Published online May 8, 2015. doi: 10.4254/wjh.v7.i7.993
Peer-review started: August 29, 2014
First decision: December 17, 2014
Revised: January 5, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: May 8, 2015
Processing time: 257 Days and 3.2 Hours
Acute kidney injury (AKI) is a frequent clinical event in patients with liver disease, compounding their prognosis. Furthermore, it is likely that the occurrence of AKI has a detrimental impact on the subsequent renal function and the long-term survival of these patients. Recently, some authors advocated the use of new diagnostic criteria for detecting acute kidney injury in patients with cirrhosis. These criteria are based on the rapidity and extent of the creatinine increase comparing to the basal creatinine and also on the kinetics of diuresis decrease. Although their validity in this population requires further studies to be clearly established, these new criteria could have two advantages: (1) to allow earlier diagnosis of AKI and, thus, hepatorenal syndrome for which earlier intervention could improve patients’ survival; and (2) to promote more intensive monitoring of renal function in these patients with high risk of AKI. Finally, recent practice guidelines about the prevention and treatment of general AKI have been published which should be useful in optimising the management of AKI in cirrhotic patients.
Core tip: Acute kidney injury (AKI) is associated with detrimental effect on early survival in hospitalised cirrhotic patients. Due to several hemodynamic modifications, both at the systemic and renal level, induced by cirrhosis, these patients are at increased risk to present acute kidney injury. Recently, new diagnostic criteria have been developed to insure early detection and allow severity assessment of AKI and to optimize the patient’s treatment. The studies available so far, showed that these criteria could have a clinical interest in the management of renal dysfunction but additional data are needed to ascertain their clinical benefit.
- Citation: Rognant N. Acute kidney injury in patients with chronic liver disease. World J Hepatol 2015; 7(7): 993-1000
- URL: https://www.wjgnet.com/1948-5182/full/v7/i7/993.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i7.993
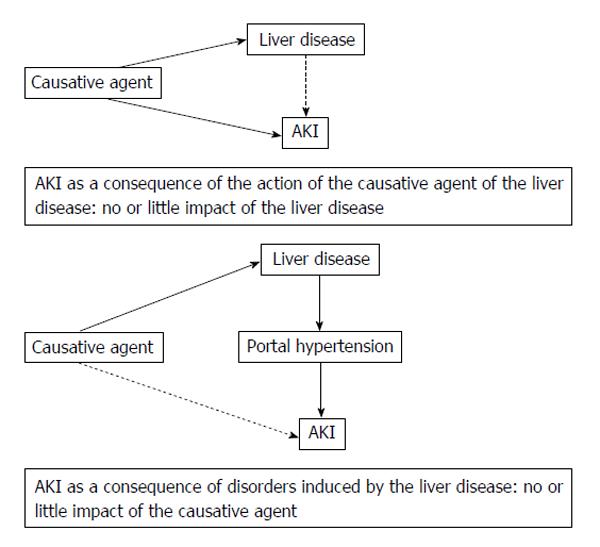
The acute kidney injury (AKI) occurring in patients with liver disease (LD) is due to an external causative agent, with little or no influence of concomitant LD, or is related to the disorders induced by the underlying LD. Because of the elevated frequency of liver cirrhosis, cirrhotic patients are, by far, the largest proportion of patients suffering from LD who experience AKI. Indeed, portal hypertension (PH) is associated with hemodynamic systemic disorders that favour AKI[1]. In this context, the occurrence of AKI negatively impacts the patient’s prognosis. This review will focus on conventional diagnostic criteria of AKI and on new criteria that have been recently proposed in order to diagnose and assess the severity of AKI. It will also address the pathophysiology, prevention and treatment of AKI in patients with cirrhosis.
AKI is defined as a brutal reduction in the renal function, which is generally reversible. Until recently, no standardised criteria existed to define this disease and its severity. This led to some heterogeneity in the clinical situations described as acute renal insufficiency or acute renal failure. This in turn caused difficulty in the interpretation of results, particularly concerning the frequency and the impact of AKI on the patients’ outcomes[2]. During the last decade, an attempt to establish a standard definition, based on evidences arising from the literature was undertaken, leading to the development of the RIFLE classification, published in 2004[3]. The main goal was to gather under the same name (AKI) some clinical situations, which present a quick and potentially reversible decrease of the renal function. Another goal was to stratify AKI by level of severity[2]. Therefore this classification proposes diagnostic criteria for AKI, and a staging system of its severity, based on changes in the levels of three renal markers: the relative increase in serum creatinine (SCr) and/or the relative decrease of the glomerular filtration rate (GFR) in the previous 7 d and the decrease of the diuresis. These RIFLE criteria were then slightly modified to produce the classification RIFLE/AKIN: in this staging system, a new criterion defined as an absolute increase in the SCr (0.3 mg/dL or 26.5 μmol/L) within a period of 48 h was introduced for diagnosing stage 1 AKI and the criterion based on relative decrease of GFR was dropped[4] (Table 1). In a study on patients hospitalised in intensive care units (ICU), the use of this additional criterion allowed to detect patients previously undetected by the RIFLE classification (especially stage 1 AKI). These newly detected patients exhibited double the early mortality rate compared to patients without AKI according to the AKIN classification[5].
| Definition of AKI | Increased SCr × 1.5 the basal value presumably to have occurred within the prior 7 d or GFR decrease > 25% or urine output < 0.5 mL/kg per hour for 6 h | Increased SCr × 1.5 the basal value or ≥ 0.3 mg/dL within 48 h or urine output < 0.5 mL/kg per hour for > 6 h | Increased SCr ≥× 1.5 the basal value presumably to have occurred within the prior 7 d or ≥ 0.3 mg/dL (26.5 μmol/L) within 48 h or urine output < 0.5 mL/kg per hour for 6 h |
| Stage 1 (risk) | Increased SCr × 1.5 the basal value or GFR decrease > 25% or urine output< 0.5 mL/kg per hour for 6 h | Increased SCr × 1.5 to 2 the basal value or ≥ 0.3 mg/dL within 48 h or urine output < 0.5 mL/kg per hour for > 6 h | Increased SCr × 1.5 to 1.9 the basal value or ≥ 0.3 mg/dL or urine output < 0.5 mL/kg per hour for 6-12 h |
| Stage 2 (injury) | Increased SCr × 2 the basal value or GFR decrease > 50% or urine output < 0.5 mL/kg per hour for 12 h | Increased SCr × 2 to 3 the basal value or urine output < 0.5 mL/kg per hour for > 12 h | Increased SCr × 2 to 2.9 the basal value or urine output < 0.5 mL/kg per hour for ≥ 12 h |
| Stage 3 (failure) | Increased SCr × 3 the basal value or GFR decrease > 75% or SCr > 4 mg/dL with acute rise of ≥ 0.5 mg/dL or urine output < 0.3 mL/kg per hour for 24 h or anuria for 12 h | Increased SCr × 3 the basal value or ≥ 4 mg/dL (353.6 μmoL/L) with acute rise ≥ 0.5 mg/dL or initiation of RRT or urine output < 0.3 mL/kg per hour for ≥ 24 h or anuria for ≥ 12 h | Increased SCr × 3 the basal value or ≥ 4 mg/dL (353.6 μmol/L) or initiation of RRT or urine output < 0.3 mL/kg per hour for ≥ 24 h or anuria for ≥ 12 h |
Concerning the clinical relevance of these classifications, various studies on hospitalised patients found an independent link between the occurrence of AKI and a higher patient early mortality, with increasing severity stages of AKI associated with decreased survival. In 2008, a systematic review, including approximately 71000 patients (mostly hospitalised in ICU) showed a relative risk (RR) of mortality of 2.4 in patients diagnosed with stage 1 AKI (also called Risk or R stage) compared to non-AKI patients. Moreover, there was a regular increase in the RR for the more severe stages of AKI: RR = 4.15 for stage 2 (also called Injury or 1 stage) and RR = 6.37 for stage 3 (or Failure or F stage)[6].
Among patients hospitalised for at least 24 h in non-ICU department, Uchino et al[7] showed that the risk of mortality was multiplied by 2.5, 5.1 and 10.4 for patients presenting stages 1, 2 and 3 of the RIFLE classification respectively, compared to patients without AKI. Lastly, by using the classification RIFLE/AKIN in patients hospitalised in ICU, AKI stages 1, 2 and 3 were independently associated with a relative risk of early mortality of 2.07, 1.93 and 2.99 respectively, compared to the patients without AKI[5].
Apart from the impact on early mortality, some studies suggest that AKI is responsible for the increased risk of chronic kidney disease (CKD) incidence or the increased development of an initial CKD[8,9]. Also, it is not excluded that AKI could have a detrimental effect on longer-term mortality[2].
In 2012, the KDIGO international initiative published guidelines concerning the diagnosis, prevention and treatment of AKI in the general population based on an exhaustive review of the literature and the opinion of some experts in the field. The results of the studies cited above (showing the clinical interest of the RIFLE/AKIN classification to predict increased mortality risk in patients with AKI) led the authors to advocate the use of the RIFLE/AKIN staging system, notwithstanding a couple of minor changes in the criteria (Table 1). However, the authors underline some limitations regarding these criteria, in particular the need to confirm their clinical relevance among patients hospitalised in non-ICU departments[2]. In these guidelines, the use of biomarkers other than the SCr is not recommended because the results of studies did not clearly prove their clinical relevance so far.
Apart from the relevance of these criteria in the early diagnosis of AKI, these guidelines stress the importance of the initial evaluation of the risk of developing AKI based on the detection of AKI susceptibility factors (that include the presence of chronic hepatic disease). Finally, they also underline the importance of determining the cause(s) of AKI in order to start specific treatment when this is possible[2].
As the presence of a chronic hepatic disease is a factor of susceptibility for AKI, the patients suffering from such a disease constitute a population with increased risk of developing AKI. Therefore, these patients require reinforced clinical and biological monitoring, particularly during hospitalisation for one or several complications, some of which are well known as triggering factors for AKI[10].
The AKI occurring in patients with cirrhosis or acute liver failure has been the most extensively studied due to the high frequency of this LD[1]. It is estimated that AKI occurs in around 20% of hospitalised cirrhotic patients[11,12]. In the context of cirrhosis or acute liver failure, several modifications of the renal perfusion, induced by PH, occur which considerably increase the sensitivity of the renal perfusion and the GFR to the changes in volemia. These are responsible for a decline in the GFR, even if only minor variations of extra-cellular fluid take place[11,13]. Moreover, these hemodynamic modifications may be accompanied by renal tissue injury related to the renal toxicity of a very high concentration of serum bilirubin, which can promote the development of AKI[14,15]. Consequently, the AKI occurring in the case of PH is often of functional origin. However, the increasing susceptibility to develop AKI is also applicable in the case of renal ischaemic or/and toxic injury, which can cause acute tubular necrosis (ATN). Finally, AKI of obstructive or vascular origin can be found as well, though this is a rare occurrence[10].
Besides these cases in which the underlying LD largely supports the development of AKI, other clinical conditions are associated with both acute hepatic and renal dysfunction, such as the infection by a leptospira[16]. In this case, the dysfunction of both organs shares the same etiologic agent with little or no influence of the LD on the development of AKI (Figure 1). Thus, this is a very different context and, in this review, we will focus on AKI arising in the context of cirrhosis.
In 2011, a working group proposed a revised classification system for diagnosing and assessing the renal dysfunction in cirrhotic patients. This meeting resulted in a publication that proposes to use the term “hepato-renal disorders” in the case of AKI or CKD arising in conjunction with advanced LD[17]. The authors advocate the use of the RIFLE/AKIN classification in order to enhance detection and management of AKI in cirrhotic patients and improve studies regarding pathophysiology, treatment and clinical consequences of AKI. The authors propose a paradigm shift regarding acute renal dysfunction in patients with LD, switching from a classification based on the triad pre-renal azotemia, ATN and hepatorenal syndrome (HRS) to a classification based on the severity of the renal dysfunction quantified with the RIFLE/AKIN criteria (Table 1). The aim of these proposals is to make the clinicians caring for cirrhotic patients aware of the broad spectrum of renal injuries that may arise in these patients because they are often focused on the occurrence of HRS. Indeed, this leads to probable under-recognition of mild AKI with possible detrimental effect on patient’s outcomes. This publication also emphasizes the interest to assess the severity of AKI in order to optimize and initiate promptly the treatment. Finally, besides the potential benefits brought by the RIFLE/AKIN classification, some authors have underlined its possible limitations in patients with cirrhosis[18]. One of these limitations is related to the value of SCr in patients with advanced cirrhosis, which is abnormally decreased compared to the level of GFR, and is a poor marker of the actual renal function. Furthermore, the ability of standardised changes in SCr to account for the actual amplitude of the renal function variations is questionable in these patients.
Several studies assessed the link between AKI and its severity, as diagnosed by the RIFLE or RIFLE/AKIN criteria, and the outcomes of hospitalised cirrhotic patients. In 2009, a study by Cholongitas et al[19] of 412 cirrhotic patients hospitalised in ICU found that the presence of AKI, as diagnosed by the RIFLE criteria, was independently associated with an increase in early mortality (OR = 2.1). Furthermore, in this study, the RIFLE score exhibited the best sensitivity (90%) to predict death in the patients. More recently, Wong et al[20] found a higher mortality in cirrhotic patients hospitalised for infection that developed AKI (according to the RIFLE criteria), compared to infected patients without AKI (34% vs 7%). In addition, the incidence of complications (transfer to ICU, need for mechanical ventilation, etc.) was also increased in patients with AKI. However, in a logistic regression model to determine factors affecting 30-d mortality of these patients, only the outcome of AKI (defined as partial or complete recovery or persistence of renal dysfunction) had an independent effect, with a worse prognosis for patients without recovery of renal function[20]. In another recent study, Belcher et al[21] included cirrhotic patients with AKI to determine the link between severity of AKI and early mortality. Interestingly, these authors have shown that instead of the increased level of severity per se (assessed by the AKIN criteria), the worsening of AKI during hospitalisation was associated with an increased early mortality (adjusted OR = 3.8), suggesting that early detection and treatment of AKI could have a beneficial impact on cirrhotic patients prognosis.
The results of two other studies suggested that a change in the AKIN classification could help to refine the prognosis of cirrhotic patients. Fagundes et al[22] tested the clinical effectiveness of the AKIN classification in hospitalised cirrhotic patients and found a higher 90-d mortality rate in patients with AKI compared to non-AKI patients (most of them presenting stage 1 AKI). However, their results showed that mortality rate was lower in stage 1 patients when it was compared to those with stage 2 or 3 AKI (whereas no difference was seen between patients with stage 2 and those with stage 3). More remarkably, among patients with stage 1 AKI, the mortality rate was significantly different according to peak SCr, with higher mortality rate occurring only when peak SCr > 1.5 mg/dL, while patients with stage 1 AKI and peak SCr below 1.5 mg/dL and non-AKI patients had similar mortality rate[22]. A study by Piano et al[23] compared the use of “conventional” criteria of AKI diagnosis (i.e., incease of SCr by 50% to a level above 1.5 mg/dL) to the new AKIN criteria in predicting hospital mortality in cirrhotic patients. These authors found that patients with stage 1 AKI did not have higher mortality rate (compared to non-AKI patients), while stage 2 or 3 AKI patients did (however with no differences in mortality between stage 2 and stage 3 patients). More importantly, in a multiple logistic regression model to predict the in-hospital mortality, they found that inclusion of AKIN criteria did not improve prediction capacity of the model whereas inclusion of conventional criteria did. Furthermore, the model that presented the best prediction ability included both AKIN criteria and the “progression” status of the AKI (defined as the change to a more severe AKI stage during hospitalisation) with “progressor” patients demonstrating lowest survival. Finally, a peak SCr higher than 1.5 mg/dL was associated with an increase of mortality rate but it was strongly linked with the progression of AKI as well. When this criterion was added to the multivariate model of death prediction, no improvement of the model predictive capacity was seen[23].
Taken together, these studies suggest that using the AKIN classification to detect and assess AKI might have a clinical interest, for example by favouring early recognition of HRS (whereas current diagnostic criteria require a SCr above 1.5 mg/dL)[13]. However, they also suggest that other parameters could be taken into account, in order to strengthen the clinical relevance of this classification, such as peak SCr level or the progression of the AKI severity during hospitalisation. Whether cirrhotic patients with stage 1 AKI and peak SCr below 1.5 mg/dL exhibit increased mortality rate compared to non-AKI patients remains to be determined by further studies. Moreover, whether less severe AKI could impact renal prognosis in these patients has to be established as well. Finally, the true impact on the patients’ prognosis of the cause of AKI should be assessed to avoid its potential confounding role, as suggested by Fagundes et al[22] and also by a study in liver transplant recipients by Nadim et al[24] (in which the cause of AKI was the single factor with independent impact on one-year post-transplantation mortality rate).
The mechanisms involved in the occurrence of AKI in cirrhosis have been extensively studied and are described in several review papers over the past 10 years[10,11,13,16]. Briefly, the primum movens of this disorder is related to the PH, which causes a decrease of effective arterial blood volume by inducing a large splanchnic vasodilation. This is followed by an increase in systemic vasodilation, related to the increased production of vasodilators in splanchnic circulation that reach the systemic circulation through the re-opening of portal-systemic shunts. This systemic vasodilation is responsible of an increase in cardiac output to maintain organs perfusion. However, the systemic and splanchnic vasodilations lead to the activation of sympathetic nervous system and the production of vasoconstrictors that decrease the renal blood flow (RBF). At some stage of the LD, this decline in RBF by renal vasoconstriction is reversible with the improvement of PH. Otherwise, the renal vasoconstriction tends to worsen due to the persistent secretion of several vasoconstrictors and also to an increased sensitivity of the kidney to these vasoconstrictors. Finally, a disturbance of the renal autoregulation also takes place in this context[13]. All these factors explain the extreme sensitivity of the renal function to the changes of volemia. In some instances, a triggering factor may elicit HRS, which can be roughly presented as a functional AKI that does not recover after adequate volume expansion. These modifications of renal hemodynamic also favour intrinsic AKI, caused by nephrotoxic compounds and/or ischemia.
Besides the renal hemodynamic changes induced by PH, the nephrotoxicity of chronic high plasma bilirubin and also some renal histological changes (like IgA glomerular deposits seen in patients with alcohol related LD) are possible worsening factors which could increase the risk of AKI[10].
Type 1 HRS (HRS-1) is an AKI occurring in patients with advanced cirrhosis, which differs from HRS-2 by its rapid occurrence. HRS-1 affects about 7% of hospitalised cirrhotic patients, both HRS-1 and HRS-2 affecting approximately 10% of cirrhotic patients with ascites[11]. A triggering factor can be found in around 50% cases (mostly, a bacterial infection)[10].
The diagnostic criteria of HRS-1 were defined in 2007 (Table 2)[25]. The criteria of HRS-1 include a two-fold increase in initial SCr, which must exceed 2.5 mg/dl (226 μmol/L)[11]. The publication, previously cited, by Wong et al[17] in 2011 suggested a possible interest to amend the HRS diagnostic criteria, particularly concerning the threshold of SCr (set at 1.5 mg/dL), by using the RIFLE/AKIN criteria. In this paper, the authors suggest that the use of these criteria could be clinically relevant because HRS is a progressive disease, that the SCr threshold doesn’t take into account. This leads to ignore patients with less increased SCr though they are already presenting HRS and could receive early treatment and benefit from improved outcomes. In 2011, a study by Boyer et al[26] found that in cirrhotic patients with HRS, initial SCr was an independent predictor of the probability to get a response to terlipressin, and also to survive at 3 mo. In this study, every 1 mg/dL of increase of SCr was associated with an increase of 50% of the risk of death. The usual triggering factors of HRS are the incidence of a bacterial infection or a syndrome of systemic inflammation that, in the context of a hyperdynamic circulatory state due to systemic vasodilation, aggravates the systemic vasodilation and the renal vasoconstriction, leading to a further decrease of the renal perfusion. All causes of effective arterial blood volume decline may also trigger HRS, with the usual causes being the excessive use of diuretics, the occurrence of diarrhoea and/or vomiting and the implementation of a large-volume paracentesis[10]. Therefore, a proper clinical (weight, diuresis and blood pressure) and biological (SCr, urine sodium) monitoring can be particularly useful in these patients.
| Cirrhosis with ascites |
| SCr > 1.5 mg/dL (133 μmol/L) |
| Doubling of basal SCr to a level greater than 2.5 mg/dL (226 μmol/L) in less than 2 wk |
| No improvement in SCr (decrease to 1.5 mg/dL or less) after at least 2 d of diuretic withdrawal and expansion of plasma volume with albumin (1 g/kg body weight/d up to a maximum of 100 g/d) |
| Absence of shock |
| No current or recent treatment with nephrotoxic drugs or vasodilators |
| Absence of parenchymal kidney disease as indicated by proteinuria > 0.5 g/d, hematuria (> 50 red blood cells per high-power field), or abnormal renal ultrasonography |
The presentation of prerenal azotemia is very close to HRS, the main difference is the absence of reversibility of AKI after an adequate expansion of plasma volume. Urinary biochemical abnormalities that can be found are similar, with, unlike ATN, the absence of epithelial cellular cast in the urine. However, in patients with advanced LD and high level of serum bilirubin, bile-stained granular casts may be found in both prerenal azotemia and HRS[11,18]. Regarding the response to volume expansion in prerenal azotemia, the improvement of the renal function may happen at variable speed depending on the initial state of effective volemia (which is difficult to assess correctly in patients with severe cirrhosis). Thus this improvement can be slower in some cases, which can be misleading for the clinician.
It accounts for about one-third to 40% of AKI in cirrhotic patients [10,11]. Toxic injury is the most frequent cause of ATN, but ischemic injury (related to shock or sepsis) is not rare. The differential diagnosis with prerenal azotemia and HRS is based on clinical examination and the analysis of plasma and urine biochemistry. Among the remarkable biochemical differences, there is the level of urine sodium, classically below 10 to 20 mEq/L in HRS and prerenal azotemia, and above 30 mEq/L in ATN. Other changes regard the value of the ratio urine on plasma for creatinine (below 20 for ATN, while above 30-40 for HRS and prerenal azotemia) and osmolarity[13,16]. These differences may however not be present or may be trivial. Furthermore, like HRS, the renal function does not respond to volume expansion in ATN. In this context, the usefulness of the new biomarkers of renal injury is not clearly established and further studies are needed, even if one study found that it could help to distinguish between ATN and other causes of AKI[27]. About the pathophysiology of ATN, ischemic ATN is secondary to extended and critical renal hypoperfusion, whether it is due to a brutal (acute bleeding, for example) or to a gradual further decrease of previously low RBF. The persistence of critical hypoperfusion causes the necrosis and loss of tubular cells with some detaching from the tubular basement membrane. The detached cells can form cellular casts able to obstruct the tubules. The ATN of toxic origin is linked to the administration of potentially nephrotoxic anti-infective agents arising in a context of extreme fragility of the renal perfusion: aminoglycosides, some penicillins and amphotericin B are frequently involved[28]. Contrast induced nephropathy (CIN) is a toxic ATN secondary to iodinated contrast agent’s administration and also a common cause of ATN in cirrhotic patients. The contrast agents, especially when a large volume of a high osmolarity agent is injected, are responsible for a decrease of the RBF, leading to an additional worsening of the renal hypoperfusion. The risk of CIN is increased in patients with CKD and/or diabetes mellitus. However, at least in the general population, the CIN can be effectively prevented by the preliminary correction of an effective hypovolemia. In addition, the administration of N-Acetylcysteine could also help the prevention, as does the use of small volumes of contrast agent and the preferential use of low or iso-osmolar contrast agents[2]. Therefore, this prevention should be implemented in cirrhotic patients because of their high risk to develop CIN.
The main preventive measure of AKI involving low effective blood volume as a mechanism is a careful monitoring of the volemia of hospitalised patients, especially those receiving diuretic therapy and/or treated with large-volume paracentesis[11]. Monitoring should be based on both clinical examination (regular assessment of weight, diuresis and perfusion) and systematic monitoring of renal function and blood and urinary biochemical parameters. In case of significant effective blood volume depletion, treatment with albumin solution (and possibly with crystalloid solution) must be undertaken promptly. Any excessive depletion of the extracellular fluid should be avoided.
About the prevention of ATN, it is based on appropriate prescription of potential nephrotoxic agents. Aminoglycosides are well-known nephrotoxic antibiotics frequently prescribed to treat gram-negative bacilli and are thus largely utilised in cirrhotic patients. KDIGO practice guidelines recommend to select an alternative agent to aminoglycosides whenever possible. If the use of aminoglycosides is required, a “once a day” injection scheme should be preferred whenever possible. From our own experience, we can also suggest that, in the presence of renal dysfunction, monitoring the aminoglycoside blood level in order to adjust the dose is helpful to prevent nephrotoxicity. Finally, the use of NSAIDs in cirrhotic patients, which is known to aggravate renal vasoconstriction, should be avoided whenever possible[2,11].
Concerning the prevention of CIN, several measures have been already cited, including the use of minimal volume of contrast agents and the importance to avoid decreased effective blood volume before the injection[2].
Finally, the prevention of HRS is based on avoiding the events able to trigger it, and also on their rapid treatment when they occur. Spontaneous bacterial peritonitis is a common circumstance that initiates HRS, and some studies suggested that the rapid introduction of a treatment by albumin infusion and antibiotic is effective to prevent HRS[29,30]. The EASL guidelines from 2010 recommend the use of intra-venous albumin in this situation and suggest that the use of norfloxacin might have an interest[31].
Recent clinical practice guidelines made recommendations regarding the treatment of AKI[2] and HRS[31].
For functional AKI, therapeutic measures include discontinuation of diuretics and/or restoration of adequate effective blood volume, which leads to recovery of renal function. Regarding the ATN, there are no specific recommendations for its management in cirrhotic patients. Nonetheless, the recommendations and suggestions from the KDIGO can be used in these patients. Before the stage of dialysis, these recommendations do not advocate to use, for the purpose of renal protection, fenoldopam, nor low-dose dopamine neither diuretic therapy (except in cases of renal sodium overload and non-oliguric AKI). When threatening electrolytes disorders arise or volume overload cannot be adequately controlled by non-dialytic treatment, renal replacement therapy (RRT) should be started rapidly. However, it can be started out of emergency, depending on the overall clinical context. The combination of several factors including, for example, multiple electrolyte disturbances, progressive deterioration of renal function with high level of blood urea nitrogen or increasing difficulty to prevent adequately volume overload can justify to start RRT. According to the KDIGO guidelines, this therapy should be continue for the patients demonstrating hemodynamic instability. Moreover, a bicarbonate-buffered dialysate instead of a lactate-buffered one should be used, particularly in hemodynamically instable patients or in patients with liver failure. Indeed, these two conditions favour the accumulation of lactate (hyperlactatemia), which may worsen both acidosis and hemodynamic instability. Dialysis should be performed without systemic anticoagulation in most of these patients owing to their high risk of bleeding. The preferred vascular access to perform RRT should be a non-tunneled catheter, primarily inserted in the right jugular vein. Finally, it is not recommended to use diuretic therapy in this setting because it does not promote renal recovery[2].
Regarding the management of HRS the recommendations from the EASL assert that furosemide and paracentesis can be used to treat ascites in this setting. However, it contraindicates the use of spironolactone because of a high risk of hyperkalemia. The first-line treatment of HRS-1 should include both the use of a vasoconstrictor and the perfusion of albumin. Among vasoconstrictors, terlipressin is the first choice agent. It must be administered with increasing dose, depending on the evolution of SCr under treatment. Alternatives to terlipressin are norepinephrine or the association midodrine-octreotide. In case of treatment failure, RRT should be started if a worthwhile indication exists. Others treatment options, such as transjugular intrahepatic portosystemic shunt, that improves the effective hypovolemia by increasing the return of blood to the heart, or systems of artificial liver support, based on the adsorption of blood toxic molecules with albumin, are not recommended. Indeed, there is a lack of evidences as regards their clinical benefits. Finally, the standard of care in the case of type HRS-1 remains orthotopic liver transplantation. This should be undertaken after initiation of the treatment of HRS. In case of prolonged renal dysfunction requiring dialysis treatment, a combined transplantation of liver and kidney should be undertaken[31].
The occurrence of AKI in patients with severe cirrhosis is a common event associated with a worsening of the prognosis. This warrants special attention in the monitoring of the renal function in these patients. Using RIFLE/AKIN classification to detect AKI and determine its severity could allow earlier diagnosis of AKI and adaptation of its treatment according to the level of severity. However, the independent association between the gravity of AKI and the early mortality rate still remains to establish in cirrhotic patients, particularly regarding the potential confounding effect of the cause of AKI. In addition, the results of recent studies suggest that this classification could be optimised in cirrhotic patients, by taking into account some other criteria like the increasing severity of AKI during hospitalisation. Lastly, regular monitoring of the renal status in cirrhotic patients is also warranted because it seems likely that the occurrence of AKI is associated with an increased risk of long term CKD development, as in the general population.
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1355] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 2. | KDIGO Clinical Practice Guideline on Acute Kidney Injury. Kidney Int Suppl. 2012;2:6-138. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4781] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 4. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 5061] [Article Influence: 266.4] [Reference Citation Analysis (0)] |
| 5. | Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 535] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 610] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 8. | Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 867] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 9. | Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol. 2012;18:3035-3049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 11. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 462] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Wong F. Acute renal dysfunction in liver cirrhosis. Gastroenterol Hepatol (N Y). 2013;9:830-832. [PubMed] |
| 13. | Davenport A. AKI in a patient with cirrhosis and ascites. Clin J Am Soc Nephrol. 2012;7:2041-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Mairiang P, Bhudhisawasdi V, Borirakchanyavat V, Sitprija V. Acute renal failure in obstructive jaundice in cholangiocarcinoma. Arch Intern Med. 1990;150:2357-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552-5559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 18. | Schrier RW, Shchekochikhin D, Ginès P. Renal failure in cirrhosis: prerenal azotemia, hepatorenal syndrome and acute tubular necrosis. Nephrol Dial Transplant. 2012;27:2625-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Cholongitas E, Calvaruso V, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24:1639-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 20. | Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280-1288.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (3)] |
| 21. | Belcher JM, Parikh CR, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis: perils and promise. Clin Gastroenterol Hepatol. 2013;11:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] |
| 26. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Hampel H, Bynum GD, Zamora E, El-Serag HB. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001;96:2206-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1007] [Article Influence: 37.3] [Reference Citation Analysis (8)] |
| 30. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 456] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 31. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1159] [Article Influence: 72.4] [Reference Citation Analysis (10)] |
P- Reviewer: Sipos F, Waisberg J, Zhang SJ S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/