Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1168
Peer-review started: August 30, 2014
First decision: October 14, 2014
Revised: January 29, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: May 28, 2015
Processing time: 264 Days and 13.2 Hours
Hepatocellular carcinoma (HCC) is the most common liver-derived malignancy with a high fatality rate. Risk factors for the development of HCC have been identified and are clearly described. However, due to the lack of tumor-specific symptoms, HCC are diagnosed at progressed tumor stages in most patients, and thus curative therapeutic options are limited. The focus of this review is on surgical therapeutic options which can be offered to patients with HCC with special regard to recent findings, not exclusively focused on surgical therapy, but also to other treatment modalities. Further, potential promising future perspectives for the treatment of HCC are discussed.
Core tip: This review presents an overview on most important knowledge on hepatocellular carcinoma (HCC) for surgeons and describes the common surgical and non-surgical therapeutic options for the treatment of HCC. Further, a perspective on novel aspect and future decision aids is given.
- Citation: Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: Surgeon's view on latest findings and future perspectives. World J Hepatol 2015; 7(9): 1168-1183
- URL: https://www.wjgnet.com/1948-5182/full/v7/i9/1168.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i9.1168
Hepatocellular carcinoma (HCC) is a very common malignant disease with more than 700000 new patients diagnosed per year. Interestingly, the incidence of HCC varies relevantly throughout the world. Whereas HCC is a very common malignant disease in sub-Saharan Africa, and central and south-east Asia with incidence rates of 20-47/100000 habitants, the incidence of HCC is comparably low in developed western countries (incidence rate 2-6/100000 habitants)[1,2]. However, diagnosis of HCC is continuously increasing in the developed countries throughout the past decades[3]. There are data from the United States showing a tripling of HCC incidence during the recent 30 years[4]. Reasons for this increase are multifactorial as follows. Besides an increasing incidence of chronic hepatitis C in developed countries, improved treatment of liver cirrhosis and cirrhosis-associated complications causes a longer survival of these patients with consequently a longer possible time period to develop HCC in the cirrhotic liver. Furthermore, screening programs for patients at risk increase the rate of newly diagnosed HCC.
When analyzing the incidence-to-mortality ratio, HCC is the second most common cause of cancer-related death. In 2012, 782000 new cases and 746000 deaths due to HCC are reported worldwide[3,5]. Risk factors for the development of HCC are well known and clearly described. The major risk factor for development of HCC is liver cirrhosis: 80%-90% of patients autopsied for HCC display signs of cirrhosis[6]. Most common causes for cirrhosis and subsequent HCC are chronic infections with hepatitis B virus (HBV) and HCV[6], chronic alcoholic liver disease, and increasingly non-alcoholic fatty liver disease[7]. According to Parkin et al[8], more than 50% of HCC are associated to HBV infection worldwide. Interestingly, in case of HBV infection, development of cirrhosis is not a prerequisite for the development of HCC, as there are up to 29% of cases of spontaneous HCC in non-cirrhotic HBV-infected livers[7,9]. Furthermore, in patients with HCV infection, HCC is not exclusively based on HCV-associated cirrhosis, as up to 54% of patients can develop HCC without having cirrhosis[9]. The risk to develop HCC in alcoholic liver disease has also been clearly demonstrated to be relevantly elevated for daily ingestion of more than 60 g alcohol[10]. Hereditary liver diseases, such as Wilson’s disease, hemochromatosis, alpha-1-antitrypsin deficiency, or autoimmune hepatitis play a minor role in the development of HCC. Interestingly, the geographic distribution of underlying diseases and risk factors for development of HCC varies, and also gender, and ethnic group display differences in the distribution of risk factors[7].
As classical tumor-associated symptoms are lacking, patients at risk with known chronic viral hepatitis benefit from screening and surveillance programs as recommended by the American Association for the Study of Liver Diseases and European Association for the Study of the Liver (EASL)-European Organization for Research and Treatment of Cancer (EORTC) practice guidelines[11,12]. The aims of surveillance programs are to detect HCC at early stages, enable the patient to obtain curative treatment, and thus reduce HCC-associated mortality. Actual EASL-EORTC Clinical Practice Guidelines recommend abdominal ultrasound every 6 mo in patients at risk[12]. Despite the advantage of cost-effectiveness and non-invasiveness, ultrasound has the disadvantage to be investigator-dependent, which compromises sensitivity. Thus, for dubious findings, additional diagnostics, such as contrast-enhanced ultrasound, computed tomography (CT) and magnetic resonance imaging offer examination tools investigating contrast agent dynamics in suspected nodules. HCC are classically characterized by an arterial hypervascularisation, thus showing typical hyperintense contrast agent accumulation in early arterial imaging phase and a washout phenomenon in portal venous imaging phase[3]. The significance for contrast enhanced ultrasound is uncertain according to the clinical practice guidelines, and nuclear imaging (PET-CT) in not appropriate for early diagnosis of HCC. Diagnosis of HCC is accepted if at least two complementary imaging techniques show classical features of HCC or HCC is proven by biopsy. This consensus is reflected by the Eurotransplant criteria for exceptional MELD application for HCC. Tumor markers are not recommended for screening routine due to the lack of sensitivity and the fact that especially early HCC do not express alpha-fetoprotein (AFP) in up to 40% of the cases[13,14]. Sensitivity and specificity of AFP are dependent on AFP serum levels[15]. According to the current EASL-EORTC and European Society for Medical Oncology clinical practice guidelines, AFP levels > 400 ng/mL are accepted to prove HCC[12,16]. Furthermore, AFP can be used as a progression parameter in case of AFP-expressing HCC after treatment, although serum AFP levels do not correlate with tumor size or tumor stage. Thus, the extent of AFP level does not allow any conclusions on the presence of metastases or vascular invasion, which might be helpful for the surgeon[17].
Prognosis for patients with HCC basically depends on the tumor stage at the time point of diagnosis, as defined by the barcelona clinic liver cancer (BCLC) classification system, as well as the fact whether the tumor is treated or not. The BCLC classification system stratifies HCC according to patients performance status, tumor size and number of nodules, tumor Okuda stage, and the presence or absence of liver function impairment and portal hypertension, and degree of cirrhosis as stratified by Child-Pugh score[18]. Cabibbo et al[19] recently reported outcome data from 320 patients with untreated HCC at different BCLC stages. Median survival in the entire cohort was 6.8 mo, whereas median survival rates ranged from 1.8 (BCLC D) to 33 mo (BCLC A). These data underline impressively the necessity for screening programs for patients at risk, since late diagnosis of HCC is associated with a very poor prognosis for these patients.
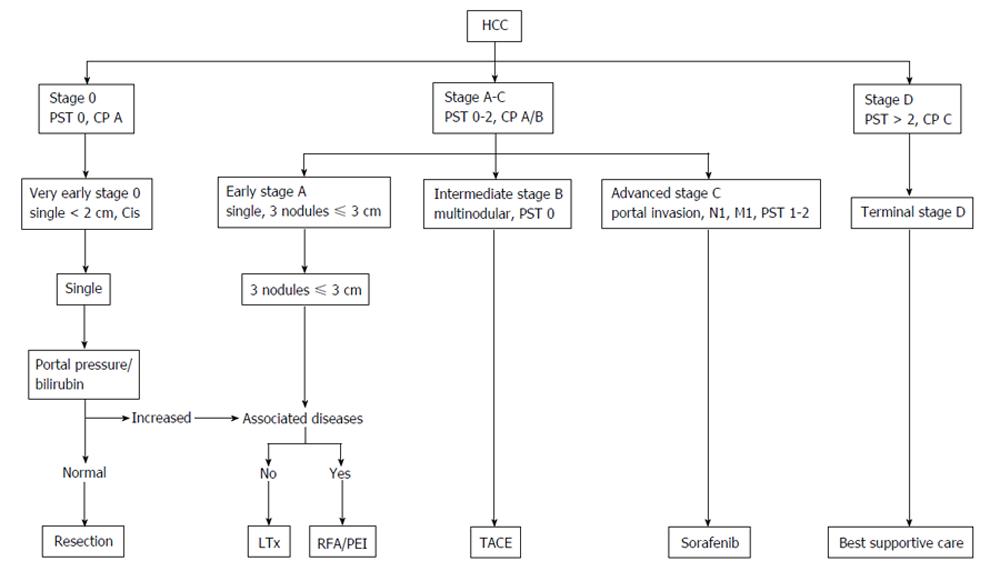
In general, treatment of patients with HCC is a multidisciplinary therapy approach. There are manifold treatment options which can be offered to our patients with HCC. The EASL-EORTC guidelines present a treatment algorithm which therapy is recommended to which patient, taking into account patient’s performance status, Child-Pugh stage, as well as tumor diameter and number of nodules (Figure 1) as given by the BCLC status[12,18]. According to this recommendation, surgical approach for HCC is restricted to very early stages of HCC, i.e., singular tumors with a diameter < 2 cm, and early stage HCC, i.e., either a single tumor < 5 cm or 3 nodules each < 3 cm (Milan criteria[20]). For patients with contraindications for liver surgery or transplantation, thus considered not suitable for a surgical approach, should be treated with non-surgical procedures, i.e., local ablative therapies, intravascular embolizing approaches, or palliative chemotherapy. Also, according to the guidelines, patients with advanced stages of HCC are considered not to profit from surgical resection of their respective tumor. The decision for the most suitable and success-promising approach for the respective patient must be defined in multidisciplinary tumor boards in which representatives of all specialist departments involved in HCC therapy including experienced hepatobiliary surgeons must be present. However, the restriction of surgery to very early and early stages of HCC is increasingly challenged with increasing evidence for broadening the indication for surgery.
As previously stated, expected survival for patients with advanced and terminal stage of HCC is very short, so that some patients actively decide not to undergo any palliative therapy due to their very limited life expectancy. For these patients, best supportive care and help through an ambulatory palliative care institution is reasonable, since patients lose relevant life expectancy under a palliative setting without any HCC-directed treatment[21,22]. Unfortunately, there are no data available on the benefit of best supportive care for these patients who decline any palliative treatment option. However, it is known that quality of life (QoL) is an independent predictor for survival in HCC patients[23], and thus it is conceivable that QoL improvement for these patients might be an effective strategy to optimize life expectancy without any tumor-directed intervention.
So far, there have been many efforts to develop effective drug treatment for HCC, either in the adjuvant setting after surgical tumor removal by liver resection or transplantation, or in the palliative setting. Unfortunately, there are no really seminal pharmacological approaches available, yet. The sole drug which has found the way into clinical practice is the multi-kinase inhibitor sorafenib[24,25]. In the setting of advanced or unresectable HCC, sorafenib has been demonstrated to prolong median survival from 7.9 to 10.7 mo and median time to progression from 2.8 to 5.5 mo, respectively. Unfortunately, sorafenib treatment is recommended to patients with Child-Pugh A and stable B cirrhosis only[26], and is associated with distinct side effects. In particular gastrointestinal side effects, hemorrhages, exanthema, hand-foot-syndrome, and cardio-vascular symptoms, which often lead to treatment discontinuation or dose reduction are common[27]. For patients with advanced cirrhosis (Child-Pugh C), sorafenib therapy is not recommended due to the limited life expectancy caused by the cirrhosis stage, a lack of evidence concerning efficacy of sorafenib treatment in this patient subgroup, and to avoid severe side effects following impaired hepatic drug metabolism. In case of sorafenib treatment failure, oxaliplatin-based treatment regimens have been demonstrated to be a suitable second-line chemotherapy achieving progression free survival and overall survival of 4.2 and 9.3 mo, respectively, with an acceptable rate of side effects[28,29]. Furthermore, numerous clinical trials have investigated the use of a variety of tyrosine kinase inhibitors, mTOR inhibitors, VEGF receptor-, FGF receptor-, and PDGF-receptor-blocking multikinase tyrosine kinase inhibitors, as well as classical chemotherapeutic drugs, such as, e.g., doxorubicin. Unfortunately, none of these trials has demonstrated a striking effect in a palliative or adjuvant treatment setting[30,31]. Despite the proven effect in the palliative setting, adjuvant systemic chemotherapy with sorafenib after liver resection for HCC showed no beneficial effects on recurrence-free survival in the current randomized, double-blind, placebo-controlled STORM-trial (NCT0069277)[32]. However, there are some promising results that interferon might improve overall and recurrence-free survival in the adjuvant setting[33,34]. But interferon therapy is also accompanied by a variety of side effects such as flu-like symptoms, fever, fatigue, myalgia, and cephalgia, which might limit the suitability of this treatment option in many patients. In conclusion, there is actually no drug for adjuvant treatment after surgical tumor resection.
According to the BCLC treatment algorithm, percutaneous tumor destruction by radio frequency ablation (RFA), or percutaneous ethanol injection (PEI) is indicated for early stages of HCC in patients which are not suitable for liver resection or liver transplantation[12]. Other percutaneous ablative techniques, such as laser-induced thermo therapy (LITT), cryotherapy, and microwave ablation (MWA) are actually not recommended in the EASL-EORTC clinical practice guidelines. This fact is due to the novelty of some of these techniques, with a consequent lack of evidence for the use and comparability of these techniques to the established and recommended techniques. However, these techniques will be presented briefly in the followings.
RFA: RFA is the most frequently used approach to destroy intrahepatic tumor masses with a diameter up to 5 cm by application of thermal energy into the tumor to induce thermal tumor necrosis. It is recommended by the EASL-EORTC guidelines for early-stages of HCC in patients who are not eligible for surgery or transplantation. According to an actual systematic Cochrane Database review[35], there is moderate evidence, that RFA is superior to percutaneous ethanol injection[36], but inferior to hepatic resection of HCC[37,38] with regard to recurrence-free and overall survival. In contrast, RFA is superior to hepatic resection with regard to procedure-related complications due to the less invasive character of the procedure[39]. Besides definite treatment of HCC in patients who are not eligible for surgery or liver transplantation, RFA can be performed both percutaneously and with a laparoscopic approach. RFA is a major bridging therapy option for patients on the waiting list for liver transplantation. Due to the very limited approach during RFA, this procedure can be performed repeatedly without causing severe intraabdominal adhesions, and thus RFA does not complicate subsequent liver transplantation[40]. Additionally, Huang et al[41] could demonstrate in a non-randomized prospective parallel cohort study comparing RFA and liver resection for small HCC < 2 cm, that RFA is well tolerated by patients, and impairs health-related quality of life significantly less than liver resection.
LITT: LITT plays obviously only a minor role, since there is only one actual large report on LITT experience in 113 HCC patients reporting both an excellent tumor response in small lesions < 2 cm after a single LITT treatment, and also in larger tumors up to 5 cm after repeated LITT sessions, with favorable 5-year survival rates of 30%[42]. Besides this report, there are only few publications with small patient cohorts using LITT for treatment of HCC. Randomized studies comparing different ablation techniques are missing in the literature. Thus, LITT seems to play a minor role among the percutaneous intervention options when compared to RFA or PEI.
Cryotherapy: The evidence for the use of cryotherapy for treatment of HCC is limited. There is only one systematic Cochrane Database review by Awad et al[43], who finish their analysis with a quite ambiguous conclusion, in that there is not enough evidence so far in favor or against cryotherapy[43]. However, there are some single center reports in the literature reporting excellent, size-dependent tumor ablation rates and even 10-year survival rates of approximately 9% in patients with cirrhosis-based HCC[44] with acceptably low procedure-related complication rates[44,45]. Interestingly, tumor response to cryotherapy as assessed by the modified Response Evaluation Criteria in Solid Tumors criteria[46] has been demonstrated to be an independent predictor of overall survival for HCC[47]. However, in contrast to the use of cryotherapy for the treatment of colorectal liver metastases[48], cryotherapy is not used routinely for the treatment of unresectable HCC. The main reason for this is certainly the advanced and expensive technique with multiple possible pitfalls due to the use of liquid nitrogen. To the author’s knowledge, there is also no ongoing trial comparing cryotherapy to the established interventional procedures. Thus, evidence in favor or against the use of cryotherapy will be owing, and some authors even expect an end of the use of cryotherapy[49]. In contrast, as stated by Awad et al[43], evidence is insufficient “to recommend or refute cryotherapy” to patients with HCC[43], and indication for cryotherapy is finally based on the experience and expertise of the respective treating physician.
PEI: PEI is an alternative very low-priced method to apply pure ethanol directly to the targeted tumor. However, the efficacy of this method is limited by the fact that ethanol spread within the tumor tissue might be altered by septa or a tumor capsule and equable ethanol distribution within the tumor is not warranted. In two recent studies from eastern Asia, PEI has been demonstrated to achieve inferior results compared to RFA[36,50], which might be attributable to an inferior rate of tumor response (or vice versa increased treatment failure) using PEI and thus resulting in decreased survival rates[36]. Accordingly, in the EASL-EORTC guidelines, PEI is recommended for small HCCs (BCLC 0 or A), and especially in cases of larger tumors up to 5 cm when RFA is not feasible due to technical reasons, as, e.g., subcapsular tumor localization or adjacent to the gallbladder, to large vessels or the hepatic hilum[12,51].
MWA: According to the actual EASL-EORTC guidelines, microwave ablation is not generally recommended and remains to be evaluated. The advantage of MWA compared to RFA is that treatment efficacy is affected in a lesser degree by the cooling effect of large blood vessels located in the proximity of the ablation area. Recently, Zhang et al[52] demonstrated comparable results for MWA and RFA with regard to overall survival, local progression, and the degree of local tumor ablation for HCC < 3 cm[52]. Additionally, MWA shows the same frequency of post-ablation syndrome, i.e., occurrence of low-grade fever, nausea, vomiting, malaise, and post-interventional pain[53] as RFA[54]. In an actual multicenter study, Groeschl et al[55] demonstrated excellent complete tumor ablation rates of approximately 94% as assessed by histology after resection of the respective pre-treated tumors, with a reported median recurrence-free survival of 25 mo. Taking into account, that MWA is by far more cost-effective compared to RFA[56], and is associated with a low complication rate of approximately 11%[57], MWA might be a promising alternative ablative technique, which even might replace RFA as standard treatment for unresectable HCC. From the surgical point of view, MWA seems not to have relevant influence on complication rate after resection of the MWA pre-treated HCC[55], but so far there are too few data to state whether neo-adjuvant MWA prior to HCC resection is really a suitable approach and will be of benefit for the patients. Equally, there is no evidence yet, whether MWA or RFA is superior in a neo-adjuvant setting with regard to overall and recurrence-free survival, as well as resection-related morbidity. This remains to be elucidated in prospective, randomized trials.
According to the EASL-EORTC guidelines, transarterial chemoembolization (TACE) is recommended for intermediate stages of HCC[12]. In contrast, selective internal radiotherapy (SIRT; synonymous: radioembolization) is a comparatively novel approach for intravascular tumor-directed therapy, which can also be used for intravascular treatment of HCC. SIRT is not recommended in the EASL-EORTC-guidelines, due to the lack of data from randomized clinical trials comparing SIRT and TACE in intermediate stages of HCC.
TACE: The rationale for TACE is, that intrahepatic malignancies and especially HCC are almost exclusively nourished via the hepato-arterial vasculature. Consequently, a catheter device is placed via femoral artery into the tumor-supplying branch of the proper hepatic artery, and a combination of high-dose chemotherapy and vessel occluding agents are applied selectively to the tumor area, whereas non-tumorous liver areas remain basically unaffected. As reviewed by Marelli et al[58], a huge variety of chemotherapeutic drugs is used, whereas doxorubicin is the most common agent. This chemotherapeutic drug is usually applied in combination with lipiodol as a carrier which offers the beneficial effect that is can be visualized by X-rays, and persists for several weeks in the liver after administration[59]. Also for vessel occlusion, a variety of embolization agents is available, whereas gelatin sponge particles, and polyvinyl alcohol particles are the most commonly used embolizing agents[58].
Besides its recommendation in the EASL-EORTC guidelines for treatment of intermediate stage HCC, TACE is also widely used as a palliative treatment approach. Interestingly, according to a recent Cochrane Database review, there is no evidence for the use of TACE for treatment of unresectable HCC[60,61]. However, due to overall heterogenous data from retrospective, and also prospective studies and the previously demonstrated capacity of TACE to prolong survival for patients with unresectable HCC in two randomized clinical trials[62,63] as well as some older reviews[64,65], TACE has been included in the EASL-EORTC guidelines and still represents the golden treatment standard for patients with unresectable intermediate stages of HCC according to the BCLC staging algorithm[12]. The recently published Canadian clinical recommendations also state that TACE probably provides benefit and thus also recommend TACE for patients with intermediate stages of HCC[66].
Furthermore, TACE is used regularly as a bridging therapy for patients with HCC on the waiting list for liver transplantation. However, there is no distinct evidence for the effectiveness of TACE to prevent patient drop-out from waiting list during the waiting time for transplantation[67] and to improve post-transplant overall and recurrence-free survival[68,69]. There is also evidence, that response of HCC to pre-transplant TACE can be an indicator for favorable outcome after liver transplantation with regard to delayed tumor recurrence[70]. In a study by Sotiropoulos et al[71], TACE even failed to reproducibly down-stage multifocal HCC or to induce entire tumor necrosis, and provided at best an “acceptable” tumor control[71]. However, the same group demonstrated, that response to TACE and TACE-induced complete tumor necrosis at the time point of liver transplantation is associated with a very low recurrence rate and improved survival after liver transplantation[68,72] thus establishing an indication for pre-transplant TACE. At least, TACE can be safely performed in patients with cirrhosis and hyperbilirubinemia who are on the waiting list for liver transplantation[73] without increasing the risk for complications during transplantation.
TACE has also been investigated as a neoadjuvant treatment option prior to liver resection for resectable HCC. There is one actual meta-analysis that demonstrates that preoperative TACE does not effectively influence postoperative overall and recurrence-free survival respectively. Furthermore, preoperative TACE has no effect on intra- or extrahepatic tumor recurrence, and is therefore not recommended as a preoperative strategy for resectable HCC[74]. Interestingly, there are some reports that adjuvant TACE, i.e., after curative resection of HCC might offer benefits with regard to disease-free and overall survival[75].
SIRT: In contrast to TACE, SIRT uses Yttrium 90 (90Y)-loaded glass microspheres which are applied via a trans-arterial catheter system into the tumor supplying arterial vasculature. 90Y decays to zirconium (90Zr) with a physical half-life of approximately 65 h. During this decay process, an average energy of approximately 0.94MeV is emitted. 90Y is a pure β radiation emitter. The corresponding β radiation penetrates into the surrounding tissue with a depth of a maximum of 11 mm, leading to tissue destruction and fibrosis[76].
So far, SIRT has not been widely used for the treatment of resectable or unresectable HCC. There are only very few studies investigating the potential beneficial effect of this treatment modality. There is a very recent review article by Sangro et al[77], who compared published outcome data from patients after SIRT for unresectable HCC. When comparing treatment efficacy of SIRT to TACE or systemic sorafenib therapy, median overall survival rates are comparably between SIRT and the other treatment modality, respectively, especially for intermediate or advanced stages of HCC[78]. SIRT has been reported to be a safe treatment option with a frequency of the so-called post-radioembolization syndrome (fatigue, nausea, vomiting, anorexia, fever, abdominal pain) of 20%-55%[79]. However, large prospective trials comparing SIRT vs TACE are lacking, yet, and also cost-effectiveness analyses for 90Y radioembolization are lacking[79]. Besides its use as a definite treatment option for patients with otherwise non-resectable HCC, SIRT has also been used as a bridging therapy prior to liver transplantation[69,80]. The emerging role of SIRT, both as a definite treatment modality as well as a curative or a bridging therapy option has been elaborated in detail in a review article by Lau et al[81]. The authors also admit, that evidence for the use of SIRT in the respective intention is quite low, due to the short time of investigation so far and the scarcity of studies investigating a potential role of SIRT in concurrence to established treatment modalities for HCC patients. However, in a recent study by El Fouly et al[82], SIRT has shown equivalent survival probabilities, less hospitalizations, less treatment sessions and a lower complication rate in patients with intermediate stage B of HCC when compared to TACE[82].
Whereas radiotherapy for treatment HCC has been considered inappropriate for a long time due to severe radiation-associated complications and liver failure, more than 600 articles have been published within the recent 5 years on this topic. This enthusiasm can be attributed to the development of novel radiotherapy technologies during the recent decade, which now allow precise application of high-dose radiation to the tumor tissue while sparing the rest of the liver and adjacent organs.
This has led to the more widespread use of radiotherapy for the treatment of HCC, especially in patients with unresectable tumors. In 2008, Tse et al[83] published their results on stereotactic body radiotherapy for patients with unresectable HCC proving the safety of this treatment option. Finally, due to the possibility to effectively use radiotherapy in advanced stages of liver cirrhosis (Child-Pugh B/C) the use of radiotherapy has been implemented in HCC treatment guidelines from the Korean Liver Cancer Study Group and the National Comprehensive Cancer Network. But despite the proven efficacy and safety, radiotherapy has not found its way into the EASL-EORTC guidelines, yet. However, Jihye et al[84] proposed a possible way of integration of radiotherapy in the BCLC guidelines. Another advantage of radiotherapy is the possibility of combination therapy with established treatment options, such as TACE[85,86] or systemic chemotherapy[87] although side effects and hepatic toxicity are critical limitations for this treatment approach. Perspectively, there are some promising experimental data on the possibility to induce radiosensitization of HCC cells using the aurora kinase inhibitor VE-465 potently suppressing tumor growth and enhancing tumor-responsiveness to radiotherapy[88]. Thus, radiotherapy opens new therapeutic options but its use is currently limited to a scarce comprehensive availability of this therapy option.
Liver resection: The surgical approach to HCC represents the only treatment option which allows entire and reliable removal of the tumor from the patient and therefore the potential of cure. Principally, there are two surgical concepts for the treatment of HCC: liver resection and liver transplantation. Latter offers the additional benefit that underlying liver disease which nourishes development of further malignancies in sense of precancerosis, is also removed. The disadvantage of liver transplantation is - despite all progresses and technical improvements of this procedure - the higher mortality risk when compared to liver resection as well as the necessity for life-long immunosuppression with all associated side effects.
When dealing with liver resection, two principle questions have to be discussed: whether to remove the HCC by anatomic or non-anatomic/atypical resection, and to perform this procedure open or laparoscopically.
The surgical approach is limited by the mandatory need to maintain sufficient functional liver remnant. In non-cirrhotic patients, maximum extent of resection can be calculated by the remnant liver volume - body weight ratio (RLV-BWR). Several reports have demonstrated that patients with a RLV-BWR ≤ 0.5%-0.8% are at high risk for postoperative hepatic dysfunction and increased mortality[89-91]. Another approach to indicate limit for liver resection is the future liver remnant (FLR), referred to the total liver volume. The safe limit is considered to be at a FLR of > 20% in patients with healthy livers[92,93]. In cirrhotic patients, parenchymal functional and regenerative capacities are relevantly reduced. Consequently, a RLV-BWR ≥ 1.4% or a FLR of at least 30%-40% are considered as critical threshold for development of postoperative complications[92,94].
Thus, from the surgical point of view, contraindications against liver surgery for HCC might be considered only to be the insufficient future liver remnant after resection. Worse prognostic factors for the outcome are vascular invasion[95], infiltration of adjacent organs[96], and presence of lymph node metastases[97] at the time of diagnosis, but represent no contraindications against surgery per se. Resection of HCC with these degrees of tumor extent can be safely resected (when respecting resection limits), and survival after resection is for sure improved when compared to best supportive therapy or palliative chemotherapy approaches.
There has been an intense debate whether anatomic or atypical resection for HCC should be preferred. Rationale for atypical resection was the idea of parenchymal-sparing surgery with an as marginal as possible loss of - in most cases - functionally altered liver parenchyma. This idea of parenchymal-preserving liver surgery is based on the limited possibilities to assess functional liver reserve after liver resection, and the fear to induce postoperative liver failure due to a too aggressive resection extent[98]. In contrast, anatomic liver resection is rationale since it is known that HCC spread along the nourishing portal venous branch distributing satellite nodules within the same anatomical segment. Thus, anatomic resection allows removal of the known tumor, as well as of potential undetectable satellite metastases[99]. Meanwhile, there is strong evidence that anatomic resection is superior to non-anatomic, i.e., atypical resection for HCC. As evaluated by several meta-analyses, anatomic resection is associated with improved survival rates, and delayed intrahepatic and systemic disease-recurrence with no differences regarding perioperative morbidity or mortality[100-102]. Interestingly, one recently published meta-analysis by Tang et al[103] could not demonstrate superiority of anatomic resection, but this seems to be explainable by the different trial selection compared to previous analyses. Thus, a general recommendation for anatomical or non-anatomical resection cannot be given. Decision on the extent of liver resection is based on the tumor location within an anatomical segment. Based on the available data, anatomic resection should be performed in non-cirrhotic livers. In patients with cirrhotic livers, potential oncologic disadvantage of a non-anatomic resection has to be accepted with regard to the necessity for maintenance of sufficient functional liver remnant volume and function.
Since the first description of laparoscopic liver resection in 1992 by Gagner et al[104] performing a non-anatomic liver resection for focal nodular hyperplasia, there has been an enormous increase of laparoscopic liver resections with increasing extent of resections up to right hemihepatectomies[105], and more and more complex procedures, such as tumor resection in the postero-superior segments (VII, VIII, IVa)[106,107]. Also, in the treatment of HCC, laparoscopic approaches have been established. But there are so far only few specialized centers worldwide reporting on laparoscopic liver resections for the treatment of HCC.
According to several meta-analyses comparing open vs laparoscopic liver resections for HCC, laparoscopic liver resections are a safe procedure with comparable overall and recurrence-free survival rates[108]. Laparoscopic liver resections are associated with reduced intraoperative blood loss and subsequent requirement for packed red blood cells. Furthermore, laparoscopic liver resections are effective to provide negative resection margins, and are associated with shorter hospitalization and less postoperative complications[108,109]. As the findings could also be observed for patients with cirrhosis, laparoscopic liver resection can be safely applied to patient with resectable HCC. However, extensive experience is necessary especially for more complex procedures, and thus, this procedure is reserved to specialized centers. With increasing experience, the extent of liver resection and localization of HCC within the liver will become more and more secondary, and only assessment of liver functional reserve as estimated predominantly by liver volumetry in western centers, and by indocyanine green excretion dynamics in eastern centers will determine the limits for laparoscopic liver resection, as it is also the case for open liver resections[110,111]. A recent review article analyzing major laparoscopic hepatectomies independent from HCC, could include a total of 29 studies from 1998 to 2011 with a total of more than 2600 patients, underlining the fast expansion of laparoscopy for liver resection[112]. However, learning curve for laparoscopic liver surgery is very flat, as demonstrated by Vigano et al[113] or Dagher et al[114], showing that at least 60 to 90 laparoscopic liver resections are necessary to perform for a surgeon, before a state of experience and standard is achieved[115]. Since laparoscopic liver resection for HCC has predominantly to be performed in cirrhotic livers, learning curve is expectedly even flatter than in non-cirrhotic livers[116]. However, meanwhile development of surgical technique is progressing, and the first reports of robotic liver resections for HCC are published. The largest study including 41 patients reports excellent morbidity and mortality rates of 7% and 0%, respectively, as well as 2-year overall and disease-free survival rates of 94% and 74%, respectively[117]. Thus, technological progress also finds its way into liver surgery with promising first results and experiences[118], providing more possibilities for further development and improvement of treatment options and thus the prognosis for our patients with HCC.
Liver transplantation: First human liver transplantation was performed at the University of Colorado in 1963 by Starzl et al[119]. The first successful liver transplantation was performed 4 years later again by Starzl et al[120] in a girl suffering from HCC. Since that time, this former experimental treatment option has developed to a widespread, highly standardized and successful therapy. Whereas the girl died 13 mo after transplantation due to tumor recurrence, patients undergoing liver transplantation for HCC have nowadays an excellent survival prognosis with 10-year survival rates of 50%[121]. These excellent outcome results have been achieved after implementation of the so-called Milan criteria described by Mazzaferro et al[20] in 1996. The Milan criteria indicate a benefit for patients undergoing liver transplantation for HCC under definite circumstances: one single nodule with a size up to 5 cm, or two or three nodules each up to 3 cm without signs of lymph node metastases and vascular invasion. Currently, these criteria which have been validated prospectively several times[122] are the selection basis for our patients for liver transplantation. However, these criteria are challenged repeatedly by attempts to expand the selection criteria for liver transplantation. For example, patients selected to the so called University of California, San Francisco (UCSF) criteria which contain larger size limits for the respective tumor nodules (single tumor < 6.5 cm, maximum of 3 total tumors with none > 4.5 cm, and cumulative tumor size < 8 cm)[123] show comparable outcome results to patients who were selected according to the Milan criteria[124]. Mazzaferro et al[125] themselves challenged their own “traditional” Milan criteria by the new Milan criteria, also called up-to-seven criteria. These criteria comprise patients with HCC in which the sum of diameters (in cm) and of the number of all HCC nodules is equal or less than seven. They showed that patients with tumor dimensions within these up-to-seven criteria had similar 5-year overall survival rates as patients with HCC within the “traditional” Milan criteria[125]. However, application of the up-to-seven criteria requires a careful patient selection. Besides these two probably most famous extended criteria of eligibility for liver transplantation for HCC, there is a multitude of further criteria with different limits for maximum tumor size of number of tumor nodules. Recently, these criteria have been reviewed, and Chan et al[126] demonstrate in their review article that these several stratification criteria yield quite similar overall survival rates[126] leading to postulations to extend criteria for liver allocation for HCC patients on the waiting list. However, the attempts to extend eligibility criteria are counteracted by the constricted availability of donor organs, as also stated by Mazzaferro et al[122] himself.
To overcome the lack of post-mortal donor organs, transplantation of partial liver grafts has been developed and advanced. The technique was first described by Pichlmayr et al[127] in 1989, and the first series of successful split liver transplantations has been published by Broelsch et al[128] in 1990. Approximately at the same time, transplantation of grafts from living donors has been developed[129,130]. However, one could assume, that implantation of a partial liver graft with subsequent liver regeneration to the extent of the recipient’s demand might represent a massive systemic proliferative stimulus[131], which might - in combination with immunosuppression-induced attenuated tumor-defense[132,133] - enhance tumor cell proliferation and promote early disease recurrence. Interestingly, a recent meta-analysis showed that there is no difference in recurrence-free and overall survival for patients after liver transplantation using living-donated partial liver grafts compared to deceased donor grafts[134]. Thus, living donor liver transplantation represents a safe method for HCC treatment, especially with the advantage, that allocation is not performed by the central allocation authorities (e.g., Eurotransplant), and not limited by tumor size criteria (e.g., Milan criteria).
According to the EASL-EORTC guidelines, there is a clear separation between the indications for liver resection and liver transplantation for HCC, respectively. However, it is a legitimate question, whether resection of HCC which should be treated with transplantation is equally effective, and vice versa if prognosis of patients treated by resection might be improved by transplantation. This is relevant in two ways. First, resection of HCC in patients which should be treated by transplantation would preserve the scarce resource donor organ, and second, transplantation instead of resection abolishes not only the main tumor, but also possible undetected additional small tumors, and removes the diseased liver which is the nutrient medium for further HCC development.
In 2013, there was a Cochrane analysis by Taefi et al[135], which concluded without any clear results due to a lack of appropriate studies. However, there are four additional meta-analyses investigating the superiority of any of these two treatment options. In mutual agreement, all publications demonstrate superiority for liver transplantation for the treatment of HCC at the early BCLC stage A[136-138] or AJCC stage I and II HCC[139]. In contrast, liver resection has been demonstrated to yield similar outcome results compared to liver transplantation in patients with incomplete cirrhosis (Ishak score 0-4), thus for these patients, liver transplantation should be avoided in this subgroup of patients[139]. Unfortunately, there are nor studies comparing liver resection and liver transplantation for patients with Child B or Child C cirrhosis. However, one might speculate that these patients might benefit from liver transplantation due to the fact that transplantation reliefs both tumor and life-limiting diseased liver.
As stated above, the EASL-EORTC guidelines represent a recommendation for HCC treatment and assign to each tumor stage a recommended - and thus assumingly best - treatment modality. In the decision algorithm, surgery is only recommended for very early and early stages of HCC (Figure 1). However, there are comparative studies investigating the value of surgical approaches for patients with more advanced tumor stages.
There is a recent meta-analysis with approximately 21000 patients which clearly demonstrates superiority of surgical resection over RFA and PEI in early stages of HCC[38], which is according to the guidelines a domain of transplantation or RFA. These findings are confirmed by a meta-analysis by Xu et al[140] also showing a significantly improved survival benefit for patients with early stages of HCC undergoing surgery instead of RFA.
When trying to further expand indication for hepatic surgery towards HCC stage B (intermediate stage), one has to compare the outcome results from surgery and TACE. So far, there are no meta-analyses available comparing this issue. There are several very recent publications investigating the role of surgery for HCC stage B. There is one prospective randomized controlled trial comparing TACE and surgery. The authors can clearly demonstrate a survival benefit for patients undergoing liver resection independent of the performance status[141]. These findings are underlined by data from two retrospective analyses enrolling together approximately 1300 patients[142,143] demonstrating a long-term survival benefit for patients undergoing hepatic resection for HCC stage B.
Systemic chemotherapy? So far, advance for systemic chemotherapy for HCC have been very disappointing. Even the gain in survival for patients treated with sorafenib, the only agent which has made its way into clinical use in palliative situations, is not groundbreaking[24,25]. In the adjuvant setting, sorafenib has failed to provide beneficial effects in a randomized, double-blind, placebo-controlled phase III study (STORM trial). As very recently reviewed in detail by Germano et al[144], a multitude of approaches for systemic HCC therapy has failed to show efficacy in the adjuvant or palliative situations, respectively. However, there are many other promising agents, and with the increased understanding of HCC cancerogenesis and cancer-related signaling pathways, molecular targeted therapy is the hope for some breakthroughs in the near future. For example, there are two recent reports for the safety and efficacy of the MET receptor tyrosine kinase inhibitor tivantinib which show promising results[145,146]. The reader might be referred to the excellent article by Germano et al[144] for further detailed information.
Expanding criteria for liver transplantation? So far, liver transplantation is accepted as a curative treatment strategy for patients with a very early stage (0) of HCC who are not suitable for liver resection due to impaired excretory liver function or portal hypertension, and for patients with early stage (A) HCC without concomitant diseases. Since the groundbreaking work by Mazzaferro et al[20] in 1996 defining tumor character limits associated with excellent patient outcome after liver transplantation for HCC, these size limits have been integrated in most guidelines (Eurotransplant, German Bundesärztekammer…). Since other tumor size criteria (e.g., UCSF, up-to-seven) have been demonstrated to show comparable overall and recurrence-free survival rates as the Milan criteria, there is an ongoing intense discussion on the extension of the very strict and limiting Milan criteria towards expanded criteria. However, this will be a relevant matter of debate since expansion of the recipient criteria might lead to a still increasing demand and consumption of the “scarce resource donor organ” and a continuative withdrawal of urgently needed donor organs to patients with other indications for liver transplantation.
Expanding indications for surgery beyond the BCLC criteria? Up to now, resection as most favorable treatment option is only recommended for the very early stage (0) of HCC in patients with normal portal venous pressure and normal serum bilirubin. However, as reviewed in detail by Guglielmi et al[147], the rigid EASL-EORTC limits for liver resection can be expanded to more advanced tumor stages. Tumor size criteria from EASL-EORTC guidelines are not considered as limits for hepatic surgery for example in eastern countries, and especially portal hypertension is not an obstacle against surgery in well selected patients. Thus, acceptable morbidity and mortality rates after hepatic resection in patients with cirrhosis and portal hypertension have been reported which are comparable to those of patients without portal hypertension[148-150].
When expanding the criteria for surgical resection, outcome results of hepatic surgery have to be compared to those of the respective treatment modality recommended by the EASL-EORTC guidelines.
When comparing liver resection vs RFA (for patients with stage A HCC who are not suitable for transplantation), there are several meta-analyses showing that hepatic resection is superior to RFA (or PEI) for HCC with regard to recurrence-free and overall survival[38,140,151,152]. Whereas this superiority of surgery is accepted especially for tumors with diameters > 3 cm, there are some contradictory results for small HCC with a diameter < 3 cm. Interestingly, a cost-effectiveness analysis by Cucchetti et al[153] demonstrated that liver resection for a HCC of 3-5 cm is more cost effective than RFA, whereas in patients with two or three tumor nodules each < 3 cm RFA is superior with regard to cost-effectiveness. Based on these insights, indication for liver resection might be extended to early stages of HCC.
For the intermediate stage of HCC (BCLC B), guidelines recommend TACE as local therapy for tumor control with an expected median overall survival of 20 mo. There are only few data available for this subgroup of patients. But there are data from two retrospective analyses[143,154] and one prospective non-randomized study[155] which clearly demonstrate that in patients with stage B of HCC with preserved liver function liver resection provides an improvement of overall survival rate when compared to TACE. However, the evidence for this is still limited, since there are no meta-analyses available, yet.
When expanding indication for surgery by another step, outcome of patients with advanced stages (stage C) of HCC with portal invasion, lymph node metastases or distant metastases, have to be compared between surgery and palliative systemic chemotherapy. The critical point is for sure the presence or absence of portal infiltration, since macrovascular invasion is known to be one of the most reliable predictors of poor prognosis. However, there are some data showing even for patients with macrovascular invasion a survival benefit after surgery compared to palliative systemic therapy[156]. However, this approach requires patient selection, and achievement of high 5-year survival rates[157] can certainly not generalized in a situation of advanced stages of HCC. But in contrast, macrovascular invasion per se has been demonstrated to allow surgical approach and thus offering a chance of improved survival to patients with otherwise severely limited prognosis[156,158].
Thus, based on the findings cited above, the rigid limits of the EASL-EORTC guidelines will be expanded slowly but constantly. In East Asian treatment algorithms, surgery is of paramount importance even for tumor stages beyond BCLC 0 and A[159]. The non-inferiority or even superiority of surgery compared to the recommended treatment modalities is most probably due to the rapid development of surgical techniques, a more profound understanding of liver anatomy and physiology, improved methods for preoperative risk assessment and liver functional remnant estimation, as well as improved intensive care regimes which overall helped surgery to a striking progress and improvement of patient safety and outcome. Ongoing research and further development in this field of research will still promote the advance of surgery, which will most probably be the most exciting and promising perspective for future HCC therapy.
Significance of HCC-causing underlying disease? There are numerous studies investigating chromosomal aberrations in HCC[160]. When comparing the patterns of chromosomal aberrations in HCC on the basis of different underlying diseases, remarkable differences in the frequency of aberrations have been demonstrated[161]. Thus, one might speculate that different underlying hepatic diseases leading to development of HCC might be associated with different degrees of chromosomal instability in the diseased liver parenchyma. Consequently, underlying disease might also be associated with the pattern of chromosomal aberrations in the respective HCCs and thus might also determine the prognosis of the patient by determining the dynamics of tumor recurrence. Indeed, a recently published large Japanese study including approximately 11950 patients after curative resection of HCC showed that patients with viral hepatitis B or C as underlying disease had a significantly worse overall and recurrence-free survival when compared to patients with non-viral underlying hepatic diseases[162]. This might be of increasing interest in the near future, since patients with different underlying diseases might require a differently close-mesh aftercare.
Since striking medical breakthroughs for the effective curative non-surgical treatment of HCCs are lacking, surgery will play a pivotal role in the multidisciplinary management of patients with HCC in the future. Up to now, surgical treatment is the only therapeutic option that can offer cure to the patient. Even for patients with cirrhosis as a kind of “precancerosis”, liver transplantation offers - within the respective given legal framework - the opportunity to relieve the patient both from the tumor burden and the tumor-favoring disease. Whether a patient can be subjected to liver surgery for HCC and will profit from surgery has to be evaluated carefully in a multidisciplinary context, to offer both best benefit to the patient, minimal risk for complications and procedure-related mortality, as well as best quality of life.
From a surgeon’s point of view, surgery is the central treatment option with regard to tumor treatment, and is thus the most effective therapy to allow the best overall survival and recurrence free survival to patients. Surgery will further gain importance since there are emerging insights that indications for liver resection for HCC can be expanded to tumor staged beyond the actual recommendations. Additional treatment options are both valuable and continuously improving tools for patients who are unsuitable for surgery, and might also further gain importance in multimodal settings with possible perioperative use of percutaneous, intravascular, or pharmacological approaches.
We appreciate the support by Oram D.
| 1. | Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076-1084. [PubMed] |
| 2. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 3. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available from: http://www.seer.cancer.gov/faststats. |
| 5. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20720] [Article Influence: 1883.6] [Reference Citation Analysis (23)] |
| 6. | Simonetti RG, Cammà C, Fiorello F, Politi F, D’Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962-972. [PubMed] |
| 7. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] |
| 8. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [PubMed] |
| 9. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 10. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [PubMed] |
| 11. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] |
| 12. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 13. | Sturgeon CM, Duffy MJ, Hofmann BR, Lamerz R, Fritsche HA, Gaarenstroom K, Bonfrer J, Ecke TH, Grossman HB, Hayes P. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56:e1-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Chen DS, Sung JL, Sheu JC, Lai MY, How SW, Hsu HC, Lee CS, Wei TC. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology. 1984;86:1404-1409. [PubMed] |
| 15. | Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [PubMed] |
| 16. | Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59-v64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 17. | Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] |
| 19. | Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, Brancatelli G, Cammà C, Craxì A, Di Marco V. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol. 2012;4:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 21. | Eun HS, Kim MJ, Kim HJ, Ko KH, Moon HS, Lee ES, Kim SH, Lee HY, Lee BS. The retrospective cohort study for survival rate in patients with advanced hepatocellular carcinoma receiving radiotherapy or palliative care. Korean J Hepatol. 2011;17:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Davila JA, Duan Z, McGlynn KA, El-Serag HB. Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States. J Clin Gastroenterol. 2012;46:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Yeo W, Mo FK, Koh J, Chan AT, Leung T, Hui P, Chan L, Tang A, Lee JJ, Mok TS. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2006;17:1083-1089. [PubMed] |
| 24. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10530] [Article Influence: 585.0] [Reference Citation Analysis (9)] |
| 25. | Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, Justice R, Pazdur R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Barni S. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. a systematic review and pooled analysis. Clin Oncol (R Coll Radiol). 2014;26:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Patrikidou A, Sinapi I, Regnault H, Fayard F, Bouattour M, Fartoux L, Faivre S, Malka D, Ducreux M, Boige V. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Invest New Drugs. 2014;32:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Peck-Radosavljevic M. Drug therapy for advanced-stage liver cancer. Liver Cancer. 2014;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Liccioni A, Reig M, Bruix J. FOLFOX-4 vs. doxorubicin for hepatocellular carcinoma: could a negative result be accepted as positive? J Hepatol. 2014;61:164-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RTP, Han KH, Tak WY. Oral Abstract Session, Gastrointestinal (Noncolorectal) Cancer. J Clin Oncol. 2014;32:5s. |
| 33. | Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol. 2013;27:351-363. [PubMed] |
| 34. | Zhuang L, Zeng X, Yang Z, Meng Z. Effect and safety of interferon for hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2013;8:e61361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;12:CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Lin ZZ, Shau WY, Hsu C, Shao YY, Yeh YC, Kuo RN, Hsu CH, Yang JC, Cheng AL, Lai MS. Radiofrequency ablation is superior to ethanol injection in early-stage hepatocellular carcinoma irrespective of tumor size. PLoS One. 2013;8:e80276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Ni JY, Xu LF, Sun HL, Zhou JX, Chen YT, Luo JH. Percutaneous ablation therapy versus surgical resection in the treatment for early-stage hepatocellular carcinoma: a meta-analysis of 21,494 patients. J Cancer Res Clin Oncol. 2013;139:2021-2033. [PubMed] |
| 39. | Feng Q, Chi Y, Liu Y, Zhang L, Liu Q. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: a meta-analysis of 23 studies. J Cancer Res Clin Oncol. 2015;141:1-9. [PubMed] |
| 40. | Feng K, Ma KS. Value of radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:5987-5998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 41. | Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, Geng WX, Zhou WP. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg. 2014;101:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Eichler K, Zangos S, Gruber-Rouh T, Vogl TJ, Mack MG. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol. 2012;46:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Awad T, Thorlund K, Gluud C. Cryotherapy for hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;CD007611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Rong G, Bai W, Dong Z, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Wang H, Gao X. Cryotherapy for cirrhosis-based hepatocellular carcinoma: a single center experience from 1595 treated cases. Front Med. 2015;9:63-71. [PubMed] |
| 45. | Chen HW, Lai EC, Zhen ZJ, Cui WZ, Liao S, Lau WY. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg. 2011;9:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3436] [Article Influence: 214.8] [Reference Citation Analysis (43)] |
| 47. | Li H, Guo Z, Si T, Wang H. EASL and mRECIST responses are independent predictors of survival in hepatocellular carcinoma patients treated with cryoablation. Eur J Gastroenterol Hepatol. 2013;25:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Schuld J, Richter S, Kollmar O. The role of cryosurgery in the treatment of colorectal liver metastases: a matched-pair analysis of cryotherapy vs. liver resection. Hepatogastroenterology. 2014;61:192-196. [PubMed] |
| 49. | Sheen AJ, Siriwardena AK. The end of cryotherapy for the treatment of nonresectable hepatic tumors? Ann Surg Oncol. 2005;12:202-204. [PubMed] |
| 50. | Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2015;45:59-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [PubMed] |
| 52. | Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8:e76119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Carrafiello G, Laganà D, Ianniello A, Dionigi G, Novario R, Recaldini C, Mangini M, Cuffari S, Fugazzola C. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas Radiol. 2007;51:550-554. [PubMed] |
| 54. | Andreano A, Galimberti S, Franza E, Knavel EM, Sironi S, Lee FT, Meloni MF. Percutaneous microwave ablation of hepatic tumors: prospective evaluation of postablation syndrome and postprocedural pain. J Vasc Interv Radiol. 2014;25:97-105.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, Martinie JB, Iannitti DA, Bloomston M, Schmidt C. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Zhang XG, Zhang ZL, Hu SY, Wang YL. Ultrasound-guided ablative therapy for hepatic malignancies : a comparison of the therapeutic effects of microwave and radiofrequency ablation. Acta Chir Belg. 2014;114:40-45. [PubMed] |
| 57. | Abdelaziz A, Elbaz T, Shousha HI, Mahmoud S, Ibrahim M, Abdelmaksoud A, Nabeel M. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28:3429-3434. [PubMed] |
| 58. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [PubMed] |
| 59. | Petersen J, Henninger B, Glodny B, Jaschke W. [Transarterial chemoembolisation in hepatocellular carcinoma]. Wien Med Wochenschr. 2013;163:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;CD004787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 61. | Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 62. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] |
| 63. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] |
| 64. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [PubMed] |
| 65. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] |
| 66. | Boily G, Villeneuve JP, Lacoursière L, Chaudhury P, Couture F, Ouellet JF, Lapointe R, Goulet S, Gervais N; Comité de l’évolution des pratiques en oncologie. Transarterial embolization therapies for the treatment of hepatocellular carcinoma: CEPO review and clinical recommendations. HPB (Oxford). 2015;17:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Seehofer D, Nebrig M, Denecke T, Kroencke T, Weichert W, Stockmann M, Somasundaram R, Schott E, Puhl G, Neuhaus P. Impact of neoadjuvant transarterial chemoembolization on tumor recurrence and patient survival after liver transplantation for hepatocellular carcinoma: a retrospective analysis. Clin Transplant. 2012;26:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, Nalesnik M, Geller DA, Steel JL, Gamblin TC. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [PubMed] |
| 71. | Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki EI, Verhagen R, Dirsch O, Gerken G, Lang H. Efficacy of transarterial chemoembolization prior to liver transplantation for hepatocellular carcinoma as found in pathology. Hepatogastroenterology. 2005;52:329-332. [PubMed] |
| 72. | Sotiropoulos GC, Malago M, Molmenti EP, Radtke A, Brokalaki EI, Nadalin S, Lang H, Frilling A, Baba HA, Kühl H. Disease course after liver transplantation for hepatocellular carcinoma in patients with complete tumor necrosis in liver explants after performance of bridging treatments. Eur J Med Res. 2005;10:539-542. [PubMed] |
| 73. | Thorat A, Lee CF, Wu TH, Chan KM, Chou HS, Lee WC. Safety of transarterial chemoembolization as bridging therapy in HCC patients with hyperbilirubinemia on the waiting list for liver transplantation: a centre experience. Hepatogastroenterology. 2013;60:2076-2079. [PubMed] |
| 74. | Zhou Y, Zhang X, Wu L, Ye F, Su X, Shi L, Li B. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Cheng X, Sun P, Hu QG, Song ZF, Xiong J, Zheng QC. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol. 2014;140:1159-1170. [PubMed] |
| 76. | Murthy R, Habbu A, Salem R. Trans-arterial hepatic radioembolisation of yttrium-90 microspheres. Biomed Imaging Interv J. 2006;2:e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 78. | Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Kim YH, Kim do Y. Yttrium-90 radioembolization for hepatocellular carcinoma: what we know and what we need to know. Oncology. 2013;84 Suppl 1:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Marin HL, Furth EE, Olthoff K, Shaked A, Soulen MC. Histopathologic outcome of neoadjuvant image-guided therapy of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2009;18:169-176. [PubMed] |
| 81. | Lau WY, Lai EC, Leung TW. Current role of selective internal irradiation with yttrium-90 microspheres in the management of hepatocellular carcinoma: a systematic review. Int J Radiat Oncol Biol Phys. 2011;81:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, Mueller S, Barakat E, Lauenstein T, Bockisch A. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657-664. [PubMed] |
| 84. | Jihye C, Jinsil S. Application of Radiotherapeutic Strategies in the BCLC-Defined Stages of Hepatocellular Carcinoma. Liver Cancer. 2012;1:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Kim SW, Oh D, Park HC, Lim do H, Shin SW, Cho SK, Gwak GY, Choi MS, Paik YH, Paik SW. Transcatheter arterial chemoembolization and radiation therapy for treatment-naïve patients with locally advanced hepatocellular carcinoma. Radiat Oncol J. 2014;32:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Chen WJ, Yuan SF, Zhu LJ, Sun XN, Zheng W. Three-dimensional conformal radiotherapy in combination with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. J BUON. 2014;19:692-697. [PubMed] |
| 87. | Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Lin ZZ, Chou CH, Cheng AL, Liu WL, Chia-Hsien Cheng J. Radiosensitization by combining an aurora kinase inhibitor with radiotherapy in hepatocellular carcinoma through cell cycle interruption. Int J Cancer. 2014;135:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [PubMed] |
| 90. | Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. “State of the art” in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 91. | Yaprak O, Guler N, Altaca G, Dayangac M, Demirbas T, Akyildiz M, Ulusoy L, Tokat Y, Yuzer Y. Ratio of remnant to total liver volume or remnant to body weight: which one is more predictive on donor outcomes? HPB (Oxford). 2012;14:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 93. | Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271-1280. [PubMed] |
| 94. | Lin XJ, Yang J, Chen XB, Zhang M, Xu MQ. The critical value of remnant liver volume-to-body weight ratio to estimate posthepatectomy liver failure in cirrhotic patients. J Surg Res. 2014;188:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Nanashima A, Tobinaga S, Kunizaki M, Miuma S, Taura N, Takeshita H, Hidaka S, Sawai T, Nakao K, Nagayasu T. Strategy of treatment for hepatocellular carcinomas with vascular infiltration in patients undergoing hepatectomy. J Surg Oncol. 2010;101:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Zhou YM, Sui CJ, Li B, Xu F, Kan T, Yang JM. Results of en bloc resection for hepatocellular carcinoma extending to adjacent organs. Can J Surg. 2012;55:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Xiaohong S, Huikai L, Feng W, Ti Z, Yunlong C, Qiang L. Clinical significance of lymph node metastasis in patients undergoing partial hepatectomy for hepatocellular carcinoma. World J Surg. 2010;34:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Cucchetti A, Cescon M, Trevisani F, Pinna AD. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol. 2012;18:6398-6408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Hasegawa K, Kokudo N. Surgical treatment of hepatocellular carcinoma. Surg Today. 2009;39:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 101. | Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y, Zhao G. Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2011;56:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Ye JZ, Miao ZG, Wu FX, Zhao YN, Ye HH, Li LQ. Recurrence after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:1771-1777. [PubMed] |
| 103. | Tang YH, Wen TF, Chen X. Anatomic versus non-anatomic liver resection for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2013;60:2019-2025. [PubMed] |
| 104. | Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor (abstract). Surg Endosc. 1992;6:99. |
| 105. | Takahashi M, Wakabayashi G, Nitta H, Takeda D, Hasegawa Y, Takahara T, Ito N. Pure laparoscopic right hepatectomy by anterior approach with hanging maneuver for large intrahepatic cholangiocarcinoma. Surg Endosc. 2013;27:4732-4733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Yoon YS, Han HS, Cho JY, Ahn KS. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc. 2010;24:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci. 2014;21:E65-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 108. | Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol. 2014;20:8274-8281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 109. | Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu XB, Hu WM. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657-6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 110. | Herman P, Coelho FF. Laparoscopic resection for hepatocellular carcinoma: eastern and western experiences. Chin J Cancer Res. 2014;26:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 111. | Kim HJ, Kim MK. Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience. Chin J Cancer Res. 2014;26:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 112. | Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 113. | Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 114. | Dagher I, O’Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, Lainas P, Laurent A, Nguyen KT, Marvin MR. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 115. | Kluger MD, Vigano L, Barroso R, Cherqui D. The learning curve in laparoscopic major liver resection. J Hepatobiliary Pancreat Sci. 2013;20:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Chan FK, Cheng KC, Yeung YP. Laparoscopic liver resection: lessons learnt after 100 cases. Hong Kong Med J. 2014;20:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg. 2013;205:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 118. | Pelletier JS, Gill RS, Shi X, Birch DW, Karmali S. Robotic-assisted hepatic resection: a systematic review. Int J Med Robot. 2013;9:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 120. | Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392-415. [PubMed] |
| 121. | Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 122. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 123. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [PubMed] |
| 124. | Lai Q, Nudo F, Mennini G, Spoletini G, Morabito V, Levi SG, Melandro F, Guglielmo N, Berloco PB, Rossi M. Expanded criteria for hepatocellular carcinoma after liver transplantation: a 20-year evolution. Hepatogastroenterology. 2013;60:2039-2041. [PubMed] |
| 125. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1617] [Article Influence: 89.8] [Reference Citation Analysis (1)] |
| 126. | Chan SC, Fan ST. Selection of patients of hepatocellular carcinoma beyond the Milan criteria for liver transplantation. Hepatobiliary Surg Nutr. 2013;2:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 127. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [PubMed] |
| 128. | Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368-375; discussion 375-377. [PubMed] |
| 130. | Strong R, Ong TH, Pillay P, Wall D, Balderson G, Lynch S. A new method of segmental orthotopic liver transplantation in children. Surgery. 1988;104:104-107. [PubMed] |
| 131. | Rupertus K, Kollmar O, Scheuer C, Junker B, Menger MD, Schilling MK. Major but not minor hepatectomy accelerates engraftment of extrahepatic tumor cells. Clin Exp Metastasis. 2007;24:39-48. [PubMed] |
| 132. | Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253-260. |
| 133. | Chak E, Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: a systematic review. Liver Int. 2010;30:1247-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 134. | Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, Shang Y, Kong Y, Guo Z, He X. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:1226-1236. |
| 135. | Taefi A, Abrishami A, Nasseri-Moghaddam S, Eghtesad B, Sherman M. Surgical resection versus liver transplant for patients with hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;6:CD006935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Zheng Z, Liang W, Milgrom DP, Zheng Z, Schroder PM, Kong NS, Yang C, Guo Z, He X. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. 2014;97:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Dhir M, Lyden ER, Smith LM, Are C. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford). 2012;14:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 138. | Rahman A, Assifi MM, Pedroso FE, Maley WR, Sola JE, Lavu H, Winter JM, Yeo CJ, Koniaris LG. Is resection equivalent to transplantation for early cirrhotic patients with hepatocellular carcinoma? A meta-analysis. J Gastrointest Surg. 2012;16:1897-1909. [PubMed] |
| 139. | Seshadri RM, Besur S, Niemeyer DJ, Templin M, McKillop IH, Swan RZ, Martinie JB, Russo MW, Iannitti DA. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford). 2014;16:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 140. | Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y, Miao Y. Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol. 2012;10:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 142. | Liu PH, Lee YH, Hsu CY, Hsia CY, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical resection is better than transarterial chemoembolization for hepatocellular carcinoma beyond Milan criteria independent of performance status. J Gastrointest Surg. 2014;18:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 144. | Germano D, Daniele B. Systemic therapy of hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol. 2014;20:3087-3099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 145. | Santoro A, Simonelli M, Rodriguez-Lope C, Zucali P, Camacho LH, Granito A, Senzer N, Rimassa L, Abbadessa G, Schwartz B. A Phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer. 2013;108:21-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 146. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 147. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Vitali M, Bertuzzo F, De Angelis M, Mantovani G, Iacono C. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol. 2014;20:7525-7533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 148. | Ishizawa T, Mise Y, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. Surgical technique: new advances for expanding indications and increasing safety in liver resection for HCC: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 149. | Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) group. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013;33:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 150. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 151. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 152. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 153. | Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 154. | Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, Chen CH. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009;16:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 155. | Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, Shi M. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 156. | Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D’Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 157. | Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 158. | Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 159. | Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, Park JW, Ichida T, Chung JW, Chow P. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81 Suppl 1:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 160. | Homayounfar K, Gunawan B, Cameron S, Haller F, Baumhoer D, Uecker S, Sander B, Ramadori G, Lorf T, Füzesi L. Pattern of chromosomal aberrations in primary liver cancers identified by comparative genomic hybridization. Hum Pathol. 2009;40:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 161. | Homayounfar K, Schwarz A, Enders C, Cameron S, Baumhoer D, Ramadori G, Lorf T, Gunawan B, Sander B. Etiologic influence on chromosomal aberrations in European hepatocellular carcinoma identified by CGH. Pathol Res Pract. 2013;209:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 162. | Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261:513-520. [PubMed] |
P- Reviewer: Chuang WL, Jin B S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/