Peer-review started: July 31, 2015
First decision: September 29, 2015
Revised: October 19, 2015
Accepted: December 18, 2015
Article in press: December 21, 2015
Published online: January 8, 2016
Processing time: 161 Days and 21.4 Hours
AIM: To identify patients with or without liver steatosis and its severity in treatment-naïve patients affected by hepatitis C virus (HCV) infection.
METHODS: We included 56 HCV infected patients, and assessed the amount of liver fat by histomorphometry, and its relationships with fat and lean mass at different parts of the body (by densitometry), hormones [insulin, homeostatic model assessment (HOMA)], adipokines (resistin, adiponectin, leptin), and cytokines (tumor necrosis factor α, interleukin-6).
RESULTS: Although the intensity of liver steatosis is related to trunk fat mass and HOMA, 33% of patients showed no liver steatosis, and this finding was not related to body mass index or genotype. Besides trunk fat mass, no other factor was related to the presence or not of liver steatosis, or to the intensity of it, by multivariate analysis. Lean mass was not related to liver steatosis. Adiponectin levels were lower among patients. No differences were observed in leptin and resistin.
CONCLUSION: Steatosis in HCV infection is common (67.2%), and closely related to trunk fat, and insulin resistance, but not with leg fat mass or adipokines.
Core tip: Pathogenesis of liver steatosis in hepatitis C virus (HCV) infection is complex and is not fully understood. For unknown reasons some patients, despite having a high body mass index (BMI), do not develop liver steatosis, whereas others with normal BMI develop intense liver fat deposition. We analyse if body fat and lean mass composition, insulin resistance and adipokine profile may help to identify patients with or without liver steatosis and its severity in treatment-naïve HCV patients. Multivariate analysis showed that only trunk fat mass and insulin resistance were independently related to liver steatosis assessed on histomorphometrical grounds and its severity.
- Citation: González-Reimers E, López-Prieto J, Quintero-Platt G, Pelazas-González R, Alemán-Valls MR, Pérez-Hernández O, de-la-Vega-Prieto MJ, Gómez-Rodríguez MA, Martín-González C, Santolaria-Fernández F. Adipokines, cytokines and body fat stores in hepatitis C virus liver steatosis. World J Hepatol 2016; 8(1): 74-82
- URL: https://www.wjgnet.com/1948-5182/full/v8/i1/74.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i1.74
Non-alcoholic steatohepatitis is observed in several clinical conditions, especially diabetes and obesity. In steatohepatitis hepatocytes become laden with fat droplets that elicit an inflammatory response which may evolve to liver cirrhosis and hepatocarcinoma. In diabetes and obesity insulin deficiency and/or resistance lead to increased mobilization of fatty acids from adipose tissue to liver. In chronic hepatitis C virus (HCV) infection, steatosis and steatohepatitis are also observed and the pathogenesis is based on complex mechanisms: although HCV by itself especially genotype 3 may lead to liver steatosis, obesity and concomitant alcohol abuse are the main factors involved[1]. However, many HCV infected patients do not drink alcohol at all, but they may develop liver steatosis. Cytokine activation and increased lipid peroxidation may contribute both to liver steatosis and to the progression of simple liver steatosis to steatohepatitis[2].
The main source of liver fat accumulation is body fat stores[3]. In this scenario, fat tissue is not only the source of fatty acids, but also produces several proinflammatory cytokines which are of paramount importance in the progression of liver disease. However, adipose tissue is heterogeneous. For instance, trunk fat is associated with increased insulin resistance and an increased vascular risk[4], whereas leg fat exerts opposite effects[5], probably due to secretion of a different cytokine profile.
The association of liver steatosis with distribution of fat stores at different parts of the body in chronic HCV infection is not well known. This is an important issue, since the heterogeneous nature of fat tissue may lead to different adipokine secretion[6]. In fact, notable controversy exists regarding serum levels of different adipokines, such as adiponectin[7-9] or leptin[10,11] and their relationship with histological changes in chronic HCV infection. In a previous report which analysed a series of patients (different from those included in this study) we found that an increased waist circumference (> 102 cm for men and > 88 cm among women) was related to increased liver fat, but we also found that 38.8% of non-obese patients also showed intense fatty infiltration[12], a result in accordance with other researchers, who have reported fatty liver among lean individuals[13]. Conversely, some HCV infected patients do not show liver steatosis, regardless of their body mass index. The mechanisms that underlie the lack of association in some cases between liver fat and body fat stores are unclear.
On the other hand, in a recent Indian study in a cohort of patients with steatohepatitis, 13% were lean patients[14], and sarcopenia has been described as an independent risk factor for steatohepatitis[15]. In addition it has been shown that interleukin-6 (IL-6) a protean cytokine also produced by muscle[16] strongly modulates liver fat accumulation[17]. Therefore, given these observations, it is important to also analyse the relationship between lean mass and liver steatosis.
Based on these facts, in the present study we analyse the association of the degree of liver steatosis with fat and lean mass stores at different parts of the body, insulin resistance, and serum adipokine levels, in treatment-naïve patients affected by HCV infection. Since we have assessed liver steatosis on histological grounds, we also look for differences in cytokine and adipokine profile, fat and lean mass distribution among HCV patients who did not show liver steatosis and those who did, in order to shed light on the reasons why some HCV patients do not develop liver steatosis.
We included 56 patients with (19 women) HCV infection, aged 41.54 ± 9.57 years. Diagnostic criteria for HCV infection were the following: (1) presence of anti-HCV and/or HCV RNA by reverse transcriptase polymerase chain reaction; and (2) Histology consistent with HCV. Most patients (43) were infected by HCV type 1 genotype, 5 by type 3 genotype, and 8 by type 4. All patients were recruited before treatment for virus C hepatitis was administered, and none of them were active drinkers. Liver function was still preserved: Liver function tests were normal, and none of them showed ascites or encephalopathy.
After informed consent was obtained, 51 patients underwent assessment of fat and lean mass at different parts of the body, such as right and left arm, trunk, right and left leg, and total body, with a LUNAR PRODIGY ADVANCE device, General Electric, Piscataway, NJ, United States. We further calculated (using the protocol established by other authors[18]) the trunk fat/(right leg + left leg fat) index, as well as the indices fat mass/lean mass at each of the body compartments mentioned before. Body mass index [BMI, as weight (kg)/height (m)2] was also recorded.
Blood samples were taken at 8:00 am in fasting conditions. Routine laboratory evaluation was performed and these analyses included, among others, prothrombin activity, serum albumin and bilirubin. Samples were immediately frozen at -20 °C. We determined the following parameters-IL-6, by chemiluminescent assay interassay variation coefficient ranging 5.3%-7.5%, recovery = 85%-104%, diagnostic products corporation (DPC), Los Angeles, CA, United States; tumour necrosis factor α (TNF-α) by immunometric chemiluminescent assay (intra-assay variation coefficient ranging 4%-6.5%, interassay variation coefficient ranging 2.6%-3.6%, recovery 92%-112%, DPC, Los Angeles, CA, United States). We also determined serum insulin, by immunoanalysis (Chemiflex); interobserver variation coefficient = 1.9%-5.2%; intraobserver variation coefficient = 1.7%-4.2%; sensitivity = 1 μU/mL; recovery = 91.1%-101.6%; (Architect system, Abbott, Wiesbaden Germany), serum resistin, by ELISA (sensitivity = 0.033 ng/mL; intra-assay variation coefficient = 2.8%-3.4%; interassay variation coefficient ranging 5.1%-6.9%, recovery = 85.2%-99.2%, Biovendor, Heidelberg, Germany), serum leptin, by ELISA (sensitivity = 0.2 ng/mL; intra-assay variation coefficient = 4.2%-7.6%; interassay variation coefficient ranging 4.4%-6.7%, recovery = 85.7%-98.0%, Biovendor, Heidelberg, Germany); serum adiponectin by ELISA (sensitivity = 26 ng/mL; intra-assay variation coefficient = 3.9%-5.9%; interassay variation coefficient ranging 6.3%-7%, recovery = 92.4%-102.9%, Biovendor, Heidelberg, Germany); insulin resistance was estimated by the homeostatic model assessment (HOMA).
Cytokine values were compared with those of a control group composed of 19 healthy hospital workers, seven of them women, aged 40.45 ± 3.57 years. As shown in Table 1, not all the variables were determined in all patients and controls.
| Patients | Controls | ||||
| n | X ± SD, median (IQ range) | n | X ± SD, median (IQ range) | ||
| Insulin (μU/mL) | 44 | 12.48 ± 15.65, 7.89 (4.63-14.32) | 10 | 8.34 ± 4.34, 7.15 (5.08-10.63) | Z = 0.43; NS |
| Resistin (ng/mL) | 44 | 4.97 ± 1.76, 4.90 (3.98-5.60) | 10 | 4.28 ± 1.42, 4.97 (3.32-5.29) | Z = 0.88; NS |
| Adiponectin (ng/mL) | 44 | 11.99 ± 8.30, 9.54 (6.04-17.16) | 16 | 24.92 ± 21.84, 19.05 (13.53-21.58) | Z = 3.18; P = 0.001 |
| Leptin (ng/mL) | 44 | 12.25 ± 15.83, 4.23 (1.15-17.78) | 10 | 18.41 ± 16.03, 12.89 (4.65-34.42) | Z = 1.78; NS |
| Tumor necrosis factor-α (pg/mL) | 56 | 10.65 ± 4.14, 10.18 (7.15-13.08) | 19 | 6.05 ± 1.90, 5.20 (4.40-8.00) | Z = 4.56; P < 0.001 |
| Interleukin-6 (pg/mL) | 53 | 4.28 ± 4.75, 2.0 (2.0-4.29) | 19 | 5.90 ± 1.64, 5.0 (5.0-6.60) | Z = 2.97; P = 0.003 |
| Body mass index (kg/m2) | 56 | 24.19 ± 3.44 | 19 | 25.20 ± 3.42 | t = 1.02; NS |
| Total fat mass (g) | 50 | 19929 ± 11944 | 19 | 21443 ± 6393 | t = 0.54; NS |
| Total lean mass (g) | 50 | 48284 ± 8848 | 19 | 50131 ± 15796 | t = 0.64; NS |
All these data were recorded the day at which the patients underwent a liver biopsy before receiving active treatment against HCV infection.
The degree of liver steatosis was determined using software based on histomorphometry (LEICAQWin, version 3.0, Wetzlar, Germany). The specimens were stained with haematoxylin-eosin and Masson trichromic and were viewed at 40 ×. This protocol has been previously described[12]. The proportion of fatty area to total area in specimens was recorded. The Knodell index and the total amount of fibrous tissue determined by histomorphometry (using Masson trichromic stain) were also measured.
The study protocol was approved by the local ethical committee of our Hospital. All patients included gave their informed consent prior to their inclusion in the study, and the study conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
We tested for normal distribution using the Kolmogorov-Smirnov test. In order to compare means between two groups or between three or more groups, we used Student’s t test and ANOVA, respectively. If the variables did not show a normal distribution, Mann-Whitney’s U and Kruskall-Wallis tests were used to compare means. Correlations between quantitative variables were established using Spearman’s r and Pearson’s r. The χ2 test was used to compare qualitative variables. We performed stepwise multiple regression analysis to establish which parameters liver steatosis depends on. All statistical analyses were performed using SPSS software (Chicago, Ill., United States).
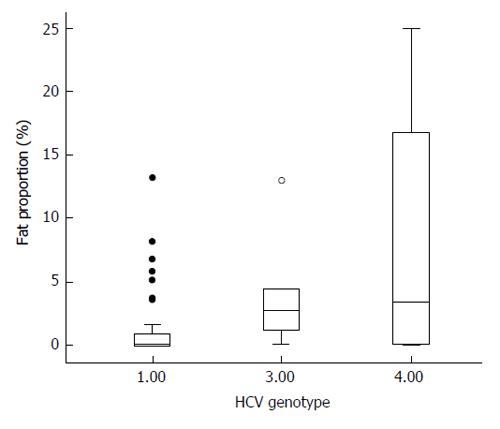
Liver steatosis was observed in 42 patients out of 56; in the remaining 14 patients, no steatosis at all was observed, and in 4 more, only very few small isolated fat droplets could be observed (fat amount < 0.05%). Median value of liver fat area was 0.20%, but 14 patients showed more than 5% of fat in their biopsies. Patients with genotype 1 showed significantly less steatosis than those with genotype 3 or 4 (Z = 2.17; P = 0.03; Figure 1). Indeed, as shown in Figure 1, patients with genotype 3 or 4 showed higher values of liver fat (fat proportion = 6.66% ± 8.42%) when compared with those with genotype 1 (fat proportion= 1.40% ± 2.78%). Only 1 (out of 5) genotype 3 patient showed no steatosis at all, compared with 13 (out of 51) affected by non-3 genotype infection, but this association was not statistically significant (χ2 = 0.07). No differences in liver fat were observed when HIV-coinfected patients were compared with non-co-infected ones (Z = 0.40; P = 0.694). Seven patients were diabetics, but although they showed a trend to more intense liver steatosis (6.66% ± 9.68%) than non-diabetics (2.05% ± 3.97%), this difference was not significant (Z = 1.31; P > 0.20). None of the diabetics showed no fat in their livers, but association between diabetes/no diabetes and presence or not of liver steatosis was not significant (P = 0.17 by exact Fisher’s test). No association was observed between viral load and proportion of liver fat.
Median proportion of fibrosis was 5.75% (interquartile range = 3.53%-8.88%). Twenty-one patients showed a Knodell index higher than 5, whereas 35 showed a Knodell index below 6.
Patients with marked steatosis (over the median) showed increased BMI and greater fat mass, especially at the trunk (t = 3.01, P = 0.004), as shown in Table 2. In addition to the finding of a significantly higher BMI among those with liver steatosis over the median (Table 2), we also found that patients with BMI over 25 kg/m2 had significantly more liver fat (Z = 2.25; P = 0.031). Only 22 patients were overweight, and only 3 of them were obese (BMI > 30 kg/m2). Three patients who were overweight showed no fat at all in their liver biopsies, vs 11 out of 33 with normal weight. This association was not statistically significant. Significant relationships were observed between fat parameters and liver steatosis, especially with trunk fat (r = 0.42; P = 0.002), right arm fat (r = 0.31; P = 0.029), left arm fat (r = 0.30; P = 0.033), and total fat (r = 0.34; P = 0.016). The significant relationship between liver steatosis and trunk fat was observed both among women (r = 0.50; P = 0.04) and men (r = 0.41; P = 0.016). In a similar way, BMI was related to liver steatosis both among women (r = 0.53; P =0.02) and men (r = 0.36; P = 0.032). However, while liver steatosis was related to arm and leg fat mass among both women and men, the correlations were not statistically significant, possibly due to the relatively low number of cases. No relationship was observed between parameters related to lean mass and liver steatosis, but when the indices fat mass/lean mass were compared with liver steatosis, the results were similar to those obtained with fat parameters (r = 0.39; P = 0.006 for the trunk, r = 0.34; P = 0.017 for the left arm, r = 0.32; P = 0.026 for the right arm, and r = 0.34; P = 0.016 for total fat). Remarkably, no association was observed when leg fat mass was compared with liver steatosis. The ratio trunk fat/legs fat was not significantly different among patients with liver steatosis below or above the median. A significant correlation was observed between liver steatosis and BMI (r = 0.41; P = 0.002).
| Steatosis over the median | Steatosis below the median | ||||
| n | X ± SD | n | X ± SD | ||
| Left arm fat mass (g) | 27 | 1345.04 ± 871.98 | 24 | 783.30 ± 577.62 | t = 2.68; P = 0.01 |
| Right arm fat mass (g) | 27 | 1396.97 ± 1084.44 | 24 | 852.37 ± 827.60 | t = 2.00; P = 0.05 |
| Trunk fat mass (g) | 27 | 12673.68 ± 6077.05 | 24 | 7939.57 ± 5027.19 | t = 3.01; P = 0.004 |
| Left leg fat mass (g) | 27 | 3919.72 ± 2533.87 | 24 | 2683.13 ± 2018.77 | t = 1.91; NS |
| Right leg fat mass (g) | 27 | 3948.29 ± 2626.75 | 24 | 2805.25 ± 1905.64 | t = 1.76; NS |
| Total body fat mass (g) | 27 | 23981.22 ± 12381.84 | 24 | 15733.66 ± 9812.41 | t = 2.61; P = 0.012 |
| Left arm lean mass (g) | 26 | 2769.64 ± 783.77 | 24 | 2899.09 ± 941.35 | t = 0.53; NS |
| Right arm lean mass (g) | 26 | 2749.02 ± 819.68 | 24 | 3064.65 ± 1649.64 | t = 0.87; NS |
| Trunk lean mass (g) | 26 | 24458.49 ± 4791.16 | 24 | 23122.30 ± 4155.06 | t = 1.05; NS |
| Left leg lean mass (g) | 26 | 7469.77 ± 1684.70 | 24 | 7011.75 ± 1945.39 | t = 0.89; NS |
| Right leg lean mass (g) | 26 | 7651.20 ± 1664.56 | 24 | 7404.65 ± 1444.64 | t = 0.56; NS |
| Total lean mass (g) | 26 | 48592.11 ± 9723.42 | 24 | 47309.62 ± 8352.70 | t = 0.50; NS |
| Body mass index (kg/m2) | 28 | 25.55 ± 2.51 | 27 | 22.79 ± 3.76 | t = 2.61; P = 0.012 |
| Trunk fat/legs fat | 27 | 1.85 ± 0.87 | 24 | 1.56 ± 0.42 | t = 1.49; NS |
Trunk fat was the only variable that was selected (P = 0.011) when a logistic regression analysis was done searching for the factors related to liver fat over or below the median values.
Similar results relative to fat mass at different parts of the body were observed when patients without liver steatosis (including those 4 with minimal steatosis) were compared with the remaining patients, although differences were less significant (t = 2.73, P = 0.009 for trunk fat, t = 2.34, P = 0.023 for left arm fat, t = 2.31; P = 0.025 for right arm fat) than when patients were classified according to the median values of liver fat. BMI was also significantly lower among those without liver steatosis (t = 2.43; P = 0.023). No differences at all were observed regarding lean mass variables. As with steatosis below or above the median, the only selected variable was trunk fat (P = 0.015) when a logistic regression was performed to discern which variables were independently related to the presence or absence of liver fat infiltration.
No associations were observed between the proportion of fibrosis in liver biopsy and any of the nutritional variables, but Knodell index was related both to fat mass variables (total fat, r = 0.37; P = 0.007; trunk fat, r = 0.32; P = 0.024); left arm and right arm fat, r = 0.47 and r = 0.45; respectively, P < 0.001; left leg and right leg, (r = 0.31 and r = 0.28, respectively, P < 0.05 in both cases), as well as to some lean mass variables (trunk lean mass, r = 0.35; P = 0.012; left leg lean mass, r = 0.30, P = 0.034).
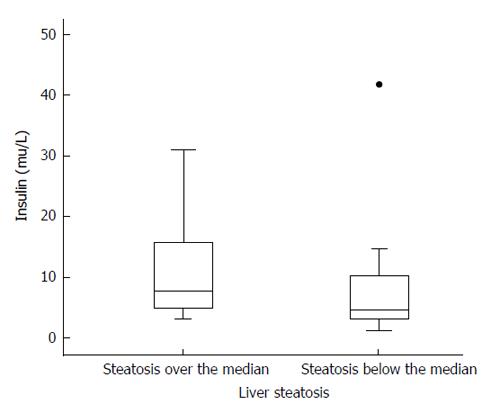
No differences were observed in any of the adipokines, HOMA, insulin, TNF-α, or IL-6 among patients with or without liver steatosis. Only HOMA, out of these parameters, was significantly higher among patients with liver fat over the median compared with those with liver fat below the median (Z = 2.15; P = 0.032); a similar trend that was not statistically significant (P = 0.059) was observed with insulin (Tables 3 and 4, Figure 2).
| Steatosis over the median | Steatosis below the median | ||||
| n | X ± SD, median (IQ range) | n | X ± SD, median (IQ range) | ||
| Insulin (μU/mL) | 24 | 15.30 ± 19.42, 11.20 (6.59-16.46) | 20 | 9.09 ± 8.72, 7.30 (3.87-11.62) | Z = 1.89; P = 0.059 |
| HOMA | 24 | 1645.68 ± 2828.28, 1068 (644-1524) | 20 | 825.01 ± 801.41, 645.5 (327.8-1082.0) | Z = 2.15; P = 0.03 |
| Resistin (ng/mL) | 24 | 4.66 ± 0.96, 4.87 (4.19-5.37) | 20 | 5.34 ± 2.38, 5.03 (3.88-6.12) | Z = 1.03; NS |
| Adiponectin (ng/mL) | 24 | 11.77 ± 6.92, 11.18 (5.45-17.62) | 20 | 12.26 ± 9.90, 8.38 (6.04-16.65) | Z = 0.21; NS |
| Leptin (ng/mL) | 24 | 10.85 ± 12.45, 6.23 (1.35-17.20) | 20 | 13.92 ± 19.35, 2.72 (0.79-32.89) | Z = 0.79; NS |
| Tumor necrosis factor-α (pg/mL) | 28 | 11.31 ± 4.90, 11.20 (6.84-14.18) | 28 | 10.00 ± 3.17, 9.56 (7.19-12.75) | Z = 1.00; NS |
| Interleukin (pg/mL) | 25 | 5.06 ± 5.17, 2.0 (2.0-5.94) | 28 | 3.59 ± 4.31, 2.0 (2.0-2.5) | Z = 1.14; NS |
| Cholesterol (mg/dL) | 28 | 167 ± 36.82 | 28 | 174.2 ± 45.56 | t = 0.65; NS |
| LDL cholesterol (mg/dL) | 27 | 95.04 ± 34.17 | 28 | 103.86 ± 36.48 | t = 1.01; NS |
| HDL cholesterol (mg/dL) | 28 | 46.71 ± 14.87 | 28 | 42.86 ± 13.82 | t = 0.92; NS |
| Triglycerides (mg/dL) | 28 | 136.25 ± 114.27 | 28 | 145.96 ± 93.04 | t = 0.35; NS |
| Leptin | Adipo-nectin | Insulin | HOMA | TNF-α | IL-6 | Resistin | |
| Trunk fat | ρ = 0.61, P < 0.001 | ρ = 0.56, P < 0.001 | ρ = 0.55, P < 0.001 | ||||
| Left leg fat | ρ = 0.70, P < 0.001 | ρ = 0.44, P = 0.005 | ρ = 0.44, P = 0.005 | ||||
| Right leg fat | ρ = 0.62, P < 0.001 | ρ = 0.42, P = 0.006 | ρ = 0.42, P = 0.006 | ||||
| Right arm fat | ρ = 0.40, P = 0.011 | ρ = 0.58 P < 0.001 | ρ = 0.57 P < 0.001 | ||||
| Left arm fat | ρ = 0.51, P < 0.001 | ρ = 0.62, P < 0.001 | ρ = 0.63, P < 0.001 | ||||
| Total fat | ρ = 0.64, P < 0.001 | ρ = 0.54, P < 0.001 | ρ = 0.53, P < 0.001 | ||||
| Total lean | ρ = -0.35, P = 0.032 | ρ = -0.31, P = 0.029 | |||||
| Left arm lean | ρ = -0.37, P = 0.02 | ρ = -0.33, P = 0.021 | |||||
| Right arm lean | ρ = -0.37, P = 0.02 | ρ = -0.29, P = 0.04 | |||||
| Left leg lean | |||||||
| Trunk lean | ρ = -0.34, P = 0.021 | ρ = -0.33, P = 0.021 | ρ = -0.34, P = 0.018 | ||||
| Right leg lean | ρ = -0.29, P = 0.039 | ||||||
| Total fat/total lean | ρ = 0.65, P < 0.001 | ρ = -0.49, P = 0.001 | ρ = 0.49, P = 0.001 | ||||
| Trunk fat/trunk lean | ρ = 0.63, P < 0.001 | ρ = -0.31, P = 0.036 | ρ = 0.52, P < 0.001 | ρ = 0.31, P = 0.036 | |||
| Right arm fat/right arm lean | ρ = 0.60, P < 0.001 | ρ = 0.55, P < 0.001 | ρ = 0.53, P < 0.001 | ||||
| Left arm fat/left arm lean | ρ = 0.69, P < 0.001 | ρ = 0.58, P = 0.001 | ρ = 0.57, P < 0.001 | ||||
| Right leg fat/right leg lean | ρ = 0.66, P < 0.001 | ρ = 0.38, P = 0.016 | ρ = 0.39, P = 0.014 | ||||
| Left leg fat/left leg lean | ρ = 0.69, P < 0.001 | ρ = 0.58, P < 0.001 | ρ = 0.57, P < 0.001 | ||||
| High density lipoprotein cholesterol | ρ = 0.56, P < 0.001 | ρ = -0.41, P = 0.012 | |||||
| Low density lipoprotein cholesterol | ρ = 0.31, P = 0.046 |
Significant relationships were observed between liver steatosis (proportion of fat) and HOMA index (r = 0.30; P = 0.046). Serum insulin (r = 0.44; P = 0.003) and HOMA (r = 0.36; P = 0.017) were directly related to Knodell index, whereas no associations were observed between any of the adipokines and cytokines and the amount of fibrosis in the liver biopsies. Selecting only those patients with liver steatosis, a significant correlation was observed between IL-6 and amount of liver fat (r = 0.49; P = 0.003).
After introducing in a multiple regression analysis the fat variables which showed a significant relationship with liver steatosis in the univariate analysis, only trunk fat (beta = 0.37; P = 0.026) was independently related to the amount of liver fat. In a similar way, trunk fat was the only selected variable when a logistic regression analysis was done searching for the factors related to liver fat over or below the median values (Table 5).
| B | E.T. | Wald | Gl | Sig. | Exp (B) | ||
| Step 1 | TrunK fat | 0.000 | 0.000 | 6.157 | 1 | 0.013 | 1.000 |
| Constant | 1.530 | 0.751 | 4.147 | 1 | 0.042 | 4.618 |
Leptin, insulin and HOMA were strongly and directly related to fat parameters, as shown in Table 4 (r > 0.40 in all the cases; P < 0.006), but not to lean mass. On the contrary, adiponectin and TNF-α were inversely related to most of the lean mass parameters. Adiponectin was also inversely related to the trunk fat mass/leg fat mass index (r = -0.33; P = 0.037).
The fat/ lean indices were also strongly related to leptin, insulin and HOMA, and also, to IL-6, in this last case only with the trunk fat/trunk lean mass index. No associations were observed between serum resistin and nutritional parameters (Table 4).
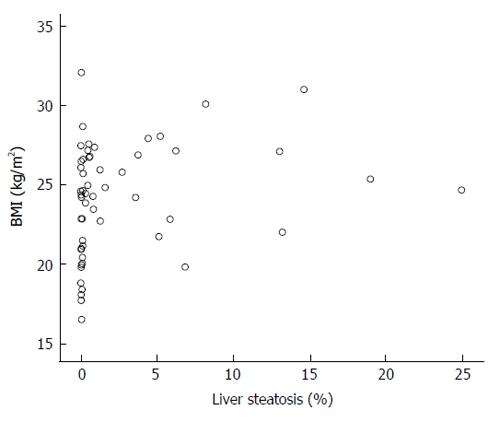
We have found that liver steatosis is frequent among patients with HCV infection (67.86%), even surpassing the prevalence data (about 50%) reported by other authors[19]. This high proportion of patients with steatosis was observed despite a BMI that was not different - even slightly lower- than that of a control population. However, as expected, liver steatosis showed a significant relationship with BMI, but it is noteworthy that some cases showed only minimal steatosis despite the fact that the patient was overweight. Some other cases showed considerable liver fat accumulation despite low BMI values (Figure 3), suggesting that factors other than BMI are involved in liver fat accumulation. This result is similar to that obtained by our group six years ago, in a different cohort of patients, in whom adiposity was assessed by waist circumference, triceps skinfold measurement, and BMI[12].
We have also shown that liver steatosis in HCV-infected patients is associated with trunk fat. This has been also reported by other authors[20,21], since, as mentioned above, it is generally accepted that trunk fat is associated with a more “noxious” adipokine secretion profile that is able to cause insulin resistance and a proinflammatory state. The opposite happens with peripheral fat. In this sense, we failed to find any relationship between liver steatosis and leg fat mass, as shown in Table 2. Therefore, in sharp contrast with trunk fat, which was clearly related to liver steatosis, liver fat accumulation seems to be independent of leg fat mass.
Regarding adipokines, adiponectin levels were significantly lower among patients than among controls, despite a similar BMI. Adiponectin was inversely related to lean mass, but not to fat mass or liver steatosis. However, it is important to highlight the inverse relationship between the trunk fat/leg fat ratio and adiponectin, fully in accordance with the observation of an inverse relationship between visceral fat and adiponectin levels in other settings[22]. Although there is little doubt about the protective role of adiponectin in steatohepatitis (it has been described that adiponectin antagonizes the effects of TNF-α[23]), in the present study, there seems to be no association between adiponectin levels and liver steatosis, despite the fact that their serum levels are lower in HCV patients in comparison to controls. This is not a universal finding. The studies on the levels of adiponectin in HCV-related steatohepatitis had been controversial[7-9,24-28]. It is also remarkable that we found, in accordance with the protective effect of adiponectin on vascular risk, a significant correlation between adiponectin and high density lipoprotein cholesterol (ρ = 0.56; P < 0.001), as other authors also did[29].
We also failed to find differences in resistin and leptin between patients and controls, or when these adipokines were compared among patients with intense or less intense steatosis. Leptin, a fat derived cytokine, may promote fibrogenesis through up-regulation of TGF-β[30], but also protects the liver from fat accumulation, by lowering the expression of SREBP-1[31]. These nearly opposite effects may explain, perhaps, disparate findings in relation to leptin levels in chronic HCV infection[32]. Indeed, there is also controversy regarding the levels of leptin in HCV-related steatohepatitis[10,11,33-35].
Hyperinsulinaemia decreases synthesis of apoB-100, thus preventing very low density lipoproteins formation and leading to liver steatosis. Moreover, transcription of lipoprotein lipase is decreased by TNF-α, leading to hypertriglyceridaemia[36]. Most of the results observed in this study sustain this hypothesis: We did find hyperinsulinemia and increased HOMA index in patients with more intense steatosis. This result is fully in accordance with the current knowledge, since insulin resistance leads to an ongoing lipolysis that overwhelms the liver capacity to metabolize them.
Genotype 3 infected patients usually show a more intense degree of steatosis, and it has been shown that it exerts a direct cytopathic effect on liver cell leading to steatosis[37]. Concordant with this, patients infected with genotype 3 showed a more intense liver steatosis than those genotype non-3 infected ones, but no significant differences were observed in nutritional anthropometric parameters among them. Also, although the number of patients infected with genotype 3 HCV was low, in one case no fat at all was observed in the liver, and this proportion was similar in HCV genotype non-3 patients. In fact, we have failed to find any difference in adipokine and/or cytokine profile between patients without fat and with fat in the liver. The only independent variable related to the intensity of liver steatosis or to the presence of liver steatosis was trunk fat. Lean mass parameters seem to play no role at all, and insulin resistance, assessed by HOMA, and IL-6 levels were also related to liver fat stores in the univariate analysis, being displaced by trunk fat mass in the multivariate analysis.
Therefore, we conclude that steatosis in chronic hepatitis C is a common event (67.86%), and is closely related to trunk fat, but not with leg fat mass; to insulin resistance, and to IL-6. The main factor involved is trunk fat, despite the normal BMI of the patients included in this study, and also despite the fact that at least 12 patients with BMI over 25 kg/m2 showed no liver steatosis, or minimal amount of it, as shown in Figure 1. The reasons for this finding are unclear, and suggest that factors other than BMI, HOMA or fat mass should be involved. The results here presented also do not support the hypothesis that lean mass plays a role in liver fat accumulation.
Authors are indebted to the nurses and staff of the Internal Medicine Unit and Infectious Diseases Section of the Hospital Universitario de Canarias.
Hepatitis C virus (HCV) infection is a common disease, ultimately leads to liver cirrhosis and hepatocarcinoma. Liver steatosis is an early finding in these patients. Mechanisms are poorly understood, although it is known that HCV genotype 3 may lead to steatosis. Possibly, trunk fat and some adipokines may be also involved.
There is a lot of controversy regarding the association of main adipokines, such as adiponectin or leptin, with liver steatosis, and their role in the progression of simple steatosis to liver inflammation. In addition, although there is general agreement in the association between obesity and liver steatosis, the relationship between fat distribution at different body compartments is not well defined. Moreover, there are some studies that also suggest a role of lean mass in liver steatosis.
In this study the authors report that liver steatosis in chronic HCV infection is a common, but not universal event (67.86%). It is closely related to trunk fat and to interleukin (IL)-6, a cytokine that may be produced by trunk fat, but not with fat at the legs, and also to insulin resistance. However, there are still some unexplained results: The relationship between liver steatosis and trunk fat was observed despite the normal body mass index (BMI) of the patients included in this study, and also at least 12 patients with BMI over 25 kg/m2 showed no liver steatosis, or minimal amount of it. In addition, their results also do not support the hypothesis that lean mass plays a role in liver fat accumulation.
This study provides new data relative to the association of liver steatosis with several adipokines and inflammatory cytokines in HCV-infected patients. As mentioned above there is considerable controversy regarding levels of some of these cytokines in HCV-infected patients, and even opposite results have been reported by several groups. In addition, this study underscores the role of trunk fat in liver steatosis, despite normal BMI, and does not support to the hypothesis that lean mass could play a role.
Cytokines are small molecules with protean effects on inflammation and immune response, among many other effects on most organs. Tumor necrosis factor alpha is one of the first cytokines described, initially as the factor responsible for tumor-induced cachexia. IL-6 is a proinflammatory cytokine, that also bears an immunomodulatory effect. Adipokines are cytokines secreted by adipose tissue.
In this manuscript, the authors described about effects of adipokines, cytokines, and body fats on liver steatosis in hepatitis C patients. The key results are very interesting to the readers of HCV and other hepatic diseases.
| 1. | Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology. 2011;140:809-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 266] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E656-E665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Snijder MB, Flyvbjerg A, Stehouwer CD, Frystyk J, Henry RM, Seidell JC, Heine RJ, Dekker JM. Relationship of adiposity with arterial stiffness as mediated by adiponectin in older men and women: the Hoorn Study. Eur J Endocrinol. 2009;160:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Kim YL, Kim TK, Cheong ES, Shin DG, Choi GS, Jung J, Han KA, Min KW. Relation of absolute or relative adiposity to insulin resistance, retinol binding protein-4, leptin, and adiponectin in type 2 diabetes. Diabetes Metab J. 2012;36:415-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ashour E, Samy N, Sayed M, Imam A. The relationship between serum adiponectin and steatosis in patients with chronic hepatitis C genotype-4. Clin Lab. 2010;56:103-110. [PubMed] |
| 8. | Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Hung CH, Lee CM, Chen CH, Hu TH, Jiang SR, Wang JH, Lu SN, Wang PW. Association of inflammatory and anti-inflammatory cytokines with insulin resistance in chronic hepatitis C. Liver Int. 2009;29:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Tiftikci A, Atug O, Yilmaz Y, Eren F, Ozdemir FT, Yapali S, Ozdogan O, Celikel CA, Imeryuz N, Tozun N. Serum levels of adipokines in patients with chronic HCV infection: relationship with steatosis and fibrosis. Arch Med Res. 2009;40:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Testa R, Franceschini R, Giannini E, Cataldi A, Botta F, Fasoli A, Tenerelli P, Rolandi E, Barreca T. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J Hepatol. 2000;33:33-37. [PubMed] |
| 12. | González-Reimers E, Castellano-Higuera A, Alemán-Valls R, Alvarez-Argüelles H, de la Vega-Prieto MJ, Abreu-González P, López-Prieto J, Santolaria-Fernández F, Valladares-Parrilla F. Relation between body fat and liver fat accumulation and cytokine pattern in non-alcoholic patients with chronic HCV infection. Ann Nutr Metab. 2009;55:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, Sun CH. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20:17932-17940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 14. | Kumar R, Rastogi A, Sharma MK, Bhatia V, Garg H, Bihari C, Sarin SK. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in Indian patients with normal body mass index: Do they differ from obese or overweight non-alcoholic fatty liver disease? Indian J Endocrinol Metab. 2013;17:665-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 16. | Bustamante M, Fernández-Verdejo R, Jaimovich E, Buvinic S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca(2+) signals and an IL-6 autocrine loop. Am J Physiol Endocrinol Metab. 2014;306:E869-E882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Vida M, Gavito AL, Pavón FJ, Bautista D, Serrano A, Suarez J, Arrabal S, Decara J, Romero-Cuevas M, Rodríguez de Fonseca F. Chronic administration of recombinant IL-6 upregulates lipogenic enzyme expression and aggravates high-fat-diet-induced steatosis in IL-6-deficient mice. Dis Model Mech. 2015;8:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Paniagua JA, Escandell-Morales JM, Gil-Contreras D, Berral de la Rosa FJ, Romero-Jimenez M, Gómez-Urbano A, Sanchez-Lopez A, Bellido E, Poyato A, Calatayud B. Central obesity and altered peripheral adipose tissue gene expression characterize the NAFLD patient with insulin resistance: Role of nutrition and insulin challenge. Nutrition. 2014;30:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] |
| 20. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 31.0] [Reference Citation Analysis (3)] |
| 21. | Brown TT, Mehta SH, Sutcliffe C, Higgins Y, Torbenson MS, Moore RD, Thomas DL, Sulkowski MS. Hepatic steatosis associated with increased central body fat by dual-energy X-ray absorptiometry and uncontrolled HIV in HIV/hepatitis C co-infected persons. AIDS. 2010;24:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, Sarmento A, Medina JL. Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis. 2014;14:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Petit JM, Minello A, Jooste V, Bour JB, Galland F, Duvillard L, Verges B, Olsson NO, Gambert P, Hillon P. Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J Clin Endocrinol Metab. 2005;90:2240-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Kara B, Gunesacar R, Doran F, Kara IO, Akkiz H. Correlation of serum adiponectin levels and hepatic steatosis in hepatitis C virus genotype 1 infection. Adv Ther. 2007;24:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Aksõz K, Unsal B, Kirci A, Alper E, Buyraç Z, Aslan F, Cekiç C, Cengiz O, Ozcan Ari F, Akpinar Z. The relationship between chronic HCV infection and the level of plasma adiponectin. Turk J Gastroenterol. 2008;19:254-257. [PubMed] |
| 27. | Siagris D, Vafiadis G, Michalaki M, Lekkou A, Starakis I, Makri M, Margaritis V, Christofidou M, Tsamandas AC, Labropoulou-Karatza C. Serum adiponectin in chronic hepatitis C and B. J Viral Hepat. 2007;14:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Liu CJ, Chen PJ, Jeng YM, Huang WL, Yang WS, Lai MY, Kao JH, Chen DS. Serum adiponectin correlates with viral characteristics but not histologic features in patients with chronic hepatitis C. J Hepatol. 2005;43:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Takahara M, Katakami N, Kishida K, Kaneto H, Funahashi T, Shimomura I, Matsunaga S, Kubo S, Fukamizu H, Otsuka A. Circulating adiponectin levels and their associated factors in young lean healthy Japanese women. J Atheroscler Thromb. 2013;20:57-64. [PubMed] |
| 30. | Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 781] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 32. | Kukla M, Mazur W, Bułdak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med. 2011;17:1397-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Giannini E, Ceppa P, Botta F, Mastracci L, Romagnoli P, Comino I, Pasini A, Risso D, Lantieri PB, Icardi G. Leptin has no role in determining severity of steatosis and fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2000;95:3211-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Myers RP, Messous D, Poynard T, Imbert-Bismut F. Association between leptin, metabolic factors and liver histology in patients with chronic hepatitis C. Can J Gastroenterol. 2007;21:289-294. [PubMed] |
| 35. | Crespo J, Rivero M, Fábrega E, Cayón A, Amado JA, García-Unzeta MT, Pons-Romero F. Plasma leptin and TNF-alpha levels in chronic hepatitis C patients and their relationship to hepatic fibrosis. Dig Dis Sci. 2002;47:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
P- Reviewer: Jin B, Yun JW S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/