Published online May 8, 2015. doi: 10.4254/wjh.v7.i7.916
Peer-review started: January 15, 2015
First decision: January 20, 2015
Revised: February 13, 2015
Accepted: March 5, 2015
Article in press: March 9, 2015
Published online: May 8, 2015
Processing time: 120 Days and 2.3 Hours
Recently, important changes have been reported regarding the epidemiology of bacterial infections in liver cirrhosis. There is an emergence of multiresistant bacteria in many European countries and also worldwide, including the United States and South Korea. The classic empirical antibiotic treatment (third-generation cephalosporins, e.g., ceftriaxone, cefotaxime or amoxicillin-clavulanic acid) is still effective in infections acquired in the community, but its failure rate in hospital acquired infections and in some health-care associated infections is high enough to ban its use in these settings. The current editorial focuses on the different epidemiology of bacterial infections in cirrhosis across countries and on its therapeutic implications.
Core tip: There is a growing prevalence of multiresistant bacteria in nosocomial and in health-care associated settings worldwide. Nowadays, it is necessary that all liver units assess the presence of antibiotic resistance in their population. The classical empirical antibiotic therapy, third generation cephalosporins, can no longer be employed in areas with high prevalence of multiresistant bacterial infections. The current editorial focuses on the different patterns of resistance across countries and on its therapeutic implications.
- Citation: Acevedo J. Multiresistant bacterial infections in liver cirrhosis: Clinical impact and new empirical antibiotic treatment policies. World J Hepatol 2015; 7(7): 916-921
- URL: https://www.wjgnet.com/1948-5182/full/v7/i7/916.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i7.916
Liver cirrhosis carries a big burden for health care worldwide. In Europe around 29 million people have a chronic liver disease, and mortality rate is 170000/year. Bacterial infection represents one of the main causes of decompensation. Patients with cirrhosis have alterations in the immune system and therefore they are more susceptible to develop bacterial infection, sepsis, and death[1-5]. Infection is present at admission or develops during hospitalization in around 25%-30% of patients[5-7]. The most frequent infections in cirrhosis are spontaneous bacterial peritonitis (SBP), urinary tract infections (UTI), pneumonia, cellulitis and spontaneous bacteremia[1-3]. Bacterial infections are not only more frequent but also more severe in cirrhosis. Infection increases the probability of death 4 fold reaching 38% at 1 mo[8]. Infection can accentuate the preexisting circulatory dysfunction present in advanced cirrhosis leading to the development of hepatorenal syndrome and can also induce an excessive pro-inflammatory response that could contribute to develop multiple organ failure (acute-on-chronic liver failure) and septic shock[9]. Thus, prompt diagnosis and appropriate treatment of infection is crucial in the management of patients with cirrhosis[10,11].
The most common origin of infection is the community-acquired (CA) setting. The definition of healthcare associated (HCA) infection is infection which occurs previous to admission or during the first two days of hospitalization in patients in contact with the hospital setting during the last 3 mo[7].
Classically, gram-negative bacilli (GNB) accounted for the vast majority of infections (80%), but this preponderance of GNB changed at the start of the new millennium and the prevalence of gram-positive cocci (GPC) increased, accounting for almost half of the infections (47%). When infections were classified according to the origin of infection, GNB were still the most frequent bacteria causing CA infections (60%), while GPC were more prevalent in nosocomial infections (60%). This fact was explained by the increasing degree of instrumentalization of the cirrhotic patient (i.e., variceal ligation, transjugular intrahepatic portosystemic shunt, and arterial chemoembolization or percutaneous ablation of hepatocellular carcinoma), and by the fact that cirrhotic patients with a critical illness are admitted into intensive care units which implies the insertion of central lines and other invasive procedures[6,7].
Furthermore, the need for long term antibiotic prophylaxis, usually with norfloxacin, also carries the risk of emerging of quinolone-resistant and cotrimoxazole-resistant bacteria. Around 50% of GNB were resistant to these antibiotics in a big study performed in 2000[7].
Multiresistant (MR) bacteria are resistant to 3 or more of the principal antibiotic families, including β-lactams[12]. The most common are extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E), Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-susceptible Enterococcus (VSE) and vancomycin-resistant Enterococcus (VRE).
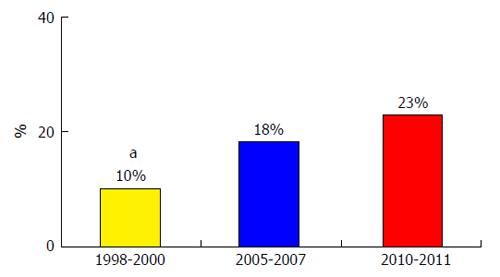
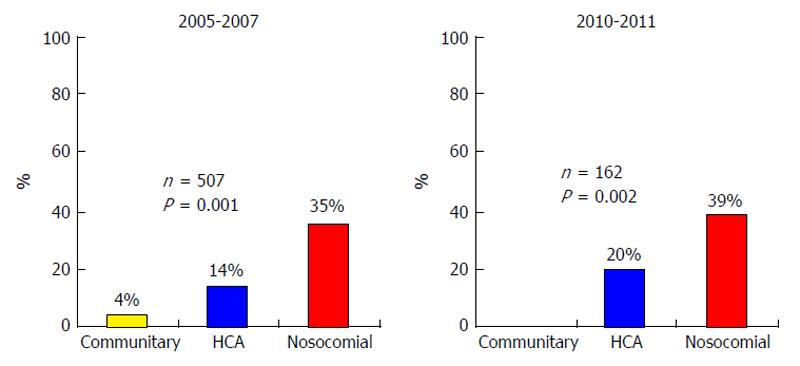
In 2000, only 1.2% of bacteria were resistant to third generation cephalosporins (TGC). Unfortunately, many recent studies from different countries show a growth in the prevalence of MR bacterial infections in cirrhosis. Fernández et al[7] reported a steady growth in the prevalence of MR bacterial infections, this prevalence rose from 10% to 23% during the period 1998-2011 in a single Spanish center[7] (Figure 1). MR bacteria were extremely frequent in the nosocomial setting, causing 35%-39% of infections, but the prevalence was very low in the CA setting, 0%-4%; and prevalence of MR bacteria in the HCA setting was intermediate, between 14% and 20%[7] (Figure 2).
Epidemiology of multiresistance differs among different countries and even among hospitals located in the same area. ESBL-E are predominant in Southern Europe and Asia[7,13-25], meanwhile MRSA and VRE are prevalent in the United States[26]. Carbapenemase-producing K. pneumoniae is a current problem described in some hospitals in Italy[27]. Table 1 shows the prevalence of different MR bacterial infections across countries in the cirrhotic population. The marked epidemiological differences observed among countries and centers suggest that local epidemiology should be evaluated regularly and that empiric antibiotic treatment of nosocomial infections in cirrhosis should be adjusted in accordance with the specific local pattern of multiresistance.
| Type of multiresistant bacteria | Prevalence rate |
| ESBL | South Korea, 4%-29%[13,14,19,20,24] |
| Italy, 8%-20%[17,21] | |
| Spain and United States, 6%-9%[7,15,26] | |
| France, Denmark and Germany, < 5%[22,23,25] | |
| MRSA | Italy, 7%[17,21] |
| United States, 5%[26] | |
| Spain, 3%-4%[7,15] | |
| France, 2%[23] | |
| Denmark, Germany, 0%[22,25] | |
| Pseudomonas aeruginosa | Spain, South Korea, 2%-3%[7,13-15,19,20,24] |
| Germany, 1%[22] | |
| Denmark, France, 0%[23,25] | |
| VSE | Denmark, 12%[25] |
| Germany, 10%[22] | |
| France, 5%[23] | |
| Spain, 1%-7%[7,15] | |
| VRE | United States, 9%[26] |
| Spain, France, Denmark, Germany, 0%[7,15,22,23,25] |
Early and appropriate antibiotic therapy is fundamental in the management of infections in patients with cirrhosis. Since the late 1980’s, TGC have been recommended as empirical antibiotic therapy of the main infections in cirrhotic population because they cover a wide variety of bacteria and they are safe[28-31].
Nevertheless, recent studies demonstrate that β-lactams are not effective in an important part of infections in cirrhosis, especially in nosocomial infections[7]. TGC have scarce efficacy in nosocomial infections (40%). This fact is reproduced in the main types of infections as SBP, UTI and spontaneous bacteremia with efficacies of 26%, 29% and 18%, respectively. Efficacy of empirical antibiotic therapy is also lower in HCA infections compared to community-acquired infections (73% vs 83%), especially in pneumonia and UTI. Other groups from Italy, Germany and Turkey have also reported a reduced efficacy of TGC in SBP, with rates of failure from 18% to 41%[21,22,32].
MR bacterial infections have a poor outcome because they entail a higher incidence of treatment failure (70% vs 92%, P < 0.0001), they lead more frequently to septic shock (26% vs 10%; P < 0.0001), and cause a much higher hospital mortality rate (25% vs 12%; P = 0.001) than those infections produced by susceptible bacteria. The delay in the initiation of an appropriate antibiotic treatment is one of the main explanations for this fact[7].
Risk factors for MR bacteria constitute an important tool to identify the group of infected patients who would benefit from changing the empirical antibiotic therapy in order to cover MR bacteria. The main risk factors for the development of MR bacterial infections in the general population are current or recent hospitalization, healthcare contact (i.e., hemodyalisis) and prior exposure to β-lactams or quinolones[33-37]. The same risk factors have been reported in cirrhosis. Hospital acquired and healthcare associated infections, long-term norfloxacin prophylaxis, recent infection by MR bacteria, recent use of β-lactams within the last 3 mo or systemic antibiotics in the past 30 d, upper gastrointestinal bleeding and diabetes mellitus[7,15,26].
Taking into account the high failure rate of TGC in a large percentage of nosocomial infections and in a subgroup of healthcare associated infections due to the emergence of MR bacteria in these settings, it is clear that empirical antibiotic treatment in cirrhosis should be chosen in accordance with severity and type of infection, and also according to the origin of infection and to the existence of risk factors for MR bacterial infection[38-40]. The AASLD guideline was updated in 2012[41] and an EASL position statement came into light in 2013[42], both recommending substantial changes to empirical antibiotic treatment in the nosocomial setting.
Regular epidemiology assessment should be carried out in all centers and policies regarding empiric antibiotic treatment in cirrhosis should be updated and tailored according to the specific epidemiological pattern of multiresistance in the area.
TGC are still effective in infections acquired in the community in cirrhosis with resolution rates of around 80%[7]. Quinolones should not be used in patients on norfloxacin prophylaxis or in zones with a high prevalence of quinolone-resistant bacteria[1,6]. For the empirical therapy of UTI we can employ trimethoprim-sulfamethoxazole, quinolones or β-lactams. β-lactams are the baseline treatment of pneumonia (in combination with levofloxacin, moxifloxacin or a macrolide) and cellulitis as well[38-42] (Table 2).
| Type of infection | Community-acquired infections | Nosocomial and HCA1 infections | Local epidemiological pattern |
| SBP, SBE and SB | Third generation cephalosporin | Piperacilin/tazobactam | Low prevalence of MR bacteria |
| Or amoxicillin/clavulanic acid | Or carbapenem | ESBL-E | |
| Plus glycopeptides (or linezolid) | MRSA and VSE (when VRE) | ||
| Urinary tract infections | Third generation cephalosporin | Without sepsis: | VSE |
| Or amoxicillin/clavulanic acid | Nitrofurantoin or fosfomycin | ||
| With sepsis: | Low prevalence of MR bacteria | ||
| Piperacilin/tazobactam | |||
| Or carbapenem | ESBL-E | ||
| Or plus glycopeptides (or linezolid) | MRSA and VSE (when VRE) | ||
| Pneumonia | Amoxicillin/clavulanic acid | Piperacilin/tazobactam | Low prevalence of MR bacteria |
| Or ceftriaxone + macrolide | Meropenem or ceftazidime + ciprofloxacin | ESBL-E and P. aeruginosa | |
| Or levofloxacin | Plus vancomycin (or linezolid) | MRSA and VSE (when VRE) | |
| Or moxifloxacin | |||
| Skin and soft tissue infections | Amoxicillin/clavulanic acid | Meropenem or ceftazidime + oxacillin | ESBL-E and P. aeruginosa |
| Or third generation cephalosporin plus oxacillin | Plus glycopeptides (or linezolid or daptomycin) | MRSA and VSE (when VRE) |
Empirical antibiotic therapy should be selected in accordance with the local epidemiological pattern of multiresistance[38-42]. In zones with a high prevalence of ESBL-E, carbapenems should be employed as empirical treatment of spontaneous infections such as SBP, spontaneous bacteremia and spontaneous empyema. A glycopeptide (vancomycin or teicoplanin) should also be added to this empirical treatment in zones with a high prevalence of MRSA or VSE. In the United States and other countries with a high prevalence of VRE, glycopeptides should be substituted for linezolid or daptomycin[38-42]. However, in zones with a low rate of MR bacteria, piperacillin-tazobactam can be employed to treat spontaneous infections[38-42] (Table 2).
Nosocomial UTI without sepsis should be treated with oral nitrofurantoin or fosfomycin. UTI with sepsis should be treated with carbapenems plus glycopeptides to cover ESBL-E and VSE (Table 2)[38-42]. Again, in zones with a scarce prevalence of MR bacteria, piperacillin-tazobactam can be employed in UTI with sepsis (Table 2).
In zones with a high prevalence of MR bacteria, nosocomial cellulitis should be covered against MRSA and Pseudomonas aeruginosa. Thus, ceftazidime or carbapenem plus a glycopeptide can be employed. In areas with low rate of MR bacteria, cloxacillin or amoxicillin-clavulanic acid can be employed[38-42] (Table 2).
Empirical treatment of hospital acquired pneumonia should follow the local guidelines recommended for the general population.
TGC and amoxicillin-clavulanic acid are effective when employed in SBP (resolution rate: 71% compared to 78% in CA infections) or cellulitis (resolution rate: 81% vs 82% in CA infections). However, their efficacy is low in HCA pneumonia (33%) and UTI (59%)[7,38]. Therefore, empirical antibiotic therapy for HCA UTI and pneumonia and for patients with HCA spontaneous infections with two or more risk factors of infection caused by MR bacteria or those patients with severe sepsis or septic shock should follow the same scheme recommended in nosocomial infections (Table 2)[38,40].
In general, de-escalation to the most suitable antibiotic should be done soon after the report from microbiological tests (about 50% of cases) in order to reduce the emergence of antibiotic resistance[38,40].
In conclusion, recent data demonstrate that TGC are not appropriate for the treatment of nosocomial infections in cirrhosis because of the high prevalence of MR bacteria in this setting. New antibiotic strategies for these infections should be adjusted in accordance with the local epidemiological pattern of multiresistance. In areas with high prevalence of ESBL-E, guidelines should include the use of carbapenems. In zones with high prevalence of MRSA and VSE glycopeptides are needed and in zones with high prevalence of VRE linezolid or daptomycin are needed as initial treatment of nosocomial infections in cirrhosis. Early de-escalation of antibiotics according to the microbiological results is also mandatory.
| 1. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 333] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 4. | Acevedo J, Fernández J. New determinants of prognosis in bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Fernandez J, Arroyo V. Bacterial infections in cirrhosis: A growing problem with significant implications. Clinical Liver Disease. 2013;2:102-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 637] [Article Influence: 26.5] [Reference Citation Analysis (16)] |
| 7. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 440] [Article Influence: 31.4] [Reference Citation Analysis (3)] |
| 8. | Fede G, D’Amico G, Arvaniti V, Tsochatzis E, Germani G, Georgiadis D, Morabito A, Burroughs AK. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Pleguezuelo M, Benitez JM, Jurado J, Montero JL, De la Mata M. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J Hepatol. 2013;5:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 12. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 9438] [Article Influence: 629.2] [Reference Citation Analysis (1)] |
| 13. | Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ, Cheong HJ. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community acquired. J Korean Med Sci. 2006;21:666-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 16. | Campillo B, Dupeyron C, Richardet JP, Mangeney N, Leluan G. Epidemiology of severe hospital-acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin Infect Dis. 1998;26:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Park YH, Lee HC, Song HG, Jung S, Ryu SH, Shin JW, Chung YH, Lee YS, Suh DJ. Recent increase in antibiotic-resistant microorganisms in patients with spontaneous bacterial peritonitis adversely affects the clinical outcome in Korea. J Gastroenterol Hepatol. 2003;18:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Song KH, Jeon JH, Park WB, Park SW, Kim HB, Oh MD, Lee HS, Kim NJ, Choe KW. Clinical outcomes of spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: a retrospective matched case-control study. BMC Infect Dis. 2009;9:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, Kim EC, Lee HS, Choe KW. Clinical outcome of bacteremic spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Korean J Intern Med. 2004;19:160-164. [PubMed] |
| 21. | Angeloni S, Leboffe C, Parente A, Venditti M, Giordano A, Merli M, Riggio O. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in the clinical practice. World J Gastroenterol. 2008;14:2757-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 23. | Piroth L, Pechinot A, Minello A, Jaulhac B, Patry I, Hadou T, Hansmann Y, Rabaud C, Chavanet P, Neuwirth C. Bacterial epidemiology and antimicrobial resistance in ascitic fluid: a 2-year retrospective study. Scand J Infect Dis. 2009;41:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 24. | Heo J, Seo YS, Yim HJ, Hahn T, Park SH, Ahn SH, Park JY, Park JY, Kim MY, Park SK. Clinical features and prognosis of spontaneous bacterial peritonitis in korean patients with liver cirrhosis: a multicenter retrospective study. Gut Liver. 2009;3:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Novovic S, Semb S, Olsen H, Moser C, Knudsen JD, Homann C. First-line treatment with cephalosporins in spontaneous bacterial peritonitis provides poor antibiotic coverage. Scand J Gastroenterol. 2012;47:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Piano S, Romano A, Rosi S, Gatta A, Angeli P. Spontaneous bacterial peritonitis due to carbapenemase-producing Klebsiella pneumoniae: the last therapeutic challenge. Eur J Gastroenterol Hepatol. 2012;24:1234-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Felisart J, Rimola A, Arroyo V, Perez-Ayuso RM, Quintero E, Gines P, Rodes J. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462. [PubMed] |
| 29. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [PubMed] |
| 30. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1159] [Article Influence: 72.4] [Reference Citation Analysis (10)] |
| 31. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 32. | Yakar T, Güçlü M, Serin E, Alişkan H, Husamettin E. A recent evaluation of empirical cephalosporin treatment and antibiotic resistance of changing bacterial profiles in spontaneous bacterial peritonitis. Dig Dis Sci. 2010;55:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Apisarnthanarak A, Bailey TC, Fraser VJ. Duration of stool colonization in patients infected with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis. 2008;46:1322-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Azap OK, Arslan H, Serefhanoğlu K, Colakoğlu S, Erdoğan H, Timurkaynak F, Senger SS. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, Azap OK, Arpin C, Pascual A, Livermore DM. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682-690. [PubMed] |
| 36. | Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, Almela M, Almirante B, Grill F, Colomina J. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Cisneros JM, Peña C, Almela M, Almirante B, Grill F, Colomina J. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48:1726-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Acevedo J, Silva A, Prado V, Fernandez J. The new epidemiology of nosocomial bacterial infections in cirrhosis: therapeutical implications. Hepatol Int. 2013;7:72-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Acevedo J, Prado V, Fernández J. Changing options for prevention and treatment of infections in cirrhosis. Curr Treat Options Gastroenterol. 2014;12:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Prado V, Acevedo J, Fernández J. Bacterial infections in cirrhosis: Prevention and treatment. Curr Hepatology Rep. 2014;13:43-49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 532] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 42. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
P- Reviewer: Kovar FM, Virk JS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/