Published online Jul 8, 2016. doi: 10.4254/wjh.v8.i19.815
Peer-review started: January 27, 2016
First decision: March 23, 2016
Revised: May 22, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: July 8, 2016
Processing time: 162 Days and 9.3 Hours
AIM: To evaluate neutrophil gelatinase associated lipocalin (NGAL) in patients infected by hepatitis C virus (HCV) before and during treatment with directly acting antivirals (DAAs).
METHODS: NGAL was measured in a group of patients with chronic HCV infection ranked, at baseline, by age, gender, anti-hypertensive therapy, HCV viral load, liver fibrosis stage and, either at baseline or after 1 year, estimated glomerular filtration rate (eGFR). Then, NGAL and eGFR evolutions were monitored in a subgroup of patients who started antiviral therapy with DAAs. Differences of median NGAL levels were evaluated through Wilcoxon-Mann-Whitney test for non-parametric data. Differences in dichotomous variables were evaluated through χ2 test. At baseline, a univariate regression analysis was conducted to verify if NGAL values correlated with other quantitative variables [age, fibrosis four (FIB-4), AST to platelet ratio index (APRI), and eGFR].
RESULTS: Overall, 48 patients were enrolled, 8 of them starting HCV treatment. At baseline, statistically significant differences were found in median NGAL values only between patients with eGFR < 60 mL/min vs patients with eGFR ≥ 90 mL/min. Differences in NGAL were not significant among patients ranked by HCV viral load, FIB-4 score and APRI, when patients with NGAL > 118.11 ng/dL were compared with those of NGAL ≤ 118.11 ng/dL, not statistically significant differences were present for age, gender, chronic kidney disease classification and liver fibrosis (P > 0.05). Linear correlation was found between NGAL and both age (P = 0.0475) and eGFR (P = 0.0282) values. Not statistically significant predictions of NGAL at baseline were demonstrated for eGFR evolution 1 year later. Interestingly, in the 8 patients treated with DAAs, median NGAL significantly increased at week 12 compared to baseline (P = 0.0239).
CONCLUSION: Our results suggest that NGAL should be further evaluated as an adjunct marker of kidney function in these patients.
Core tip: For the first time, we evaluated the evolution of neutrophil gelatinase associated lipocalin (NGAL), a novel biomarker of renal impairment, in patients infected by hepatitis C virus before and during treatment with directly acting antivirals (DAAs). In our study, we documented a significant increase of NGAL during the first 12 wk of therapy with DAAs and a correlation of NGAL with both age and estimated glomerular filtration rate before starting treatment. In a context of paucity of information about safety of the new DAAs, we believe that these data are both informative and novel, provoking urgent investigations.
- Citation: Strazzulla A, Coppolino G, Di Fatta C, Giancotti F, D’Onofrio G, Postorino MC, Mazzitelli M, Mammone SV, Gentile I, Rivoli L, Palella E, Gravina T, Costa C, Pisani V, De Maria V, Barreca GS, Marascio N, Focà A, Fuiano G, Gulletta E, Torti C. Is neutrophil gelatinase associated lipocalin useful in hepatitis C virus infection? World J Hepatol 2016; 8(19): 815-824
- URL: https://www.wjgnet.com/1948-5182/full/v8/i19/815.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i19.815
Estimated prevalence of renal insufficiency is increased by 40% in patients infected by hepatitis C virus (HCV) compared to the HCV negative population[1]. Renal disease, ranging from mild to end-stage renal disease (ESRD), often complicates prognosis and treatment of HCV infection with haemodialysis being required in some cases. Moreover, in the context of liver disease, kidney function is one of the key predictors of death and serum creatinine, is a component of both King’s college criteria and model for end-stage liver disease scoring systems that are used for prognostic stratification in patients with acute and chronic liver failure[2].
Virus related kidney diseases mainly show up as a glomerular impairment, predominantly a membrano-proliferative glomerulonephritis with type 2 crioglobulinaemia and sub-endothelial or intra luminal deposits of IgG, IgM and complement components[3]. Moreover, HCV core proteins were isolated in both glomerular and tubular tissues, suggesting the presence of a parallel tubular-interstitial damage[4].
Treatment of HCV infection in patients with renal impairment has always been challenging due to the side effects of the past treatment used as standard, an association of pegylated-interferon (PEG-IFN) and ribavirin (RBV), which was more difficult to manage in patients with renal impairment than in patients with normal renal function. In 2014, with the advent of first generation directly acting antivirals (DAAs), efficacy of anti HCV treatment was substantially improved. However, the first generation DAAs, boceprevir and telaprevir (TVR), were not recommended in patients with severe renal impairment or ESRD because these patients were excluded from registrational trials[5]. Moreover, in real-life patients without renal impairment, an increase of serum creatinine was observed during treatment with TVR, even if it was not associated with more severe renal impairment[6]. In March 2015, second generation DAAs were available in Italy for the treatment of HCV. Even if second generation DAAs are better tolerated, there is a paucity of information about the possible impact of these drugs on renal function[7]. Therefore, the evidence of kidney impairment may make clinicians less comfortable to begin an antiviral treatment.
Estimated glomerular filtration rate (eGFR) can be calculated by creatinine values, however in circumstances such as toxic drug dosage, kidney disease improving global outcome (KDIGO) guidelines suggest to add at least another biomarker. In fact, GFR estimating equation alone is biased in many situations, such as acute kidney disease (AKI), high GFR, non-GFR determinants of serum creatinine and interferences with creatinine assays[8]. Additionally, creatinine-based measures are even more limited in cirrhotic patients due to a decrease in hepatic synthesis of creatinine, reduced skeletal muscle mass and increased tubular secretion[9].
Among novel kidney biomarkers, one of the most promising is neutrophil gelatinase associated lipocalin (NGAL) either in urine and serum (or plasma)[10]. This is a small glycoprotein in three different forms: A monomer (25 kDa), a homodimer (45 kDa) and a heterodimer (135 kDa). It is secreted by many human cells, such as epithelial cells (liver, kidney, lungs) and blood cells (neutrophils, monocytes and macrophages), filtered in the glomerulus and reabsorbed by the proximal tubules[11,12]. It is removed by hemodyalisis[13]. In general, NGAL urinary levels increase after a tubular injury and reveal a kidney damage earlier than the increased levels of creatinine[14]. On the other hand, plasmatic or serum NGAL are more extensively adopted in AKI contexts, because their measurement is less limited by availability of samples when patients are anuric[15]. Remarkably, an increase of plasmatic NGAL is considered to be an early predictor of AKI in various critical settings such as cardiac surgery, septic shock, contrast induced nephropathy, renal and liver transplantation[16-22]. However, NGAL is increased also in case of epithelial damage or inflammation outside the kidney[10].
In cirrhotic patients, NGAL is a marker of AKI and urinary NGAL that can help distinguish among different causes of renal impairment[10]. Recently, a significant difference in plasmatic NGAL levels amongst HCV positive patients with cirrhosis and eGFR < 60 mL/min vs ≥ 60 mL/min has been demonstrated[23]. Also, recent data showed that NGAL is a good marker of renal damage due to drug toxicity. For example, urinary NGAL is a good predictor of tacrolimus induced AKI in liver transplanted patients and nonsteroidal anti-inflammatory drug (NSAID) associated AKI in cirrhotic patients[24,25]. To our best knowledge, no published data on NGAL during HCV treatment with DAAs are available so far.
The objectives of this study were to explore: (1) whether there is a difference in plasmatic NGAL between HCV positive patients and HCV negative people; (2) whether there is a difference in plasmatic NGAL among HCV positive patients ranked by age, gender, viral load, eGFR and liver fibrosis stage; (3) whether NGAL levels at baseline correlate with modification of eGFR after 1 year; and (4) the evolution of renal function in patients treated with DAA including regimens.
A prospective study was conducted. Patients with chronic hepatitis C who attended the Outpatient Service of the Infectious Diseases Unit and the Hepatology Unit of the “Mater Domini” Teaching Hospital in Catanzaro (Italy) from February 1, 2014 to April 30, 2014 were included in this study. Exclusion criteria included: Leukocytosis (leukocyte count higher than 12000 cells/μL), variceal bleeding, primary kidney diseases (glomerular nephropathy), KDIGO classification of chronic kidney disease (CKD) ≥ G4 (eGFR < 30 mL/min), ongoing HCV therapy (with or without interferon or DAA). Approval from local ethical committee was obtained. All enrolled patients signed an informed consent.
All patients underwent physical examination and history taking at baseline. The following blood tests were collected: AST, ALT, total and fractioned bilirubin, albumin, γGT, alkaline phosphatase, prothrombin time, total blood cell count (including neutrophil and platelet count) and urea. Serum creatinine levels were measured at baseline and after 1 year. For patients who started anti-viral therapy, serum creatinine levels and GFR were studied at week 4 and week 12 after baseline.
Glomerular filtration rate was estimated through Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI, 2009) since CKD-EPI is less biased and more accurate for eGFR ≥ 60 mL/min than MDRD (Modification of Diet in Renal Disease) and Cockcroft-Gault formulas[7]. The following formula was used: 141 × min (SCr/k, 1)α× max (SCr/k, 1)-1.209× 0.993Age× (1.018 if female or 1.159 if black), where SCr is serum creatinine (in mg/dL), k is 0.7 for females and 0.9 for males, a is 0.329 for females and 0.411 for males, min is the minimum of SCr/k or 1, and max is the maximum of SCr/k or 1.
Liver fibrosis was estimated at baseline by either fibrosis four (FIB-4) score or AST to platelet ratio index (APRI) which are the most used formulas for estimating stage of liver disease. FIB-4 has a negative predictive value of 94.7% to exclude severe fibrosis with a sensitivity of 74.3% when < 1.45 and a positive predictive value to confirm the existence of a significant fibrosis (F3-F4) of 82.1% with a specificity of 98.2% when ≥ 3.25. The following formula was used: Age × AST/(platelets ×√ALT) where AST and ALT were measured as IU/L, platelets were measured as number × 106/μL and age was measured in years[26]. An APRI value ≤ 0.5 rules out significant fibrosis and cirrhosis while values ≥ 1.5 indicates significant fibrosis[27]. More specifically, when APRI score is greater than 1.0 it has a sensitivity of 76% and a specificity of 72% for predicting cirrhosis[28]. The following formula was used: [(AST/AST upper normal limit)/platelets] × 100 where AST was measured as IU/L, platelets were measured as number × 106/μL and AST upper normal limit was fixed at 35 IU/L.
Peripheral venous blood samples were taken from each patient at baseline and then processed for NGAL measurement. Plasmatic NGAL was measured in all patients at baseline. For patients who started anti-viral therapy, NGAL was measured at different endpoints (baseline, week 4 and 12). NGAL assay was performed using human NGAL Rapid Elisa Kit (BioPorto Diagnostic). A 96-well microtiter plate coated with purified anti-human NGAL monoclonal antibody was used. In each well 50 μL of each sample, diluted 1:100 with sample diluting buffer, undiluted calibrators and controls were added; then 50 μL of horseradish peroxidase -conjugated NGAL antibodies were added. During this first step of incubation at room temperature for 30 min, NGAL bind either the specific antibody adsorbed to microwells or the second antibody; an aspiration-washing step (3 times with 300 μL of wash solution) was performed to remove excess and unbounded reagents; 100 μL of tetramethyl-benzidine solution were added to each well and a second step of incubation performed at room temperature for 15 min; at end of incubation 100 μL of stop solution was added and the color developed in each well was measured at 450 nm. The values of samples were determined on the basis of a standard curve. The methodology was performed using a Triturus automatic analyzer. The commercial kit, that is validated by Pedersen et al[29] is used in our study. The reference range (41.19-118.11 ng/mL) had been previously validated in our laboratory on a group of healthy volunteers.
Differences of NGAL values were evaluated in patients ranked by age (< 65 years vs ≥ 65 years), gender (female vs male), anti-hypertensive therapy (present vs absent), HCV viral load (< 1000000 copies/mL vs ≥ 1000000 copies/mL), FIB-4 score (≤ 1.45 vs 1.45 to 3.25 vs ≥ 3.25), APRI (≤ 0.5 vs 0.5 to 1.5 vs ≥ 1.5), and eGFR (≥ 90 mL/min vs ≥ 60 mL/min to < 90 mL/min vs < 60 mL/min). Patients were ranked by eGFR worsening vs stable/improved with respect to baseline values after 1 year. Any reduction of eGFR was considered as worsening. Viral load cut-off was set at 1000000 HCV RNA copies/mL because this value is commonly considered to be high[30]. Cut-off for age was set at 65 years because that is the threshold discriminating adulthood and elderly life in many western countries[31]. We analyzed eGFR as a continuous measure and did not consider any specific-cut off for change of eGFR.
Differences in dichotomous variables (gender, FIB-4 ≥ 3.25, APRI ≥ 1.5, KDIGO CKD classification ≥ G3a, KDIGO CKD classification ≥ G2, age ≥ 65 year) were evaluated in patients ranked at baseline by NGAL values (> 118.11 ng/dL vs ≤ 118.11 ng/dL).
Evolution of NGAL at different time points (baseline, week 4 and week 12) was evaluated separately in patients who started antiviral treatment with DAA containing regimens during the first twelve weeks of therapy.
Differences of distribution NGAL levels from baseline to one year in the overall population and differences of NGAL and eGFR distribution in patients prescribed HCV therapy (evaluated at baseline, week 4 and week 12) were evaluated through Wilcoxon-Mann-Whitney test for non parametric data. Also, at baseline, a Spearman correlation analysis was conducted to verify if NGAL values correlated with other quantitative variables (age, FIB-4, APRI, and eGFR). Differences in dichotomous variables (age, gender, CKD classification and liver fibrosis) in patients with NGAL > 118.11 ng/dL or NGAL ≤ 118.11 ng/dL were evaluated through χ2 test. Statistical analysis was performed using Graphpad Prism 6.01 (GraphPad Software, La Jolla, CA, United States). Nominal statistical significance was set at P < 0.05.
Forty-eight HCV RNA positive patients were enrolled with median NGAL of 68.5 ng/dL (range: 136-27). Main characteristics of the population are summarized in Table 1. Seventeen (35%) patients were < 65 years old, 25 (52%) patients were females, 10 (21%) patients suffered from blood hypertension. Median NGAL was: 63 ng/dL (111-27) for patients < 65 years old and 77 ng/dL (136-28) for patients ≥ 65 years old (P = 0.1353); 70 ng/dL (132-27) for males and 63 ng/dL (111-27) for females (P = 0.7822); 72.5 ng/dL (102-28) for patients with hypertension and 66.5 ng/dL (136-27) for patients with normal blood pressure (P = 0.7756).
| Patients’ characteristics | Overall population (n = 48) | Patients treated with DAAs (n = 8) |
| Qualitative variables | ||
| Gender | ||
| Female | 25 (52) | 1 (12) |
| Male | 23 (48) | 7 (88) |
| HCV RNA genotype | ||
| 1a | 3 (6) | 0 (0) |
| 1b | 29 (61) | 8 (100) |
| 2a/2c | 4 (8) | 0 (0) |
| 3 | 1 (2) | 0 (0) |
| 4 | 2 (4) | 0 (0) |
| Not available | 9 (19) | 0 (0) |
| Quantitative variables, median (range) | ||
| Age (yr) | 67.0 (36.0-84.0) | 63.5 (51.0-69.0) |
| eGFR (mL/min) | 90.0 (30.0-111.7) | 93.0 (77.0-109.0) |
| FIB-4 | 3.0 (0.5-18.3) | 3.7 (1.5-9.0) |
| AST (IU/L) | 44.0 (17.0-180.0) | 70.0 (28.0-103.0) |
| ALT (IU/L) | 45.0 (12.0-268.0) | 53.5 (35.0-171.0) |
| Albumin (mg/dL) | 4.2 (3.0-4.8) | 4.3 (3.3-4.6) |
| Total bilirubin (mg/dL) | 0.8 (0.3-2.2) | 0.6 (0.3-1.0) |
| PLT (n/μL) | 141000 (13500-312000) | 132000 (71000-229000) |
| WBC (cells/μL) | 5560 (2220-11500) | 6245 (2790-9200) |
| HCV-RNA (copies/mL) | 776500 (90100-19500000) | 2330000 (793000-3250000) |
Differences in NGAL were not significant among patients ranked by HCV viral load, FIB-4 score and APRI. Quantitative HCV RNA was available in 36 patients, 19 (53%) of them having HCV RNA < 1000000 copies/mL (i.e., low HCV RNA group). Median NGAL at baseline was 70 ng/dL (range: 132-27) vs 63 ng/dL (121-28), respectively. For FIB-4, 8 (17%) patients with FIB-4 ≤ 1.45 had median NGAL of 60.5 ng/dL (111-27), 16 (33%) with FIB-4 from 1.45 to 3.25 had median NGAL of 82 ng/dL (136-36) and 24 (50%) with FIB-4 ≥ 3.25 had median NGAL of 74.5 ng/dL (132-28). Regarding APRI, 10 (21%) patients were ≤ 0.5, 22 (46%) were between 0.5 and 1.5, and 16 (33%) were ≥ 1.5. Median NGAL values were 60.5 ng/dL (range: 136-27), 74.5 ng/dL (124-36) ng/dL and 80 ng/dL (132-28) in the three APRI groups, respectively.
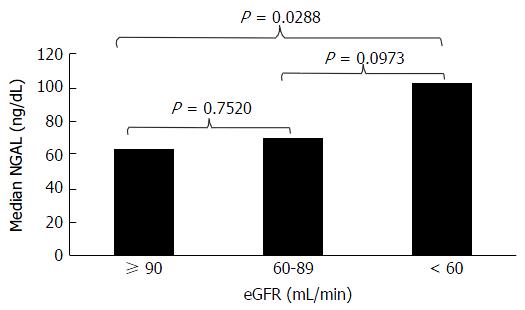
According to eGFR ranking, 25 (52%) patients had eGFR ≥ 90 mL/min, 19 (40%) had Egfr ≥ 60 but less than 90 mL/min and 4 (8%) had eGFR < 60 mL/min. Median NGAL was 63 ng/dL (range: 136-36), 70 ng/dL (124-27) and 102.5 ng/dL (132-88) in the three groups, respectively. Statistical analysis showed significant differences in median NGAL values only in patients with eGFR < 60 mL/min vs patients with eGFR ≥ 90 mL/min (Figure 1; P = 0.0288).
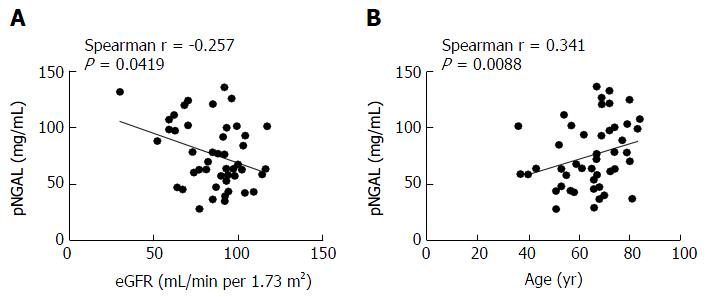
Spearman correlation analysis showed statistically significant positive correlation between NGAL and age (Spearman r = 0.341; 95%CI: 0.05450-0.5759; two-tailed P = 0.0088) and a negative correlation between NGAL and eGFR values (Spearman r = -0.257; 95%CI: -0.5164 to 0.04421; two-tailed P = 0.0419) (Figure 2). Not statistically significant results were obtained when NGAL was correlated FIB-4 score (P = 0.413) or APRI (P = 0.7430).
In 6 (12.5%) patients, NGAL exceeded the upper limit of the reference interval (NGAL > 118.11 ng/mL). When patients with NGAL > 118.11 ng/dL were compared with patients with NGAL ≤ 118.11 ng/dL, no statistically significant differences were present for age, gender, CKD classification and liver fibrosis (P > 0.05). In fact: 6/6 (100%) patients with NGAL > 118.11 ng/mL vs 25/42 (60%) patients with NGAL ≤ 118.11 ng/mL were ≥ 65 years old; 3/6 (50%) patients with NGAL > 118.11 ng/mL vs 20/42 (48%) patients with NGAL ≤ 118.11 ng/mL were males; 1/6 (17%) patients with NGAL > 118.11 ng/mL vs 7/42 (17%) patients with NGAL ≤ 118.11 ng/mL had a KDIGO CKD ≥ G3a; 4/6 (67%) patients with NGAL > 118.11 ng/mL vs 26/42 (62%) patients with NGAL ≤ 118.11 ng/mL had a KDIGO CKD classification ≥ G2; 4/6 (67%) patients with NGAL > 118.11 ng/mL vs 19/42 (45%) patients with NGAL ≤ 118.11 ng/mL had a FIB-4 ≥ 3.25; 3/6 (50%) patients with NGAL > 118.11 ng/mL vs 13/42 (31%) patients with NGAL ≤ 118.11 ng/mL had an APRI ≥ 1.5.
Serum creatinine and eGFR were collected after 1 year for 40 patients, while the remaining 8 were analyzed separately because they started HCV treatment in the meanwhile. No statistically significant differences were demonstrated in median NGAL values at baseline between patients with worsening eGFR vs patients with stable/improved eGFR at year 1. Indeed, median NGAL at baseline was 71.5 ng/dL (range: 136-36) in patients with worsening eGFR after 1 year, while it was 73.5 ng/dL (132-27) in patients with stable/improved eGFR after 1 year (P = 0.4898).
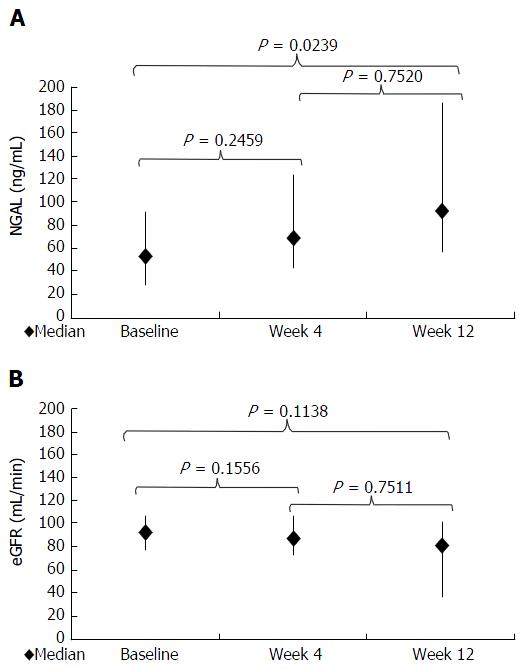
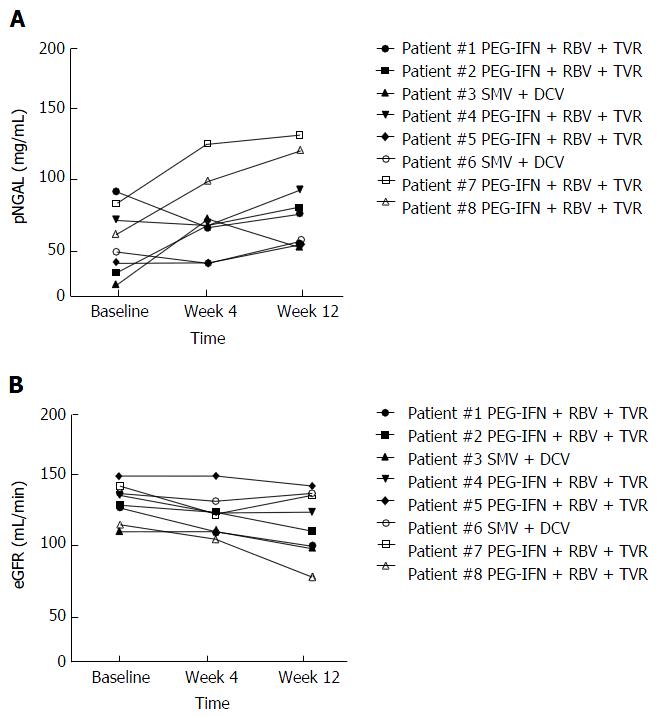
A separate prospective analysis was conducted in the 8 patients who started with antiviral therapy. Baseline characteristics of these patients are summarized in Table 1. Two patients started simeprevir (SMV) + daclatasvir (DCV) for 24 wk, while 6 patients started PEG-IFN + RBV + TVR for 12 wk followed by PEG-IFN + RBV for other 24 wk (36 wk overall). Three patients were relapser to previous treatments with PEG-IFN + RBV, while 5 were partial responders. At week 4, 2 patients had negative but detectable HCV RNA (≤ 15 UI/mL) while 6 had undetectable HCV RNA. At week 12, HCV RNA was undetectable in all patients. Median NGAL was 53 ng/dL (range: 92-28) at baseline, 69 ng/dL (125-43) at week 4, and 93 ng/dL (186-56) at week 12 (P = 0.0239 compared with baseline value) (Figures 3A and 4A). Median eGFR was 93 mg/dL (109-77) at baseline, 86.5 mg/dL (108-72) at week 4, and 82.5 mg/dL (103-72) at week 12; no statistically significant differences were found for eGFR along the time points of the study (Figures 3B and 4B).
This is the first study which investigated NGAL, both before and during the first twelve weeks of therapy with DAA including regimens. Until now, other investigators evaluated NGAL but only in HCV positive patients with cirrhosis and before treatment with DAA containing regimens[23].
We found that, in patients not exposed to DAA, eGFR was below normality around 50%, while NGAL was in the range of normality in most individuals. Moreover, among the six patients with increased NGAL, two cases had eGFR normal. These results suggest that a discordance between the two methods exists when interpretation is “categorical”. Despite these findings, in the overall population plasmatic NGAL was statistically correlated with eGFR. Particularly, NGAL was significantly higher in patients with eGFR < 60 mL/min than those with eGFR ≥ 90 mL/min. It is difficult to explain apparent discrepancies with the current literature data because Alhaddad et al[23] previously demonstrated that HCV positive cirrhotic patients with eGFR < 60 mL/min had a significantly lower plasmatic NGAL than HCV positive cirrhotic patients with eGFR ≥ 60 mL/min. Inclusion of both cirrhotic and non-cirrhotic patients in our study could be an explanation. In conclusion, we think that further studies should evaluate the rate of concordance between the two methods in diverse stages of liver disease. Also, it has to be seen whether these markers provide useful insights on the glomerular (especially detected by e-GFR) or tubular (especially detected by NGAL) damage in these conditions.
We were not able to find any correlations explaining the variability in NGAL values apart from age and incontrast with Bolignano et al[11] who, however, selected patients with non-terminal CKD of various etiologies. The correlation found in our study (increasing NGAL with increase in age) was consistent with the inverse and expected correlation between eGFR and age. Importantly, NGAL was not correlated with stage of liver disease or burden of HCV RNA, suggesting that further mechanisms are implicated. Similarly, in their study, Gungor et al[22] did not find differences between cirrhotic patients and healthy controls but, at the same time, they found that a high plasmatic NGAL was associated with a higher risk of mortality in cirrhotic patients. Our data support the fact that NGAL is not influenced by HCV RNA, suggesting that glomerular and tubular damages have different pathogenic and clinical significances.
We did not find any signals pointing to a predictive role of NGAL for eGFR evolution during the follow-up. Indeed, contrary to this hypothesis, patients with a reduction of eGFR at 1 year had lower NGAL than patients with an improvement of eGFR at 1 year. Bolignano et al[11] found that patients with non-advanced CKD who progressed in their kidney disease during the follow-up period (median 18.5 mo, range 1-20) had a significantly higher urinary and serum NGAL levels at baseline than patients with non-advanced CKD who did not progress. Our patients did not suffer from significant CKD and renal function did not decrease significantly during the follow-up. These considerations, together with the small sample size and limited length of follow-up may have limited our possibility to demonstrate a significant prediction in this study.
We measured NGAL for the first time during therapy with DAA. Importantly, NGAL increased significantly from baseline to week 12, whereas eGFR did not change significantly, suggesting that tubular damage induced by drugs is not accompanied by glomerular impairment. An alternative hypothesis could be that NGAL increase is induced by a pro-inflammatory status, so this increase in NGAL would be a consequence, at least in part, of inflammation due to activity of antiviral drugs and consequent reduction of HCV RNA. Until now, data about relationship of HCV RNA and inflammation have been controversial. Indeed, some studies pointed to a pro-inflammatory role of HCV RNA, highlighting the enhanced activity of tumor necrosis factor-alpha and the consequent increase of some pro-inflammatory interleukins (ILs), such as IL-10, in presence of detectable HCV RNA[32,33]. Instead, other studies showed that a higher HCV RNA load was correlated with a lower value of C-reactive protein (CRP) and that levels of other ILs, such as IL-6, were correlated with liver fibrosis rather than with HCV RNA load[34]. Lastly, successful IFN-free regimens resulted in improved functional responses by natural killer cells (such as degranulation and TRAIL expression) to in vitro stimulation with IFNα[35]. Further studies are necessary to understand evolutions of immunologic or inflammatory markers (including NGAL) after DAA treatment and the underlying mechanisms.
According to this hypothesis that increase of NGAL during HCV treatment is due to drug toxicity, it is likely that our results were scarcely affected by the use of PEG-IFN and RBV. In fact, these two molecules did not demonstrate significant nephrotoxicity, while the reverse may be true because renal impairment reduces excretion of both PEG-IFN and RBV, leading to an increased risk of side effects, such as hemolytic anaemia due to intra-erythrocyte accumulation of RBV[5,36].
All regimens analyzed in our study included a NS3/4A protease inhibitor (i.e., TVR or SMV). Currently, far more data are available about renal safety of TVR than renal safety of SMV or other DAAs. The available data showed that TVR causes a significant but reversible reduction of eGFR which may be associated with an increase of side effect related to the drug[37,38]. Our study showed that, aside reduction of eGFR, there was also an increase of NGAL which could be a consequence of tubular damage. Currently, there is a paucity of data about renal safety of interferon free regimens including DAAs different from TVR. Particularly, it is unknown if second or third generation DAAs have a direct nephrotoxic effect. In our study, both patients treated with SMV + DCV experienced an increase of NGAL with a concomitant eGFR decrease during the first 12 week of treatment. Our results need to be confirmed by further and larger studies, however.
Several limitations may affect the study conclusions: (1) sample size was small and length of follow-up was limited; (2) correlations of NGAL with other factors which could have impaired renal function, such as diabetes, cryoglobulinaemia, treatment with non NSAIDs or diuretics were not evaluated; and (3) we did not measure urinary NGAL or inflammatory molecules such as CRP and ILs which could confirm or disprove the hypotheses above. Notwithstanding these limitations, we feel that our results are important and should provoke further investigations. In particular, we hypothesize that NGAL reveals kidney damage earlier than eGFR during DAA containing regimens.
However, many questions still remain to be answered. Indeed, it has to be established whether a clinical cut-off of NGAL may guide clinical decisions (e.g., dosage modification or stopping of the offending drug). Also, it has to be evaluated whether NGAL could predict AKI during HCV treatment, especially in most-at-risk patients such as those with advanced cirrhosis and a high risk of renal complications (e.g., hepato-renal syndrome). Lastly, cost-effectiveness studies need to be conducted to verify the hypothesis that NGAL should be routinely used to monitor kidney function during HCV treatment instead of (or in addition to) creatinine.
Neutrophil gelatinase associated lipocalin (NGAL) is a novel biomarker of renal impairment but also a marker of inflammation. For the first time, the authors evaluated the evolution of NGAL in patients infected by hepatitis C virus (HCV) before and during treatment with directly acting antivirals (DAAs).
Kidney toxicity of new DAAs has not been established until now. The authors’ results suggest that NGAL could provide information complementary to estimated glomerular filtration rate in monitoring the kidney toxicity of DAAs.
The literature suggests that NGAL is a good marker of acute kidney injury but no data are available about NGAL in HCV positive patients. This article adds to literature data about evolution of NGAL before and during HCV treatment with DAA containing regimens.
This study serves as an additional evidence supporting the fact that NGAL can reveal the presence of a kidney impairment before creatinine.
NGAL is a small glycoprotein secreted by multiple human cells, such as epithelial cells (liver, kidney, lungs) and blood cells (neutrophils, monocytes and macrophages), filtered in the glomerulus and reabsorbed by the proximal tubules. An increase of plasmatic NGAL is considered to be an early predictor of acute kidney disease in various critical settings.
This study examined the measures of a novel biomarker for kidney function, NGAL, before and after HCV treatment with direct acting antivirals among 48 patients. The authors have performed a good study, the manuscript is interesting.
| 1. | Ozer Etik D, Ocal S, Boyacioglu AS. Hepatitis C infection in hemodialysis patients: A review. World J Hepatol. 2015;7:885-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46 Suppl 5:S165-S173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Sansonno D, Lauletta G, Montrone M, Grandaliano G, Schena FP, Dammacco F. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol. 2005;140:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Bunchorntavakul C, Maneerattanaporn M, Chavalitdhamrong D. Management of patients with hepatitis C infection and renal disease. World J Hepatol. 2015;7:213-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Matsui K, Kamijo-Ikemori A, Sugaya T, Ikeda H, Okuse C, Shibagaki Y, Yasuda T, Kimura K. Does elevation of serum creatinine in patients with chronic hepatitis C under therapy of telaprevir mean renal impairment? Nephrology (Carlton). 2015;20:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Fabrizi F, Messa P. Therapy of hepatitis C by direct-acting anti-virals: the end of HCV in dialysis population? Expert Rev Clin Pharmacol. 2015;8:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. |
| 9. | Davenport A, Cholongitas E, Xirouchakis E, Burroughs AK. Pitfalls in assessing renal function in patients with cirrhosis--potential inequity for access to treatment of hepatorenal failure and liver transplantation. Nephrol Dial Transplant. 2011;26:2735-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 10. | Firu SG, Streba CT, Firu D, Tache DE, Rogoveanu I. Neutrophil Gelatinase Associated Lipocalin (NGAL) - a biomarker of renal dysfunction in patients with liver cirrhosis: Do we have enough proof? J Med Life. 2015;8 Spec Issue:15-20. [PubMed] |
| 11. | Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Bolignano D, Coppolino G, Aloisi C, Romeo A, Nicocia G, Buemi M. Effect of a single intravenous immunoglobulin infusion on neutrophil gelatinase-associated lipocalin levels in proteinuric patients with normal renal function. J Investig Med. 2008;56:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Bolignano D, Coppolino G, Romeo A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology (Carlton). 2010;15:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, Formentini I, Kientsch-Engel R, Eckardt KU, Willam C. Comparison of Plasma and Urine Biomarker Performance in Acute Kidney Injury. PLoS One. 2015;10:e0145042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1694] [Cited by in RCA: 1713] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 17. | Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176-c181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297-1303. [PubMed] |
| 20. | Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 347] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Portal AJ, McPhail MJ, Bruce M, Coltart I, Slack A, Sherwood R, Heaton ND, Shawcross D, Wendon JA, Heneghan MA. Neutrophil gelatinase--associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl. 2010;16:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Gungor G, Ataseven H, Demir A, Solak Y, Gaipov A, Biyik M, Ozturk B, Polat I, Kiyici A, Cakir OO. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int. 2014;34:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Alhaddad OM, Alsebaey A, Amer MO, El-Said HH, Salman TA. Neutrophil Gelatinase-Associated Lipocalin: A New Marker of Renal Function in C-Related End Stage Liver Disease. Gastroenterol Res Pract. 2015;2015:815484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Tsuchimoto A, Shinke H, Uesugi M, Kikuchi M, Hashimoto E, Sato T, Ogura Y, Hata K, Fujimoto Y, Kaido T. Urinary neutrophil gelatinase-associated lipocalin: a useful biomarker for tacrolimus-induced acute kidney injury in liver transplant patients. PLoS One. 2014;9:e110527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Elia C, Graupera I, Barreto R, Solà E, Moreira R, Huelin P, Ariza X, Solé C, Pose E, Baiges A. Severe acute kidney injury associated with non-steroidal anti-inflammatory drugs in cirrhosis: A case-control study. J Hepatol. 2015;63:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1665] [Article Influence: 87.6] [Reference Citation Analysis (1)] |
| 27. | Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350-357. [PubMed] |
| 28. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 815] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 29. | Pedersen KR, Ravn HB, Hjortdal VE, Nørregaard R, Povlsen JV. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Invest. 2010;70:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Saracco G, Ciancio A, Ghisetti V, Rocca G, Cariti G, Andreoni M, Tabone M, Roffi L, Calleri G, Ballaré M. Treatment with interferon-alpha2b of naive non-cirrhotic patients with chronic hepatitis C according to viraemia and genotype. Results of a randomized multicentre study. The North West Italian Hepatological Group. Eur J Gastroenterol Hepatol. 2001;13:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Delanaye P, Glassock RJ, Pottel H, Rule AD. An Age-Calibrated Definition of Chronic Kidney Disease: Rationale and Benefits. Clin Biochem Rev. 2016;37:17-26. [PubMed] |
| 32. | Vanis N, Mehmedović A, Mesihović R. Use of serum levels of proinflammatory cytokine IL-1α in chronic hepatitis C. Coll Antropol. 2015;39:75-79. [PubMed] |
| 33. | Pircher J, Czermak T, Merkle M, Mannell H, Krötz F, Ribeiro A, Vielhauer V, Nadjiri J, Gaitzsch E, Niemeyer M. Hepatitis C virus induced endothelial inflammatory response depends on the functional expression of TNFα receptor subtype 2. PLoS One. 2014;9:e113351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Shah S, Ma Y, Scherzer R, Huhn G, French AL, Plankey M, Peters MG, Grunfeld C, Tien PC. Association of HIV, hepatitis C virus and liver fibrosis severity with interleukin-6 and C-reactive protein levels. AIDS. 2015;29:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, Ghany M, Rehrmann B. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Jain AB, Eghtesad B, Venkataramanan R, Fontes PA, Kashyap R, Dvorchik I, Shakil AO, Kingery L, Fung JJ. Ribavirin dose modification based on renal function is necessary to reduce hemolysis in liver transplant patients with hepatitis C virus infection. Liver Transpl. 2002;8:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Kozielewicz D, Dybowska D, Karwowska K, Wietlicka-Piszcz M. Renal impairment in patients with chronic hepatitis C treated with first generation protease inhibitors. Expert Opin Drug Saf. 2015;14:1815-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Loustaud-Ratti V, Carrier P, Vong C, Essig M. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology. 2014;59:2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Grant JL, Leidner AJ S- Editor: Gong ZM L- Editor: A E- Editor: Li D