Published online May 8, 2015. doi: 10.4254/wjh.v7.i7.1001
Peer-review started: January 16, 2015
First decision: February 7, 2015
Revised: February 21, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: May 8, 2015
Processing time: 119 Days and 0.9 Hours
AIM: To identify a mean platelet volume (MPV) cutoff value which should be able to predict the presence of bacterial infection.
METHODS: An observational, analytic, retrospective study. We evaluated medical records of cirrhotic patients who were hospitalized from January 2012 to January 2014 at the Gastroenterology Department of “Hospital General de México Dr. Eduardo Liceaga”, we included 51 cirrhotic patients with ascites fluid infection (AFI), and 50 non-infected cirrhotic patients as control group. Receiver operator characteristic curves were used to identify the best cutoff value of several parameters from hematic cytometry, including MPV, to predict the presence of ascites fluid infection.
RESULTS: Of the 51 cases with AFI, 48 patients (94.1%) had culture-negative neutrocytic ascites (CNNA), 2 (3.9%) had bacterial ascites, and one (2%) had spontaneous bacterial peritonitis. Infected patients had greater count of leucocytes and polymorphonuclear cells, greater levels of MPV and cardiac frequency (P < 0.0001), and lower mean arterial pressure compared with non-infected patients (P = 0.009). Leucocytes, polymorphonuclear count, MPV and cardiac frequency resulted to be good or very good predictive variables of presence of AFI in cirrhotic patients (area under the receiving operating characteristic > 0.80). A cutoff MPV value of 8.3 fl was the best to discriminate between cirrhotic patients with AFI and those without infection.
CONCLUSION: Our results support that MPV can be an useful predictor of systemic inflammatory response syndrome in cirrhotic patients with AFI, particularly CNNA.
Core tip: To suspect and recognize promptly those patients with ascites fluid infection (AFI) is crucial. Systemic inflammatory response syndrome and clinical signs are not always present in cirrhotic patients with AFI, and gold standard tests for diagnosis, such as neutrophils count and ascites cultures are not always quickly available in many clinical settings. The mean platelet volume (MPV) is a very good predictor of systemic inflammatory response. A MPV cutoff value equal or greater than 8.3 fl predicts the presence of AFI particularl culture-negative neutrocytic ascites.
- Citation: Gálvez-Martínez M, Servín-Caamaño AI, Pérez-Torres E, Salas-Gordillo F, Rivera-Gutiérrez X, Higuera-de la Tijera F. Mean platelet volume as a novel predictor of systemic inflammatory response in cirrhotic patients with culture-negative neutrocytic ascites. World J Hepatol 2015; 7(7): 1001-1006
- URL: https://www.wjgnet.com/1948-5182/full/v7/i7/1001.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i7.1001
Bacterial infections are considered one of the main cause of death in decompensated cirrhotic patients, they are also related to the development of other complications, such as hepatic encephalopathy, hepatorenal syndrome, and recurrence of variceal bleeding[1,2].
Spontaneous bacterial peritonitis (SBP) is the most common infection in patients with cirrhosis, counting for 10% to 30% of all bacterial infections, and representing around 10% to 50% off in-hospital mortality, meanwhile mortality at 1 year has been reported around 31% to 93%[3,4].
According to the definition of ascites fluid infection (AFI) in the cirrhotic patient there are three main categories: Culture-negative neutrocytic ascites (CNNA) is defined by greater or equal count of polymorphonuclear (PMN) to 250 cells/mm3 but without a positive culture in the appropriate setting, which includes: The fluid must be cultured in blood culture bottles, no previous antibiotic therapy, and no other explanation for an elevated PMN count.
Bacterial ascites is defined as a positive result of culture, without increment of polymorphonuclear cell count and it has been reported in 2%-3% of ambulatory patients and 11% in hospitalized cirrhotic patients.
Spontaneous bacterial peritonitis is found in patients where besides the increase of polymorphonuclear counting, they present a positive result in culture.
CNNA has the same mortality rate as SBP and must be treated promptly[5-10]. The PMN count is not always quickly available in clinical practice and the culture result usually takes 72 h or more, these situations could delay the start of antibiotic therapy, therefore it is important to search biomarkers wide and quickly available that help to predict ascites infection[11-15].
Platelet size is a determinant factor of platelet pro-inflammatory functions[16]. Several studies have found relationship between the mean platelet volume (MPV) and pro-inflammatory conditions, particularly acute infections, such as, pyelonephritis[17] and endocarditis[18,19]. Thus they may predict the severity of sepsis[20]. Recently, two different studies have found increase in MPV levels in cirrhotic patients with AFI and proposed it as an accurate diagnostic test to predict AFI, nevertheless, these two studies differ in their propose cutoff values, and found different sensitivity, specificity and predictive values[21,22]. Therefore, our objective was to determine if there is difference between MPV value in cirrhotic patients without infection and cirrhotic patients with AFI and to identify a MPV cutoff value which could be able to predict the presence of bacterial infection in cirrhotic patients.
Observational, analytic, retrospective study. We evaluated medical records of cirrhotic patients who were hospitalized from January 2012 to January 2014 at the Gastroenterology Department of “Hospital General de México Dr. Eduardo Liceaga”, México City, we included 51 cirrhotic patients who met the diagnosis of SBP, CNNA and bacterial ascites according to the latest guidelines of the American Association for the Study of Liver Disease[10], and we included 50 non-infected cirrhotic patients as control group. We excluded patients with conditions that could affect the MPV value, such as other bacterial infections, diabetes, hypertension, history of cerebrovascular event, cardiac failure, dyslipidemia, recent hemorrhage, or antibiotic use in the previous month.
We also collected the following data: age, gender, cause of cirrhosis, Child-Pugh class, parameters which are part of the systemic inflammatory response syndrome (SIRS); such as, cardiac frequency, respiratory frequency, temperature, leukocytes count. Additionally we addressed the mean arterial pressure (MAP) and we collected other relevant data from the hematic cytometry report: PMN count, MPV, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH).
The distribution of numerical variables was analyzed trough kurtosis, asymmetry and Kolmogorov-Smirnov test, in case of non-normal distribution we performed base 10 logarithmic transformation. Numerical variables were expressed as median and standard deviation, and categorical variables were expressed as proportion and percentages. To compare between groups, student’s t test, chi square test or Fisher’s exact test were used as appropriate. Receiver operator characteristic (ROC) curves were used to identify the best cutoff value of several parameters from hematic cytometry, including MPV, to predict the presence of AFI.
The statistical methods of this study were reviewed by Fatima Higuera de la Tijera, MD, MSc. from “Hospital General de Mexico, Dr. Eduardo Liceaga’’.
We included 101 cirrhotic patients, 50 of them were used as controls without bacterial infection (group A), 51 of them were cases with AFI (group B), of them, 48 patients (94.1%) had CNNA, 2 (3.9%) had bacterial ascites, and one (2%) had SBP. Eighty eight patients were women who corresponded to 87.1% of our study population.
The media and standard deviation of age was 55.7 ± 10.3 years. According to Child-Pugh class, 59 patients (58.4%) were class C, 33 patients (32.7%) were class B, and 9 patients (8.9%) were class A. The most frequent cause of cirrhosis was alcohol intake, other etiologies are summarized in Table 1.
| Variable | Group An= 50 | Group Bn= 51 | P | 95%CI |
| Age (yr) | 57 ± 9.8 | 55 ± 10.8 | 0.30 | -1.9 to 6.2 |
| Gender (female), n (%) | 35 (70) | 27 (53) | 0.08 | |
| Child-Pugh A/B/C | 9/26/15 | 0/7/44 | < 0.0001 | |
| Cause of cirrhosis: | ||||
| Alcohol | 18 | 27 | 0.34 | |
| NASH | 9 | 4 | ||
| CHC | 13 | 9 | ||
| Autoimmune | 2 | 2 | ||
| Cryptogenic | 8 | 9 |
When we compared between groups the different parameters of the SRIS and parameters from the hematic cyotmetry, patients in the group B had greater count of leucocytes and PMN, also greater levels of MPV, MCV, MCH and cardiac frequency (P < 0.0001). Furthermore, patients in the group B had lower MAP compared with patients in the group A (P = 0.009). Other hematological and clinical variables which are part of the SRIS, such as, temperature and respiratory frequency were not different between groups (Table 2).
| Variable | Group An= 50 | Group Bn= 51 | P | 95%CI |
| Leukocytes (103/mcl) | 4.9 ± 2.1 | 12.4 ± 7.2 | < 0.0001 | -9.6 to -5.4 |
| PMN (103/mcl) | 3.0 ± 1.6 | 10.7 ± 6.8 | < 0.0001 | -9.6 to -5.7 |
| Platelets (103/mcl) | 106.7 ± 56.0 | 110.0 ± 69.0 | 0.80 | -28.1 to 21.6 |
| MPV (fl) | 7.7 ± 0.5 | 9.0 ± 0.8 | < 0.0001 | -1.5 to -0.9 |
| Hemoglobin (g/dL) | 11.6 ± 2.5 | 11.1 ± 2.2 | 0.33 | -0.5 to 1.4 |
| MCV(fl) | 89.7 ± 13.3 | 98.5 ± 9.4 | < 0.0001 | -13.3 to -4.3 |
| MCH (pg) | 30.1 ± 4.3 | 33.0 ± 3.7 | < 0.0001 | -4.5 to -1.3 |
| RDW (%) | 17.7 ± 3.1 | 18.3 ± 4.6 | 0.47 | -2.1 to 1.0 |
| MAP (mmHg) | 81.9 ± 10.3 | 74.7 ± 16.1 | 0.009 | 1.8 to 12.5 |
| CF (beats/min) | 73 ± 16 | 96 ± 18 | < 0.0001 | -30.0 to -16.0 |
| RF (breaths/min) | 20 ± 2 | 24 ± 14 | 0.04 | -8.4 to -0.2 |
| Temperature (°C) | 36.4 ± 0.5 | 36.7 ± 0.8 | 0.12 | -0.5 to 0.6 |
| SIRS, n (%) | 0 (0) | 38 (74.5) | < 0.0001 | NA |
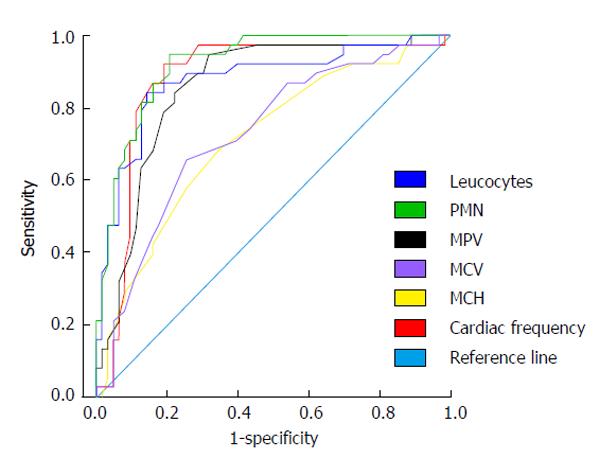
The variables that were different between groups: leucocytes and PMN count, MPV, MCV, MCH, and cardiac frequency (P < 0.0001) were tested trough ROC curves. From all of them, leucocytes and PMN count, MPV and cardiac frequency resulted to be good or very good predictive factors of presence of AFI in cirrhotic patients [area under the receiving operating characteristic (AUROC) > 0.80]. MCV and MCH were regular predictors of presence of AFI in cirrhotic patients (AUROC > 0.60 and < 0.75) (Figure 1 and Table 3).
| Variable | AUROC | 95%CI | P |
| Leukocytes | 0.89 | 0.82-0.95 | < 0.0001 |
| PMN | 0.94 | 0.90-0.99 | < 0.0001 |
| MPV | 0.90 | 0.84-0.96 | < 0.0001 |
| MCV | 0.73 | 0.63-0.83 | < 0.0001 |
| MCH | 0.72 | 0.61-0.82 | < 0.0001 |
| CF | 0.86 | 0.78-0.94 | < 0.0001 |
We determined sensitivity, specificity, positive predictive value, negative predictive value and accuracy for different cutoff MPV values. A cutoff MPV value of 8.3 fl was the best to discriminate between cirrhotic patients with AFI and those without infection (Table 4).
| Cutoff value (fl) | Sensibility % (95%CI) | Specificity % (95%CI) | PPV % (95%CI) | NPV % (95%CI) | Accuracy |
| 8.0 | 92 (84-100) | 62 (48-76) | 71 (60-83) | 89 (77-100) | 77 (69-86) |
| 8.1 | 88 (78-98) | 78 (66-90) | 80 (69-92) | 87 (76-98) | 83 (75-91) |
| 8.2 | 84 (73-95) | 80 (68-92) | 81 (70-93) | 83 (72-95) | 82 (74-90) |
| 8.3 | 84 (73-95) | 82 (70-94) | 83 (72-94) | 84 (72-95) | 83 (75-91) |
| 8.4 | 78 (66-91) | 88 (78-98) | 87 (76-98) | 80 (69-91) | 83 (75-98) |
| 8.5 | 76 (64-89) | 88 (78-98) | 87 (76-98) | 78 (67-90) | 82 (74-90) |
AFI is a severe condition with a high mortality rate if is not diagnosed and treated promptly. Although, gold standard for diagnosis is based on the determination of PMN cells count equal or greater than 250 cells/mm3 of ascites, with or without a positive culture[9,10], in many settings the results from these gold standard tests are not quickly available, causing the delay in the diagnosis and early treatment. For this reason, other methods, more rapid and widely available have been proposed; nevertheless, some of them, such as the use of reagent strips have a low diagnostic accuracy for the diagnosis of AFI[23]. In addition, many patients with AFI may have not classic symptoms or signs of peritonitis[6,23] nor develop classic manifestations of sepsis. Several characteristics of the cirrhotic patients may difficult the diagnosis of SIRS and sepsis, for example: Baseline reduced PMN count due to hypersplenism, baseline elevated heart rate because of the hyperdynamic circulatory syndrome, baseline hyperventilation due to hepatic encephalopathy or blunted elevation of body temperature that is often observed in cirrhotic patients[24]. In our study we found that although temperature and respiratory frequency are variables which integrate the SIRS, there were not differences between them in patients with and without AFI. On the other hand, patients with AFI had greater count of leucocytes and PMN, but also greater levels of MPV, MCV, MCH and cardiac frequency, and they had also lower MAP compared with patients without AFI.
In our study, leucocytes and PMN count, MPV and cardiac frequency resulted to be good or very good predictive variables of presence of AFI in cirrhotic patients (AUROC > 0.80). Leucocytes and PMN count and cardiac frequency are variables already well known predictors of systemic inflammatory response, however, the novelty of our study is that the MPV resulted on being an additional variable of great utility to predicting systemic inflammatory response. Several conditions, such as, thrombotic events, endothelial dysfunction and inflammation are associated with platelet activation and consumption[25,26]. Besides their hemostatic function, platelets also play a role in recruiting neutrophils to sites of injury and infection; platelets also help to the activation of neutrophils via engagement of neutrophil and endothelial cell receptors and release of chemokines[27].
There are two previous studies conducted in patients with cirrhosis and AFI, which demonstrated that MPV could be a predictor of SIRS. In the first study by Suvak et al[21], the authors found that MPV increased the response to AFI in patients with cirrhosis, compared with those patients without AFI and with healthy controls. These authors proposed a cutoff value of 8.45, with a sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) of 70.7%, 67.5%, 75.4% and 62.1% respectively (area under the curve: 0.768). A recently study by Abdel-Razik et al[22], also found a greater level of MPV in cirrhotic patients with AFI compared with cirrhotic patients without AFI and healthy controls. These authors found that MPV had 95.9% sensitivity and 91.7% specificity for detecting AFI (area under the curve: 0.964) at a cutoff value of 8.77. In our study, we tested different cutoff values, and we found that the best was the cutoff value of 8.3 fl, with sensitivity, specificity, PPV, NPV and accuracy of 84%, 82%, 83%, 84% and 83% respectively.
Our results support that MPV can be an useful predictor of SIRS in cirrhotic patients with AFI, particularly CNNA, better than other variables such as temperature or respiratory frequency.
Bacterial infections are considered one of the main causes of death in decompensated cirrhotic patients, being the spontaneous bacterial peritonitis the most common infection, clinical data and laboratories, such as polymorphonuclear count, or ascites culture, are not always available and close to 30% of patients remain asymptomatic. A diagnosis and timely treatment of the infection of ascites fluid is able to reduce the mortality rate of 80% to 20%. Hence the reason for using non invasive markers, that are fast and easy to apply, which can help to predict the development of ascites infection.
Platelet size is a determinant factor of platelet pro-inflammatory functions. Several studies have found relationship between the mean platelet volume (MPV) and pro-inflammatory conditions, particularly acute infections, such as, pyelonephritis and endocarditis.
Recently, two different studies have found increase in MPV levels in cirrhotic patients with ascites fluid infection (AFI) and proposed it as an accurate diagnostic test to predict AFI, nevertheless, these two studies differ in their propose cutoff values, and found different sensitivity, specificity and predictive values, being that the main reason why the authors attempted to determine if there is any difference between MPV value in cirrhotic patients without infection and cirrhotic patients with ascites fluid infection and to identify a MPV cutoff value, able to predict the presence of bacterial infection in cirrhotic patients.
The study results suggest that MPV can be an useful predictor of systemic inflammatory response syndrome in cirrhotic patients with AFI, particularly culture-negative neutrocytic ascites (CNNA), better than other variables such as temperature or respiratory frequency.
According to the definition of spontaneous bacterial peritonitis there are three main categories: CNNA is defined by greater or equal count of polymorphonuclear to 250 cells/mm3 but without a positive culture. Bacterial ascites is defined as a positive result of culture, without increment of polymorphonuclear cell count. Spontaneous bacterial peritonitis is found in patients where besides the increase of polymorphonuclear counting, they present a positive result in culture.
Interesting study.
| 1. | Strauss E. The impact of bacterial infections on survival of patients with decompensated cirrhosis. Ann Hepatol. 2014;13:7-19. [PubMed] |
| 2. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [PubMed] |
| 4. | Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Castellote J, Girbau A, Maisterra S, Charhi N, Ballester R, Xiol X. Spontaneous bacterial peritonitis and bacterascites prevalence in asymptomatic cirrhotic outpatients undergoing large-volume paracentesis. J Gastroenterol Hepatol. 2008;23:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Jeffries MA, Stern MA, Gunaratnam NT, Fontana RJ. Unsuspected infection is infrequent in asymptomatic outpatients with refractory ascites undergoing therapeutic paracentesis. Am J Gastroenterol. 1999;94:2972-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 510] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1159] [Article Influence: 72.4] [Reference Citation Analysis (10)] |
| 10. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 11. | Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol. 2004;41:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Stassen WN, McCullough AJ, Bacon BR, Gutnik SH, Wadiwala IM, McLaren C, Kalhan SC, Tavill AS. Immediate diagnostic criteria for bacterial infection of ascitic fluid. Evaluation of ascitic fluid polymorphonuclear leukocyte count, pH, and lactate concentration, alone and in combination. Gastroenterology. 1986;90:1247-1254. [PubMed] |
| 13. | Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082-1088. [PubMed] |
| 14. | Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012;32:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Koulaouzidis A, Leontiadis GI, Abdullah M, Moschos J, Gasem J, Tharakan J, Maltezos E, Saeed AA. Leucocyte esterase reagent strips for the diagnosis of spontaneous bacterial peritonitis: a systematic review. Eur J Gastroenterol Hepatol. 2008;20:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Thompson CB, Jakubowski JA, Quinn PG, Deykin D, Valeri CR. Platelet size as a determinant of platelet function. J Lab Clin Med. 1983;101:205-213. [PubMed] |
| 17. | Tekin M, Konca C, Gulyuz A, Uckardes F, Turgut M. Is the mean platelet volume a predictive marker for the diagnosis of acute pyelonephritis in children? Clin Exp Nephrol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Cho SY, Jeon YL, Kim W, Kim WS, Lee HJ, Lee WI, Park TS. Mean platelet volume and mean platelet volume/platelet count ratio in infective endocarditis. Platelets. 2014;25:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Icli A, Tayyar S, Varol E, Aksoy F, Arslan A, Ersoy I, Akcay S. Mean platelet volume is increased in infective endocarditis and decreases after treatment. Med Princ Pract. 2013;22:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Catal F, Tayman C, Tonbul A, Akça H, Kara S, Tatli MM, Oztekin O, Bilici M. Mean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants. Clin Lab. 2014;60:1193-1200. [PubMed] |
| 21. | Suvak B, Torun S, Yildiz H, Sayilir A, Yesil Y, Tas A, Beyazit Y, Sasmaz N, Kayaçetin E. Mean platelet volume is a useful indicator of systemic inflammation in cirrhotic patients with ascitic fluid infection. Ann Hepatol. 2013;12:294-300. [PubMed] |
| 22. | Abdel-Razik A, Eldars W, Rizk E. Platelet indices and inflammatory markers as diagnostic predictors for ascitic fluid infection. Eur J Gastroenterol Hepatol. 2014;26:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thévenot T, Silvain C, Bureau C, Nouel O, Pilette C, Paupard T, Vanbiervliet G, Oberti F, Davion T, Jouannaud V, Roche B, Bernard PH, Beaulieu S, Danne O, Thabut D, Chagneau-Derrode C, de Lédinghen V, Mathurin P, Pauwels A, Bronowicki JP, Habersetzer F, Abergel A, Audigier JC, Sapey T, Grangé JD, Tran A. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275-1281. [PubMed] |
| 24. | Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 285] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 836] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 26. | Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2003;497-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Andrews RK, Arthur JF, Gardiner EE. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb Haemost. 2014;112:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
P- Reviewer: Allam N, Li YY S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/