]. Hemodynamic disorders such as hepatic venous obstruction and portal thrombosis may develop if no response is observed after diuretic induction[72].
The most frequent cause of morbidity and mortality after LT is not immunological rejection but infection-related complications[73-75]. Some infections are usually intractable in patients with LC, including bacterial cholangitis[76], spontaneous bacterial peritonitis[77], spontaneous bacterial empyema[78], viral infection[79], aspergillosis[80], and Pneumocystis jirovecii pneumonia (formerly known as Pneumocystis carinii pneumonia)[81]. Because the postoperative risk of complications is associated with the pretransplant conditions[82,83], these infections should be ruled-out and/or treated beforehand. Even a subtle infection will trigger severe complications after LT[73-75,83,84]. Evaluation of LT candidates should be carefully performed[83,85,86]; pretransplant infections may greatly impair the clinical course and outcomes after LT[83,87,88]. Transplant physicians should never forget that intentional pretransplant control of infections, including bacterial, viral, and fungal infections, has a large influence on allograft function and survival[89,90]. Uncontrolled infections will have catastrophic effects[83,87,88], and any infections should therefore be treated before LT.
Glycemic control also has an influence on the clinical course after LT[91]. Good glycolytic activity and glycemic control in the perioperative period will help to ensure adequate liver regeneration[92,93].
Hepatopulmonary syndrome (HPS) and portopulmonary hypertension (PPHTN) are cardiopulmonary complications[3,94-97] that are frequently seen in patients with LC[54,94-98]. Both conditions result from a lack of hepatic clearance of vasoactive substances produced in the splanchnic territory[95]. These substances mainly cause subsequent pulmonary vascular remodeling. In previous studies, some degree of vasoconstriction in patients with PPHTN resulted in pulmonary arterial hypertension (PAH) and right ventricular dysfunction[54,98]. The current definition of PPHTN includes secondary PAH due to portosystemic shunts[98]. In patients with HPS, these vasoactive mediators cause intrapulmonary shunts with hypoxemia[97]. The HPS is accompanied by abnormal pulmonary gas exchange and evidence of intrapulmonary vascular dilatation that results in a right-to-left intrapulmonary shunt[98]. These entities are both clinically and pathophysiologically distinct[3,94,95], and PPHTN and HPS should be considered as different pathological states[98]. HPS is characterized by abnormal pulmonary vasodilation and right-to-left shunting that result in gas exchange abnormalities[3,54,94,95,97,98], whereas PPHTN is caused by pulmonary artery vasoconstriction that leads to hemodynamic failure[3,94-96]. Both HPS and PPHTN are associated with significantly increased morbidity and mortality[3,94,95,97], although these patients are commonly asymptomatic. All candidates for LT should be actively screened for the presence of these two complications[54,94,95,97,98].
Although LT results in the disappearance of HPS within 1 year[95,99], the effect of LT on PPHTN is highly unpredictable[54,95,98-101]. PPHTN with PAH has historically been a contraindication for LT[54,98-100]. However, the diagnosis and treatment of PPHTN have advanced during the past two decades[54]. Assessment of patients’ preoperative reactivity and response to pharmacological therapies for moderate-to-severe PPHTN is important to ensure excellent survival rates after LT[102]. Prostaglandin I2 has drastically improved outcomes[103] and is currently considered a key drug in the control of PPHTN[103]. Modern strategies in managing HPS and PPHTN rely on a thorough screening and grading of the disease severity to tailor the appropriate therapy and select only the patients who will benefit from LT[54,95,97-101]. Hemodynamic and respiratory modifications in the perioperative period must be avoided through continuation of the preoperatively initiated drugs, appropriate intraoperative monitoring, and proper hemodynamic and respiratory therapies[54,95,98,99]. The most reliable monitoring factor for PPHTN with PAH during the perioperative period is the mean pulmonary arterial pressure[54,98], though supplemental oxygen and monitoring of oxygen saturation during the perioperative period are adequate for monitoring of HPS[97,104,105].
The systemic hyperdynamic state causes vessel dilation and collateral development, and the venous endothelium becomes damaged[4,65]. An intact endothelial barrier is important, especially in critical situations such as sepsis and thrombotic microangiopathy[106,107]. High mobility group box 1 (HMGB1) is an evolutionarily conversed nuclear protein that is passively released by almost all cells during cellular necrosis and is actively secreted from activated macrophages, monocytes, and endothelial cells[108]. Once secreted into the extracellular space, HMGB1 serves as a dangerous signal that stimulates inflammatory reactions[108]. Thrombomodulin (TM) is an endothelial anticoagulant cofactor that promotes thrombin-mediated formation of activated protein C[109]. TM plays an anti-inflammatory role through inactivation of HMGB1[109,110]. Recombinant human soluble TM (rTM) has recently become available[111], and this novel drug is effective for sepsis[110]. Thrombotic microangiopathy and a positive lymphoid cross-match combination will result in poor outcomes after LT, especially in adult recipients[112,113]. Intrahepatic and vascular conditions pathophysiologically overlap. Pathophysiologically, rTM is effective for sepsis and thrombotic microangiopathy in LT recipients[107,111], although there are no reports of its usefulness for ABO incompatibility in patients undergoing LT. Vascular alterations including endothelial injury still remain even after LT. Based on our experience, the dose of rTM should be reduced to two-thirds of the regular dose in LT recipients with LC, although one-half of the regular dose loses any effects.
Insurance systems are different in each country[114,115], and every country has its own limitations of medical resources[116]. Hence, transplant physicians should always consider a cost-benefit analysis if they want to continue an effective LT program[116,117]. Dialysis treatment, plasma exchange, blood derivatives, and direct-acting antivirals are very expensive[62,118,119]. Notably, attempts to perform blood transfusion and infusion of fraction products are ill-advised because they are very detrimental to the medical economy[62,116,117,119]. A shorter intensive care unit (ICU) stay has benefits for patients[120], although expensive and intensive care during the ICU stay is needed for post-transplant management. Longer hospital stay impairs quality of life and spoil social status after hospital discharge[121,122].
It is necessary to standardize CO against BV for precise evaluation of preload[1]. Considering that cirrhotic hyperdynamics are consolidated in patients with a large BV and high CO under a low TPR[3,6,8-10] and that the concept of preload is focused on the balance between CO and BV[35], we can now use the new concept of the CO/BV ratio by making the best use of available devices that can noninvasively measure BV[1,2,15]. The PDD guarantees noninvasive vigilance of the balance between CO and BV as an index for precise assessment of the systemic hemodynamic state in LT recipients with LC[1]. The CO/BV ratio expresses the CO per min corresponding to a fraction of the BV, which represents how the heart efficiently ejects the BV that should be circulated[1,2]. Interestingly, previous studies revealed no differences in the CO/BV among recipients with LC, recipients without LC, and healthy individuals[1,2]. This variable has potential as a reliable clinical marker after LT. Subtle instabilities that do not appear when comparing absolute values themselves are simply indicated by the balance between CO and BV[1,2]. It seems reasonable that tachycardia resulted in a lower CO in recipients with poor outcomes (Figure 4D) and that the decreased CO could not circulate the large BV in these recipients (Figure 5B).
In LDLT, the CIT is short and the hepatocytes are well preserved[41]. Therefore, division by the graft weight is a simple method that allows the kICG to reflect only the PVF, by taking advantage of the shorter CIT in LDLT[41]. Strategic values in ICG kinetics are used to set the optimal PVF during LDLT and to evaluate the optimal systemic hemodynamics after LT[1,2,32,41,42]. ICG kinetics reflects the functional hepatocyte volume and effective PVF[31,49-52]. Advanced selection criteria of a graft with an GRWR of ≥ 0.6 and establishment of a target PVP of ≤ 15 mmHg during LDLT are currently documented for successful LDLT[123-126]. This defiant set-up with lower GRWR has advantages for donor pool and safety, although these grafts may cause critical problems[40]. ICG kinetics is useful to set-up of adequate PVF during LDLT with lower GRWR. Conversely, in deceased-donor LT, although PVF is a major determinant of kICG in the normal liver[32,34,49,51,53], the kICG value may be affected by damaged hepatocytes due to the longer CIT. The decreased kICG may not indicate only an inadequate PVF in deceased-donor LT because ICG kinetics is dually factorial.
LT recipients with LC exhibit peculiar hemodynamics (i.e., systemic hyperdynamic syndrome and PH). Vascular alterations do not easily disappear despite restorations of PH and liver function in recipients with LC, and PVF impacts liver regeneration after LT[43]. Stability of characteristic systemic hyperdynamics is indispensable for adequate PVF and successful LT[1,2]. Even a subtle disorder of the systemic hyperdynamics dictates PVF[1,2]. ICG kinetics is useful to set an adequate PVF during LDLT and evaluate the optimal systemic hemodynamics after LT[1,2,32,41,42]. Perioperative management has a large influence on the postoperative course and outcome. Transplant physicians should fully understand the peculiarities of cirrhotic hemodynamics. We hope that this review will be informative for transplant physicians.
| 1. | Hori T, Yagi S, Iida T, Taniguchi K, Yamagiwa K, Yamamoto C, Hasegawa T, Yamakado K, Kato T, Saito K. Optimal systemic hemodynamic stability for successful clinical outcomes after adult living-donor liver transplantation: prospective observational study. J Gastroenterol Hepatol. 2008;23:e170-e178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Hori T, Yagi S, Iida T, Taniguchi K, Yamagiwa K, Yamamoto C, Hasegawa T, Yamakado K, Kato T, Saito K. Stability of cirrhotic systemic hemodynamics ensures sufficient splanchnic blood flow after living-donor liver transplantation in adult recipients with liver cirrhosis. World J Gastroenterol. 2007;13:5918-5925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Licata A, Mazzola A, Ingrassia D, Calvaruso V, Cammà C, Craxì A. Clinical implications of the hyperdynamic syndrome in cirrhosis. Eur J Intern Med. 2014;25:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Ho HL, Huang HC. Molecular mechanisms of circulatory dysfunction in cirrhotic portal hypertension. J Chin Med Assoc. 2015;78:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Stanley MM. Pathogenesis of ascites in cirrhosis. A unitary hypothesis. ASAIO Trans. 1989;35:161-163. [PubMed] |
| 6. | Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 403] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120-1126. [PubMed] |
| 8. | Henderson JM, Mackay GJ, Hooks M, Chezmar JL, Galloway JR, Dodson TF, Kutner MH. High cardiac output of advanced liver disease persists after orthotopic liver transplantation. Hepatology. 1992;15:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358-367. [PubMed] |
| 11. | Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, Soupison T, Sogni P, Degott C, Durand F. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458-465. [PubMed] |
| 12. | Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit. 1997;13:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Haruna M, Kumon K, Yahagi N, Watanabe Y, Ishida Y, Kobayashi N, Aoyagi T. Blood volume measurement at the bedside using ICG pulse spectrophotometry. Anesthesiology. 1998;89:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Hori T, Yamamoto C, Yagi S, Iida T, Taniguchi K, Hasegawa T, Yamakado K, Hori Y, Takeda K, Maruyama K. Assessment of cardiac output in liver transplantation recipients. Hepatobiliary Pancreat Dis Int. 2008;7:362-366. [PubMed] |
| 16. | Fujita Y, Yamamoto T, Fuse M, Kobayashi N, Takeda S, Aoyagi T. Pulse dye densitometry using indigo carmine is useful for cardiac output measurement, but not for circulating blood volume measurement. Eur J Anaesthesiol. 2004;21:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Ishigami Y, Masuzawa M, Miyoshi E, Kato M, Tamura K, Kanda M, Awazu K, Taniguchi K, Kurita M, Hayashi N. Clinical applications of ICG Finger Monitor in patients with liver disease. J Hepatol. 1993;19:232-240. [PubMed] |
| 18. | Erickson JR, Mccormick JB, Seed L. An improved method for the determination of blood volume using radioactive iodinated human serum albumen. Science. 1953;118:595-596. [PubMed] |
| 19. | Strumia MM, Colwell LS, Dugan A. The measure of erythropoiesis in anemias. I. The mixing time and the immediate post-transfusion dissappearance of T-1824 dye and of Cr-51-tagged erythrocytes in relation to blood volume determination. Blood. 1958;13:128-145. [PubMed] |
| 20. | Reba RC, Eckelman WC, Albert SN. Tc-99m labeled red blod cells: a new radiopharmaceutical for the determination of total blood volume and blood pool scanning. Med Ann Dist Columbia. 1973;42:1-3. [PubMed] |
| 21. | Bradley EC, Barr JW. Determination of blood volume using indocyanine green (cardio-green) dye. Life Sci. 1968;7:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Iijima T, Iwao Y, Sankawa H. Circulating blood volume measured by pulse dye-densitometry: comparison with (131)I-HSA analysis. Anesthesiology. 1998;89:1329-1335. [PubMed] |
| 23. | Imai T, Mitaka C, Nosaka T, Koike A, Ohki S, Isa Y, Kunimoto F. Accuracy and repeatability of blood volume measurement by pulse dye densitometry compared to the conventional method using 51Cr-labeled red blood cells. Intensive Care Med. 2000;26:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Paulsen AW, Klintmalm GB. Direct measurement of hepatic blood flow in native and transplanted organs, with accompanying systemic hemodynamics. Hepatology. 1992;16:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, Bernuau J, Belghiti J, Gayet B, Erlinger S. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175-178. [PubMed] |
| 26. | Henderson JM, Mackay GJ, Kutner MH, Noe B. Volumetric and functional liver blood flow are both increased in the human transplanted liver. J Hepatol. 1993;17:204-207. [PubMed] |
| 27. | Plevak DJ. Hyperdynamic circulatory state after liver transplantation. Transplant Proc. 1993;25:1839. [PubMed] |
| 28. | Textor SC, Wiesner R, Wilson DJ, Porayko M, Romero JC, Burnett JC, Gores G, Hay E, Dickson ER, Krom RA. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Chezmar JL, Redvanly RD, Nelson RC, Henderson JM. Persistence of portosystemic collaterals and splenomegaly on CT after orthotopic liver transplantation. AJR Am J Roentgenol. 1992;159:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Liang YY, Wang J, Shan H, Yan RH, Hu B, Jiang ZB, He BJ, Liu JJ, Ren LL, Shao S. [To evaluate the role of OLT on splenomegaly of portal hypertension by the radiological changes of splenic morphology and collaterals]. Zhonghua Yi Xue Za Zhi. 2012;92:3058-3061. [PubMed] |
| 31. | Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ, Starzl TE. Indocyanine green elimination test in orthotopic liver recipients. Hepatology. 1996;24:1165-1171. [PubMed] |
| 32. | Hori T, Iida T, Yagi S, Taniguchi K, Yamamoto C, Mizuno S, Yamagiwa K, Isaji S, Uemoto S. K(ICG) value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl. 2006;12:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Wong F, Liu P, Tobe S, Morali G, Blendis L. Central blood volume in cirrhosis: measurement with radionuclide angiography. Hepatology. 1994;19:312-321. [PubMed] |
| 34. | Hashimoto M, Watanabe G. Simultaneous measurement of effective hepatic blood flow and systemic circulation. Hepatogastroenterology. 2000;47:1669-1674. [PubMed] |
| 35. | Sakka SG, Reinhart K, Wegscheider K, Meier-Hellmann A. Comparison of cardiac output and circulatory blood volumes by transpulmonary thermo-dye dilution and transcutaneous indocyanine green measurement in critically ill patients. Chest. 2002;121:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Magder S. Understanding central venous pressure: not a preload index? Curr Opin Crit Care. 2015;21:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Hartleb M, Rudzki K, Karpel E, Becker A, Waluga M, Boldys H, Nowak A, Nowak S. Cardiovascular status after postural change in compensated cirrhosis: an argument for vasodilatory concept. Liver. 1997;17:1-6. [PubMed] |
| 38. | Yagi S, Iida T, Hori T, Taniguchi K, Yamamoto C, Yamagiwa K, Uemoto S. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation. 2006;81:373-378. [PubMed] |
| 39. | Yagi S, Iida T, Taniguchi K, Hori T, Hamada T, Fujii K, Mizuno S, Uemoto S. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Ogura Y, Hori T, El Moghazy WM, Yoshizawa A, Oike F, Mori A, Kaido T, Takada Y, Uemoto S. Portal pressure & lt; 15 mm Hg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl. 2010;16:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Hori T, Ogura Y, Ogawa K, Kaido T, Segawa H, Okajima H, Kogure T, Uemoto S. How transplant surgeons can overcome the inevitable insufficiency of allograft size during adult living-donor liver transplantation: strategy for donor safety with a smaller-size graft and excellent recipient results. Clin Transplant. 2012;26:E324-E334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Hori T, Ogura Y, Yagi S, Iida T, Taniguchi K, El Moghazy WM, Hedaya MS, Segawa H, Ogawa K, Kogure T. How do transplant surgeons accomplish optimal portal venous flow during living-donor liver transplantation? Noninvasive measurement of indocyanine green elimination rate. Surg Innov. 2014;21:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Eguchi S, Yanaga K, Sugiyama N, Okudaira S, Furui J, Kanematsu T. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547-551. [PubMed] |
| 44. | Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958;99:11-14. [PubMed] |
| 45. | Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531-554. [PubMed] |
| 46. | Kwon AH, Ha-Kawa SK, Uetsuji S, Inoue T, Matsui Y, Kamiyama Y. Preoperative determination of the surgical procedure for hepatectomy using technetium-99m-galactosyl human serum albumin (99mTc-GSA) liver scintigraphy. Hepatology. 1997;25:426-429. [PubMed] |
| 47. | de Graaf W, Bennink RJ, Veteläinen R, van Gulik TM. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J Nucl Med. 2010;51:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Kaibori M, Ha-Kawa SK, Maehara M, Ishizaki M, Matsui K, Sawada S, Kwon AH. Usefulness of Tc-99m-GSA scintigraphy for liver surgery. Ann Nucl Med. 2011;25:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Groszmann RJ. The measurement of liver blood flow using clearance techniques. Hepatology. 1983;3:1039-1040. [PubMed] |
| 50. | Jiao LR, El-Desoky AA, Seifalian AM, Habib N, Davidson BR. Effect of liver blood flow and function on hepatic indocyanine green clearance measured directly in a cirrhotic animal model. Br J Surg. 2000;87:568-574. [PubMed] |
| 51. | Niemann CU, Yost CS, Mandell S, Henthorn TK. Evaluation of the splanchnic circulation with indocyanine green pharmacokinetics in liver transplant patients. Liver Transpl. 2002;8:476-481. [PubMed] |
| 52. | Niemann CU, Roberts JP, Ascher NL, Yost CS. Intraoperative hemodynamics and liver function in adult-to-adult living liver donors. Liver Transpl. 2002;8:1126-1132. [PubMed] |
| 53. | Huet PM, Villeneuve JP. Determinants of drug disposition in patients with cirrhosis. Hepatology. 1983;3:913-918. [PubMed] |
| 54. | Ogawa E, Hori T, Doi H, Segawa H, Uemoto S. Living-donor liver transplantation for congenital biliary atresia with porto-pulmonary hypertension and moderate or severe pulmonary arterial hypertension: Kyoto University experience. Clin Transplant. 2014;28:1031-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | McCullough AJ, Mullen KD, Kalhan SC. Measurements of total body and extracellular water in cirrhotic patients with and without ascites. Hepatology. 1991;14:1102-1111. [PubMed] |
| 56. | Vitale GC, Neill GD, Fenwick MK, Stewart WW, Cuschieri A. Body composition in the cirrhotic patient with ascites: assessment of total exchangeable sodium and potassium with simultaneous serum electrolyte determination. Am Surg. 1985;51:675-681. [PubMed] |
| 57. | Nakayama M, Yamauchi M, Kanaya N, Namiki A. [Utility of bicarbonated Ringer’s solution as an intraoperative fluid during long-term laparotomy]. Masui. 2007;56:1334-1338. [PubMed] |
| 58. | Fukuta Y, Kumamoto T, Matsuda A, Kataoka M, Kokuba Y. [Effects of various Ringer’s solutions on acid-base balance in rats in hemorrhagic shock and with hepatic dysfunction]. Masui. 1998;47:22-28. [PubMed] |
| 59. | Satoh K, Ohtawa M, Okamura E, Satoh T, Matsuura A. Pharmacological study of BRS, a new bicarbonated Ringer’s solution, in partially hepatectomized rabbits. Eur J Anaesthesiol. 2005;22:624-629. [PubMed] |
| 60. | Bernardi M, Ricci CS, Santi L. Hyponatremia in Patients with Cirrhosis of the Liver. J Clin Med. 2014;4:85-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Liu H, Gaskari SA, Lee SS. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol. 2006;12:837-842. [PubMed] |
| 62. | Alessandria C, Elia C, Mezzabotta L, Risso A, Andrealli A, Spandre M, Morgando A, Marzano A, Rizzetto M. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis. 2011;43:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (4)] |
| 64. | Gracia-Sancho J, Maeso-Díaz R, Bosch J. Pathophysiology and a Rational Basis of Therapy. Dig Dis. 2015;33:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (14)] |
| 66. | Vincent JG. Use of autologous pericardium for ventricular aneurysm closure. Ann Thorac Surg. 1989;48:146-147. [PubMed] |
| 67. | Hayakawa M, Gando S, Kameue T, Morimoto Y, Kemmotsu O. Abdominal compartment syndrome and intrahepatic portal venous gas: a possible complication of endoscopy. Intensive Care Med. 2002;28:1680-1681. [PubMed] |
| 68. | Ahmed K, Atiq M, Richer E, Neff G, Kemmer N, Safdar K. Careful observation of hepatic portal venous gas following esophageal variceal band ligation. Endoscopy. 2008;40 Suppl 2:E103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Li G, Thabane L, Cook DJ, Lopes RD, Marshall JC, Guyatt G, Holbrook A, Akhtar-Danesh N, Fowler RA, Adhikari NK. Risk factors for and prediction of mortality in critically ill medical-surgical patients receiving heparin thromboprophylaxis. Ann Intensive Care. 2016;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Kloek JJ, Heger M, van der Gaag NA, Beuers U, van Gulik TM, Gouma DJ, Levi M. Effect of preoperative biliary drainage on coagulation and fibrinolysis in severe obstructive cholestasis. J Clin Gastroenterol. 2010;44:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Kogiso T, Tokushige K, Hashimoto E, Ikarashi Y, Kodama K, Taniai M, Torii N, Shiratori K. Safety and efficacy of long-term tolvaptan therapy for decompensated liver cirrhosis. Hepatol Res. 2016;46:E194-E200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Thomas MN, Sauter GH, Gerbes AL, Stangl M, Schiergens TS, Angele M, Werner J, Guba M. Automated low flow pump system for the treatment of refractory ascites: a single-center experience. Langenbecks Arch Surg. 2015;400:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Arslan H. Infections in liver transplant recipients. Exp Clin Transplant. 2014;12 Suppl 1:24-27. [PubMed] |
| 74. | Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211-6220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 75. | Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, McDiarmid SV, Anand R, Song C. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant. 2008;8:396-403. [PubMed] |
| 76. | van Delden C. Bacterial biliary tract infections in liver transplant recipients. Curr Opin Organ Transplant. 2014;19:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Coons SJ. Promoting the appropriate use of medications by older adults; the pharmacist’s role. J Ky Med Assoc. 1989;87:571-573. [PubMed] |
| 78. | Chen TA, Lo GH, Lai KH. Risk factors for spontaneous bacterial empyema in cirrhotic patients with hydrothorax. J Chin Med Assoc. 2003;66:579-586. [PubMed] |
| 79. | Takino T, Ogasawara T, Okuno T, Takahashi T. Disseminated cytomegalic inclusion disease in an adult with cirrhosis of liver and review of literatures. Gastroenterol Jpn. 1976;11:347-355. [PubMed] |
| 80. | Jeurissen S, Vogelaers D, Sermijn E, Van Dycke K, Geerts A, Van Vlierberghe H, Colle I. Invasive aspergillosis in patients with cirrhosis, a case report and review of the last 10 years. Acta Clin Belg. 2013;68:368-375. [PubMed] |
| 81. | Valand AG, Deshpande V, Pandya BS. Pneumocystis carinii pneumonia in immunocompromised host--an autopsy report of three cases. Indian J Pathol Microbiol. 2007;50:38-40. [PubMed] |
| 82. | Mueller AR, Platz KP, Kremer B. Early postoperative complications following liver transplantation. Best Pract Res Clin Gastroenterol. 2004;18:881-900. [PubMed] |
| 83. | Wiklund RA. Preoperative preparation of patients with advanced liver disease. Crit Care Med. 2004;32:S106-S115. [PubMed] |
| 84. | Paya CV, Hermans PE. Bacterial infections after liver transplantation. Eur J Clin Microbiol Infect Dis. 1989;8:499-504. [PubMed] |
| 85. | Mah A, Wright A. Infectious Considerations in the Pre-Transplant Evaluation of Cirrhotic Patients Awaiting Orthotopic Liver Transplantation. Curr Infect Dis Rep. 2016;18:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Carrion AF, Aye L, Martin P. Patient selection for liver transplantation. Expert Rev Gastroenterol Hepatol. 2013;7:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, Durazo F, Honda H, Gornbein J, Wu V. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014;259:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 88. | Morell B, Dufour JF. [Liver transplantation - when and for whom it should be performed]. Ther Umsch. 2011;68:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant. 2015;15:3024-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 90. | Balogh J, Gordon Burroughs S, Boktour M, Patel S, Saharia A, Ochoa RA, McFadden R, Victor DW, Ankoma-Sey V, Galati J. Efficacy and cost-effectiveness of voriconazole prophylaxis for prevention of invasive aspergillosis in high-risk liver transplant recipients. Liver Transpl. 2016;22:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, Wang T, Liang J, He S, Gao J. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Burnstock G, Vaughn B, Robson SC. Purinergic signalling in the liver in health and disease. Purinergic Signal. 2014;10:51-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Amaya MJ, Oliveira AG, Guimarães ES, Casteluber MC, Carvalho SM, Andrade LM, Pinto MC, Mennone A, Oliveira CA, Resende RR. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Raevens S, Geerts A, Van Steenkiste C, Verhelst X, Van Vlierberghe H, Colle I. Hepatopulmonary syndrome and portopulmonary hypertension: recent knowledge in pathogenesis and overview of clinical assessment. Liver Int. 2015;35:1646-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Aldenkortt F, Aldenkortt M, Caviezel L, Waeber JL, Weber A, Schiffer E. Portopulmonary hypertension and hepatopulmonary syndrome. World J Gastroenterol. 2014;20:8072-8081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Porres-Aguilar M, Mukherjee D. Cardiopulmonary hemodynamics for accurate diagnosis of portopulmonary hypertension: a redefinition to consider. Hepatology. 2015;61:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Pastor CM, Schiffer E. Therapy Insight: hepatopulmonary syndrome and orthotopic liver transplantation. Nat Clin Pract Gastroenterol Hepatol. 2007;4:614-621. [PubMed] |
| 98. | Ogawa E, Hori T, Doi H, Segawa H, Uemoto S. Living-donor liver transplantation for moderate or severe porto-pulmonary hypertension accompanied by pulmonary arterial hypertension: a single-centre experience over 2 decades in Japan. J Hepatobiliary Pancreat Sci. 2012;19:638-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M, Marotta P, Uemoto S, Stoffel MP. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174-182. [PubMed] |
| 100. | Kuo PC, Plotkin JS, Gaine S, Schroeder RA, Rustgi VK, Rubin LJ, Johnson LB. Portopulmonary hypertension and the liver transplant candidate. Transplantation. 1999;67:1087-1093. [PubMed] |
| 101. | Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450. [PubMed] |
| 102. | Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, Ramsay M, Davis GL. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258-1264. [PubMed] |
| 103. | Krowka MJ. Pulmonary hypertension: diagnostics and therapeutics. Mayo Clin Proc. 2000;75:625-630. [PubMed] |
| 104. | Møller S, Bendtsen F. Complications of cirrhosis. A 50 years flashback. Scand J Gastroenterol. 2015;50:763-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Grace JA, Angus PW. Hepatopulmonary syndrome: update on recent advances in pathophysiology, investigation, and treatment. J Gastroenterol Hepatol. 2013;28:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 107. | Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, Klein E, Wagner R, Long C, Thacker J. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 108. | Matthay MA. Severe sepsis--a new treatment with both anticoagulant and antiinflammatory properties. N Engl J Med. 2001;344:759-762. [PubMed] |
| 109. | Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267-1274. [PubMed] |
| 110. | Li YH, Kuo CH, Shi GY, Wu HL. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci. 2012;19:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 111. | Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304:H1585-H1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 112. | Hori T, Uemoto S, Takada Y, Oike F, Ogura Y, Ogawa K, Miyagawa-Hayashino A, Yurugi K, Nguyen JH, Hori Y. Does a positive lymphocyte cross-match contraindicate living-donor liver transplantation? Surgery. 2010;147:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Hori T, Kaido T, Oike F, Ogura Y, Ogawa K, Yonekawa Y, Hata K, Kawaguchi Y, Ueda M, Mori A. Thrombotic microangiopathy-like disorder after living-donor liver transplantation: a single-center experience in Japan. World J Gastroenterol. 2011;17:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | de Paiva Haddad LB, Decimoni TC, Turri JA, Leandro R, de Soárez PC. Economic evaluations in gastroenterology in Brazil: A systematic review. World J Gastrointest Pharmacol Ther. 2016;7:162-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 115. | Dan YY, Wong JB, Hamid SS, Han KH, Jia JD, Liu CJ, Piratvisuth T, Lok AS, Lim SG. Consensus cost-effectiveness model for treatment of chronic hepatitis B in Asia Pacific countries. Hepatol Int. 2014;8:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y). 2011;7:661-671. [PubMed] |
| 117. | Axelrod DA. Economic and financial outcomes in transplantation: whose dime is it anyway? Curr Opin Organ Transplant. 2013;18:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Cortesi PA, Mantovani LG, Ciaccio A, Rota M, Mazzarelli C, Cesana G, Strazzabosco M, Belli LS. Cost-Effectiveness of New Direct-Acting Antivirals to Prevent Post-Liver Transplant Recurrent Hepatitis. Am J Transplant. 2015;15:1817-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Katz PP, Showstack JA, Lake JR, Brown RS, Dudley RA, Colwell ME, Wiesner RH, Zetterman RK, Everhart J. Methods to estimate and analyze medical care resource use. An example from liver transplantation. Int J Technol Assess Health Care. 1999;15:366-379. [PubMed] |
| 120. | Mor E, Cohen J, Erez E, Grozovsky A, Shaharabani E, Bar-Nathan N, Yussim A, Micowiz R, Shapira Z, Zinger P. Short intensive care unit stay reduces septic complications and improves outcome after liver transplantation. Transplant Proc. 2001;33:2939-2940. [PubMed] |
| 121. | Head SJ, Osnabrugge RL, Howell NJ, Freemantle N, Bridgewater B, Pagano D, Kappetein AP. A systematic review of risk prediction in adult cardiac surgery: considerations for future model development. Eur J Cardiothorac Surg. 2013;43:e121-e129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 122. | Baztán JJ, Gálvez CP, Socorro A. Recovery of functional impairment after acute illness and mortality: one-year follow-up study. Gerontology. 2009;55:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Uemura T, Wada S, Kaido T, Mori A, Ogura Y, Yagi S, Fujimoto Y, Ogawa K, Hata K, Yoshizawa A. How far can we lower graft-to-recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery. 2016;159:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 124. | Hammad A, Kaido T, Ogawa K, Fujimoto Y, Tomiyama K, Mori A, Uemura T, Uemoto S. Perioperative changes in nutritional parameters and impact of graft size in patients undergoing adult living donor liver transplantation. Liver Transpl. 2014;20:1486-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 125. | Kaido T, Mori A, Ogura Y, Hata K, Yoshizawa A, Iida T, Yagi S, Uemoto S. Lower limit of the graft-to-recipient weight ratio can be safely reduced to 0.6% in adult-to-adult living donor liver transplantation in combination with portal pressure control. Transplant Proc. 2011;43:2391-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 126. | Kaido T, Ogawa K, Fujimoto Y, Ito T, Tomiyama K, Mori A, Ogura Y, Uemoto S. Section 7. A new therapeutic strategy on portal flow modulation that increases donor safety with good recipient outcomes. Transplantation. 2014;97 Suppl 8:S30-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bubnov RV, Giorgio A S- Editor: Qiu S L- Editor: A E- Editor: Li D