Published online Nov 28, 2015. doi: 10.4254/wjh.v7.i27.2716
Peer-review started: June 29, 2015
First decision: July 28, 2015
Revised: October 25, 2015
Accepted: November 13, 2015
Article in press: November 17, 2015
Published online: November 28, 2015
Processing time: 152 Days and 14.9 Hours
Inflammatory bowel disease (IBD) is composed of Crohn’s disease and ulcerative colitis and is manifested by both bowel-related and extraintestinal manifestations. Recently the number of therapeutic options available to treat IBD has dramatically increased, with each new medication having its own mechanism of action and side effect profile. A complete understanding of the hepatotoxicity of these medications is important in order to distinguish these complications from the hepatic manifestations of IBD. This review seeks to evaluate the hepatobiliary complications of non-steroid based IBD medications and aide providers in the recognition and management of these side-effects.
Core tip: Recently the number of medical therapies for inflammatory bowel disease (IBD) has greatly increased. Each medication has its own mechanism of action and side effect profile. This review article discusses the hepatic side effects of medications used to treat IBD enabling physicians to better recognize and manage these complications.
- Citation: Hirten R, Sultan K, Thomas A, Bernstein DE. Hepatic manifestations of non-steroidal inflammatory bowel disease therapy. World J Hepatol 2015; 7(27): 2716-2728
- URL: https://www.wjgnet.com/1948-5182/full/v7/i27/2716.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i27.2716
Inflammatory bowel disease (IBD) is primarily composed of Crohn’s disease (CD) and ulcerative colitis (UC). The North American incidence is 19.2 cases per 100000 person years and 20.2 cases per 100000 person years for UC and CD, respectively[1]. Through a combination of genetic and environmental factors, IBD appears to be caused by an inappropriate and overactive response of the body’s immune system directed primarily at the gastrointestinal tract. Though most disease manifestations are bowel-related, multiple extraintestinal manifestations including dermatologic (pyoderma gangrenosum, erythema nodosum), ophthalmic (uveitis and episcleritis), joint (large and small joint arthritis and sacroiliitis) as well hepatobiliary complications may occur. Additionally, biliary and hepatic manifestations are common, with up to 29% of IBD patients developing abnormal liver tests[2].
Recently the number of therapeutic options available to treat IBD has increased dramatically. Current guidelines emphasize a move away from short term corticosteroid based treatment and towards IBD targeted medications proven to either induce and/or maintain clinical remission. Each new class of medication has its own mechanism of action and side effect profile. While multiple reviews have evaluated the hepatobiliary manifestations of IBD, few have focused in detail on the hepatic side effects of these medications. A complete understanding of the prevalence, characteristics and management of the hepatoxicity of these medications is important to distinguish these effects from IBD itself, limit their toxicity and maximize their therapeutic benefit. This review evaluates the hepatobiliary complications, mainly drug induced liver injury (DILI), of currently available non-steroid based IBD therapies.
Sulfasalazine is a pro-drug composed of 5-aminosalicylic acid (5-ASA) linked to sulfapyridine by an azo bond, and has been used in the treatment of IBD for over 70 years[3]. The primary efficacy of sulfasalazine in IBD is through the 5-ASA moiety and although the exact mechanism of action is not known, 5-ASA has been shown to have an array of anti-inflammatory properties including effects on reactive oxygen species, nuclear factor kappa B, and cytokines[4-6]. Though mainly demonstrating benefit for UC, it is commonly used for CD. Common side effects include headache, nausea, dyspepsia, or allergic reactions, and are generally attributed to the sulfa component. The overall incidence of liver toxicity has been estimated to be between 0.4%-2.9%, depending upon the study group and underlying disease[7,8]. Though most liver test abnormalities are minor and reversible with drug discontinuation, previous studies have described severe hepatotoxicity to sulfasalazine due to a systemic hypersensitivity reaction characterized by high fevers, generalized lymphadenopathy, a maculopapular rash, elevated liver enzymes, eosinophilia and immune complexes[9]. Granulomatous hepatitis has been described in patients on sulfasalazine presenting with an elevated alkaline phosphatase and bilirubin along with the presence of granulomas on liver biopsy. This has been described in patients without CD and appears to be medication rather than disease related[10-12]. Also reported are rare cases of fatal fulminant hepatic necrosis from drug rash with eosinophilia and systemic symptoms, a devastating complication with an estimated mortality of 10% if not appropriately diagnosed and treated with high dose corticosteroids[13-20].
Due to adverse effects of the sulfapyridine moiety, 5-ASA formulations without sulfa, the mesalamine derivatives, were developed in the 1970s, and are now more widely used than sulfasalazine. A systematic review of the short-term adverse effects of mesalamine show that this class of medication is generally well tolerated, with rates of adverse events similar to placebo controls[21]. The most common side effects associated with mesalamine include flatulence, nausea, headache, dyspepsia, and diarrhea. Despite the absence of the sulfa moiety and limited systemic absorption of mesalamine, a randomized trial comparing mesalamine to sulfasalazine showed rates of DILI to be similar between the two medications, with 2.6% of patients taking mesalamine experiencing liver injury[8]. One case report described possible drug-induced autoimmune hepatitis in a patient with CD treated with mesalamine. This patient developed chronic hepatitis with evidence of fibrosis on liver biopsy, as well as an elevated anti-nuclear antibody and anti-smooth muscle antibody, which resolved with discontinuation of the medication[22]. Other case reports have linked mesalamine to systemic hypersensitivity reactions similar to sulfasalazine as well as to the development of hepatocellular cholestasis[23,24].
When signs of liver injury develop secondary to sulfasalazine or mesalamine, the dosage should be decreased or the medication stopped completely depending upon the severity of the liver test elevation and the patient’s condition. While no formal guidelines exist specifically for sulfasalazine or mesalamine, cases suspicious for DILI should be evaluated and managed according to currently accepted guidelines[25].
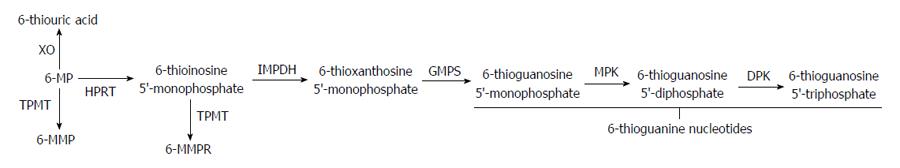
The thiopurine immunomodulators azathioprine (AZA) and its principle metabolite, 6-mercaptopurine (6-MP), have shown efficacy in the maintenance of steroid induced remission for both CD and UC[26]. AZA is converted to its active metabolite 6-MP through non-enzymatic reactions by compounds such as glutathione. Intracellular metabolism of 6-MP is dictated by the activity of several enzymatic pathways resulting in both the therapeutically active metabolite 6-thioguanine (6-TG), which is associated with myelosuppression, as well as the non-therapeutic metabolites 6-methylmercaptopurine (6-MMP) and 6-methylmercaptopurine ribonucleotide (6-MMPR) which have been associated with hepatotoxicity (Figure 1)[27].
AZA and 6-MP have been shown to result in both a hepatocellular and cholestatic hepatitis, with a number of studies evaluating the incidence of liver test abnormalities occurring in IBD patients treated with these medications. A large study of 3931 patients from the prospective Spanish nationwide database found that 4% of patients on thiopurines developed hepatotoxicity[28]. Another study that followed 786 patients with IBD, of whom 138 received either AZA or 6-MP, found that 7.1% of those treated per patient year developed elevated liver tests that were 1-2 times the upper limit of normal. Approximately 2.6% of those treated per patient year developed liver tests greater than twice the upper limit of normal[29]. A large systematic review of 3485 patients from 2007 evaluating the incidence of liver injury in patients with IBD exposed to AZA or 6-MP showed an overall prevalence of abnormal liver tests of 3.3%. When follow-up time was included, 2992 patients received treatment during a total of 6952 years of follow-up, with the average rate of drug-induced liver test elevation being 1.4% per patient year. These liver test abnormalities were generally noted to occur within the first few months of drug initiation[30].
The primary hepatic manifestations include hypersensitivity reactions, cholestasis, peliosis hepatitis, Disse space fibrosis, veno-occlusive disease and nodular regenerative hyperplasia (NRH)[31]. In hypersensitivity syndromes, symptoms usually develop within 2-3 wk of initiation with an elevated bilirubin and alkaline phosphatase, as well as with moderate elevations of aminotransferases. At the histologic level, parenchymal cell necrosis is noted[30,31]. NRH, peliosis hepatitis, fibrosis, and veno-occlusive disease have been shown to be dose-dependent injuries and likely secondary to damage to the endothelial cells lining the sinusoids and terminal hepatic venules[30,32]. This is often noted between 3 mo and 3 years of initiating treatment[30].
Thiopurine methyltransferase (TPMT) activity plays an important role in determining AZA and 6-MP metabolism and the development of side effects. Approximately 0.3% of the population has low or absent activity, 11% of the population has intermediate activity as they are heterozygote’s, and 89% of the population possess the wild type with normal activity[33]. In general, 6-TG levels greater than 230-260 pmol/8 × 108 erythrocytes are associated with clinical efficacy, while levels over 450 pmol/8 × 108 erythrocytes are associated with bone marrow toxicity. Though high levels of 6-TG can also result in liver damage, particularly the development of NRH, most liver toxicity is believed to be related to 6-MMP and 6-MMPR, particularly at levels over 5700 pmol/8 × 108 erythrocytes[27,30,34].
Though no commercial testing is available for high TPMT activity, this appears to occur in up to 15% of the population with a preferential formation of the hepatotoxic 6-MMP and 6-MMPR metabolites[35]. Despite this association, routine monitoring for 6-MMP and 6-MMPR is not widely recommended. In patients who develop abnormal liver tests associated with the overproduction of 6-MMP and 6-MMPR the thiopurine may be stopped, the dose reduced or allopurinol may be used to inhibit xanthine oxidase. While an examination of the metabolic pathways involved would suggest this approach to result in an increase of both 6-TG and 6-MMP/6-MMPR levels, in fact, the effect is one of preferentially increased 6-TG over 6-MMP/6-MMPR. Typically AZA or 6-MP dosing is cut to one third or half the prior dose when used in conjunction with allopurinol, and the patient’s blood work monitored closely.
As no formal guidelines exist, and most cases of liver toxicity occur within the first few months of therapy, we recommend following the protocol outlined by the United States Food and Drug Administration (FDA) (http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205919s000lbl.pdf), which suggests checking serum transaminase levels, alkaline phosphatase, and bilirubin levels weekly for the first month[30]. Following this initial therapy we agree with repeat testing monthly for the next 3 mo, then every three months thereafter, following along with the currently recommended monitoring of white blood cell counts. This sequence should be repeated with any dose increase. It is common for patients to have a slight elevation of liver enzymes with normalization on repeat testing. However, if elevations become persistent or marked, the dose of AZA or 6-MP may be reduced in half with continued close monitoring, or by finer dose adjustments guided by metabolite levels. With normalization, careful resumption of the previous dose can be reattempted[30]. In one prospective study that evaluated this method, slightly less than half of patients were able to resume their previous dosage[36]. Patients whose liver tests fail to normalize should have the medication discontinued. If severe cholestatic jaundice occurs, the medication should be withdrawn with continued close monitoring of the liver tests for improvement. Patients should consider undergoing liver biopsy if liver tests remain persistently elevated after medication withdrawal[30].
Methotrexate (MTX) was discovered in the 1940’s and was first used to treat leukemia in 1948. It is a structural analogue of folate which competitively inhibits the enzyme dihydrofolate reductase and thereby the production of the intracellular metabolite folinic acid and the synthesis of purines and pyrimidines. Clinically, it has demonstrated effectiveness in the treatment of CD but not UC[37,38]. Generally, it is given intramuscularly at a dose of 25 mg weekly in combination with another medication used to induce remission, such as steroids or a biologic agent. Once a clinical response is observed, patients are switched to subcutaneous or intramuscular injections at a dose of 15 mg weekly. Multiple side effects have been reported including infections, pneumonitis, bone marrow suppression and liver test abnormalities.
There is extensive literature relating to MTX’s effects on the liver with significant variability in reports of liver tests abnormalities and histologic changes on biopsy. While the mechanism resulting in hepatotoxicity is not fully understood, it is likely related to MTX induced folate deficiency, as supplementation has been shown to reduce hepatic adverse events[39,40]. Much of the evidence relating to its heptotoxicity comes from its use in rheumatoid arthritis and psoriatic arthritis, where it has been shown to have a variety of effects on the liver ranging from hepatic steatosis to fibrosis. The CORRONA database is a large cohort of patients which followed 2104 patients with rheumatoid arthritis and psoriatic arthritis exposed to methotrexate. Overall, approximately 22% of patients exposed to MTX with rheumatoid arthritis developed an elevated aspartate aminotransferase (AST)/alanine aminotransferase (ALT), while 35% of patients with psoriatic arthritis exposed to MTX had an AST/ALT > 1 × the upper limit of normal[41].
Similar results were found in patients with IBD on chronic low dose MTX. A retrospective study evaluating 87 subjects with a mean duration of therapy of 81 wk and an average dose of 1813 mg found that 24% of patients developed elevated liver tests. Of these patients, 44% had underlying risk factors for liver disease. While 23% of patients in the study had abnormal liver tests at baseline, 45% had normalization while on MTX, while 45% experienced a worsening of their liver tests[42]. A meta-analysis evaluating 12 studies of 457 children with IBD exposed to MTX found that 10.2% of patients developed abnormal liver biochemistry with 6.4% of patients requiring dose reduction and 4.5% of patients requiring discontinuation of the medication[43].
Beyond elevated liver tests, long term MTX use may lead to the development of fibrosis and cirrhosis. The histologic feature of methotrexate-induced liver toxicity tends to resemble nonalcoholic steatohepatitis[44]. Earlier studies reported up to a 50% chance of developing fibrosis, though there is concern that this may be an overestimation secondary to confounding factors[45-47]. A more recent meta-analysis of 15 studies examined the relationship between long term low-dose MTX and biopsy evidence of liver fibrosis. It showed that patients have a 6.7% chance of progressing at least one histologic grade of fibrosis for every gram of MTX taken and that the rate of progression of liver disease was associated with the cumulative dose of the medication. In addition, it revealed a 5.0% chance of having advanced pathologic changes on biopsy[48].
In general, measures to prevent or identify hepatotoxic effects should be employed prior to chronic use and risk factors for the development of hepatoxicity identified. These risk factors, created by the American Academy of Dermatology, allow for stratification and guidance in how aggressive liver enzymes should be monitored and whether a patient should be considered for liver biopsy[44]. Patients should be ruled out for chronic viral infections such as hepatitis B and hepatitis C, and educated to avoid hepatotoxic agents. In addition, all patients should undergo periodic monitoring of their liver tests. While there are no formal recommendations for laboratory monitoring by gastrointestinal societies, the American College of Rheumatology recommends monitoring liver tests every 2-4 wk for the first 3 mo, then every 8-12 wk for the next 3 mo. After 6 mo of therapy liver tests should be checked every 12 wk[49].
With regards to liver biopsy, the most recent set of American College of Gastroenterology guidelines recommend consideration of a biopsy prior to initiation of MTX in patients with elevated liver tests at baseline, patients with one or more risk factor for hepatotoxicity and in patients who are suspected to have chronic liver disease[26]. Given the lack of biopsy data in patients with CD, current guidelines defer to the American Rheumatology Associations guidelines which recommend a liver biopsy for patients who develop elevations in AST in 5 of 9 blood samples in a 12-mo period, 6 of 12 samples if performed monthly, or if there is a decrease in serum albumin below the normal range[26,50]. The role for current noninvasive technologies such as transient or MRI elastography, or serum non-invasive markers of fibrosis remains unclear.
Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine that plays a role in the immunopathology of IBD[51]. Infliximab (REMICADE®) is a chimeric monoclonal anti-TNF-α antibody which was first approved to treat CD in 1998 and UC in 2005[52]. Its most common side effects are headache, rash and cough, while infusion reactions have been seen in 5% of patients[53]. In addition, through its immunosuppressive properties, it has been associated with an increased risk of infection based on large cohort studies[54].
Infliximab has been associated with several forms of DILI. A large, prospective cohort study found that 6.7% of patients treated with infliximab experienced hepatocellular cytolytic injury, with mild elevations in AST/ALT > 1 × the upper limit of normal, but discontinuation rates were not significant compared to those who did not have liver enzyme elevations[55]. In another retrospective cohort study, 6% of patients, the vast majority who were on low-dose infliximab, developed mild idiopathic liver enzyme elevations. In up to 50% of these patients, other possible causes (medication, alcohol, metabolic, viral) were identified. The mean time to ALT elevation was 29 wk, and resolution occurred after a median of 17 wk in 82% of patients despite continuation of therapy[56]. In this study, a small subset of patients were found to have positive autoimmune markers, and underwent liver biopsies which demonstrated features of autoimmune hepatitis, requiring cessation of the initial anti-TNF-α treatment.
Although infliximab has been used in several studies to treat refractory autoimmune hepatitis, autoimmune hepatitis due to infliximab is becoming an increasingly recognized form of hepatocellular injury[57-59]. In a review of 34 cases of DILI secondary to TNF-α antagonists, 16/26 patients treated with infliximab had autoimmune antibodies or liver biopsies suggestive of autoimmune hepatitis, with interface hepatitis, piecemeal necrosis, and portal lymphocytic inflammation with plasma cells[60]. Patients with autoimmune features had a latency period of 16 wk before liver injury was apparent and higher peak ALT values. In the reported cases, patients often recovered with discontinuation of infliximab and corticosteroid therapy.
Cholestatic liver injury has been described in several case reports of patients treated with infliximab. Patients developed jaundice after infliximab infusions, with markedly elevated alkaline phosphatase and bilirubin. One patient who underwent liver biopsy demonstrated bland cholestasis. Cholestatic liver injury resolved in most cases within 6-8 wk with supportive therapy and withdrawal of infliximab[61,62]. In a severe case of acute cholestatic hepatitis after eight months of treatment with infliximab for rheumatoid arthritis, one patient progressed to liver failure requiring liver transplant. This patient’s liver biopsy showed ductal proliferation with collapse and enucleation of the hepatocytes[63].
Apart from DILI, infliximab has also been shown to cause reactivation of hepatitis B, in patients with a positive surface antigen (HBsAg) and patients with anti-core antibodies (HBcAb) without HBsAg. In a systematic review of HBsAg-positive patients, 2/14 patients treated with infliximab experienced hepatitis B reactivation, characterized by jaundice with elevated liver enzymes and a positive HBV DNA[64]. The outcomes for patients with reactivation range from recovery with lamivudine treatment to fatal fulminant hepatitis[65]. Patients with
HBsAg-positive or anti-HBc positive serology are considered to be at moderate risk (1%-10%) for reactivation (Table 1)[66]. Given the poor outcomes associated with hepatitis B and the availability of prophylactic antivirals, both the AGA and AASLD recommend screening for hepatitis B with HBsAg and anti-HBcAb prior to initiating therapy[66,67]. If a positive result is obtained, HBV DNA should be checked before initiating prophylaxis. Antiviral prophylaxis has been associated with an 87% relative risk reduction of reactivation and an 84% relative risk reduction of HBV-associated hepatitis flares. Tenofovir or entecavir is recommended for long-term antiviral prophylaxis given high resistance rates with lamivudine. In contrast to the experience with hepatitis B, hepatitis C has not been shown to be affected by infliximab usage, and screening for hepatitis C is not currently recommended.
| Risk group | Medication | Hepatitis B virus status |
| High risk (> 10%) | Corticosteroids > 4 wk (moderate-high dose) | HBsAg+/HBcAb+ |
| Moderate risk (1%-10%) | TNF-α inhibitors | HBsAg+/HBcAb+ (1%-10%) |
| Ustekinumab | HBsAg-/HBcAb+ (1%) | |
| Vedolizumab | ||
| Natalizumab | ||
| Corticosteroids > 4 wk | HBsAg+/HBcAb+ (1%-10%) (low dose) | |
| HBsAg-/HBcAb+ (1%) (moderate-high dose) | ||
| Low-risk (< 1%) | Azathioprine | HBsAg+/HBcAb+ |
| 6-mercaptopurine | HBsAg-/HBcAb+ | |
| Methotrexate |
Adalimumab (HUMIRA®) is a fully human monoclonal anti-TNF-α antibody, and was FDA-approved for use in CD in 2007 and UC in 2012. It has also been associated with DILI, though less seldom than infliximab. In a population-based study from Iceland, the absolute risk of DILI with adalimumab was reported to be 1/270 patients, compared to 1/120 infliximab users[68]. Idiosyncratic liver enzyme elevations can be seen with adalimumab, albeit at a lower incidence than infliximab. In a study of 1753 IBD patients treated with anti-TNF therapy, 33% of patients were initiated on adalimumab; however, only 6% of the 102 patients with elevated liver enzymes were adalimumab users (three times the number on infliximab)[56]. Resolution of mild liver enzyme elevations generally occurred despite continued use of adalimumab.
Two cases of drug-induced autoimmune hepatitis have been reported in patients being treated with adalimumab, including one patient with CD[69,70]. Liver enzyme elevations and autoantibodies were seen after 10-12 wk of treatment. Liver biopsies in both patients showed typical morphologic features of autoimmune hepatitis. Both patients recovered with cessation of the medication and initiation of corticosteroids. Interestingly, two cases of patients who developed autoimmune hepatitis with infliximab with subsequent treatment with adalimumab have also been described in the literature. In both cases, autoimmune hepatitis resolved after infliximab was discontinued, with no evidence of recurrence after adalimumab was initiated, suggesting that DILI from one anti-TNF-α treatment does not preclude therapy with another drug in the same class[71,72].
One case of severe cholestatic injury due to adalimumab has been reported[73]. A female patient with CD being treated with adalimumab developed severe jaundice after seven months. Bilirubin and alkaline phosphatase were elevated, and liver biopsy demonstrated cannalicular cholestasis associated with large intracannicular bile thrombi throughout the parenchyma without other significant inflammation. Spontaneous recovery was seen 5 wk after discontinuation of adalimumab.
As with infliximab, adalimumab has been implicated in hepatitis B reactivation, including fatal hepatic failure in a patient with clinically resolved hepatitis B infection[74,75]. Consequently, hepatitis B screening and prophylaxis is recommended prior to starting patients on adalimumab. Certolizumab and golimumab are the remaining anti-TNF-α treatments which have been FDA-approved for use in CD and UC, respectively. Due to limited use, data on hepatoxicity is scarce, though the same recommendations for hepatitis B screening apply.
In addition to direct drug-related injury or injury by viral reactivation, there is evidence of an increased risk of malignancy affecting the liver in patients on TNF-α treatment. Nineteen cases of hepatosplenic T-cell lymphoma (HSTCL) have been associated with infliximab use[76]. This rare but aggressive malignancy is an extranodal form of non-Hodgkin’s lymphoma that predominantly affects young males, and often has a poor prognosis. Presenting symptoms include hepatomegaly, splenomegaly, systemic B-type symptoms, elevated liver enzymes and signs of hepatitis. The exact causal relationship between infliximab and HSTCL is unclear, as patients were also treated with azathioprine, 6-mercaptopurine, or steroids. More long-term studies are needed to delineate the relationship between infliximab and HSTCL.
The use of anti-integrin therapy for moderate to severe UC or CD that is refractory to anti-TNF agents or immunomodulators has increased with the approval of vedolizumab in May 2014. Integrins modulate the adhesion of leukocytes to endothelial cells and are responsible for a key step in the inflammatory cascade whereby activated leukocytes anchor to the endothelial wall prior to transmigration. Two anti-integrin therapies are currently approved to treat IBD, natalizumab and vedolizumab, while a third, ertolizumab, is currently in phase 3 testing.
Natalizumab (TYSABRI®) is a monoclonal antibody directed to the alpha-4 integrin. Initially used for multiple sclerosis, it was subsequently approved to induce and maintain clinical remission in patients with moderate to severe active CD in patients who failed conventional therapies and anti-TNF-α agents[77,78]. Its use has been significantly limited in CD due to morbidity and mortality in postmarketing cases of progressive multifocal leukencephalopathy that developed secondary to the JC virus. Initial trials examining natalizumab’s efficacy in treating IBD did not mention hepatotoxicity[77,79-82]. Generally elevation of aminotransferases have occurred in approximately 5% of patients on natalizumab compared with 3%-4% of controls[83,84]. In the post marketing setting, clinically significant liver injury, including liver failure, has been reported with at least 59 cases of hepatic injury reported to the FDA’s Adverse Event Reporting System. In a report of 6 cases of liver injury, there was a significant hepatocellular component with ALT’s reaching 2212 IU/L in one patient. Serum bilirubins ranged from 4.5-16 g/dL and occurred as early as 6 d after treatment. In addition, there were varying degrees of autoantibodies that were positive[85]. The etiology of the liver injury that occurs is unclear but is likely immunologically mediated.
Vedolizumab (ENTYVIO®) and ertolizumab are newer anti-integrin therapies with less data relating to their hepatotoxic effects. Vedolizumab is a monoclonal antibody to the α4β7 integrin that modulates lymphocyte migration into the gut. In the phase 3 trials examining its efficacy, three patients developed elevated transaminases with or without bilirubin elevation after 2-5 doses of vedolizumab. One further case was reported in the open labeled trial. Some patients developed autoantibodies and were treated with systemic steroids[86-90]. Currently, screening for hepatitis B prior to treatment is not routinely recommended.
Ertolizumab is a monoclonal antibody that binds the β7 subunit of α4β7 and αEβ7 integrins. Phase 1 and phase 2 studies in UC have been performed while phase 3 studies are underway. No reported adverse events related to the liver have been reported[91,92].
Ustekinumab (STELARA®) is a human IgG1k monoclonal antibody that targets interleukin (IL)-12 and IL-23 activity by blocking its receptors[93]. It was approved in 2009 for use in moderate to severe psoriasis and psoriatic arthritis. While it has demonstrated benefit in patients with CD, it currently does not have FDA approval for this indication. In the two published trials evaluating its efficacy in CD, there were two hepatobiliary disorders reported as biliary colic and cirrhosis, which did not differ significantly in number from the control group[94,95]. Elevated liver enzymes were a relatively rare occurrence, occurring in up to 1.4% of patients through 64 wk of follow up in one study. In patients treated for up to 5 years with ustekinumab for moderate-severe psoriasis, hepatobiliary disorders were rare, representing only 0.2 events per 100 patient-years for up to 5 years of follow up[96-98].
IL-12 plays an important role in controlling hepatitis B viral infections through its facilitation of type 1 T helper lymphocytes and by inhibiting HBV replication through interferon-gamma production[99-101]. Given its mechanism of action, reactivation rates between 1%-10% would be expected (Table 1)[66]. There is therefore a theoretical risk of reactivation of hepatitis B with the use of ustekinumab. One retrospective study in patients with psoriasis reported 11 patients who where hepatitis B surface antigen positive and surface antibody negative who were started on ustekinumab. Out of the 7 patients in whom prophylaxis was not given, two experienced reactivation. Of the 4 patients in whom prophylaxis was initiated, no cases of reactivation were observed[101]. Assuming a 5% rate of reactivation of hepatitis B in patients exposed to ustekinamab, prophylaxis would result in 44 fewer reactivation events per 1000 patients treated and should be considered[66].
Cyclosporine prevents the activation of T-lymphocytes by inhibiting the production and release of IL-II. It is used to induce remission in refractory UC and serve as a bridge to medications such as AZA or 6-MP for maintenance therapy. Traditionally given as an intravenous infusion at a rate of 4 mg/kg, the lower 2 mg/kg dose has been shown to be equally effective with fewer reported side effects[102-104]. Over 80% of steroid refractory patients will respond to therapy and in many cases, cyclosporine provides an alternative to colectomy[105]. While side effects such as nephrotoxicity, hypertension and infections are more prevalent, hepatotoxicity is rare.
Animal studies evaluating its effects on the liver reveal increased bile acid and bilirubin concentrations when given at high doses, with a corresponding reduction of the biliary secretion of bile acids, cholesterol and phospholipids. Cholestasis was secondary to a decrease in bile acid dependent and independent bile flow fractions[106,107]. Case series that evaluated dosages ranging from 2-10 mg/kg per day found mild abnormalities in liver tests consistent with mild increases in alkaline phosphatase levels as well as slight increases in bilirubin and aminotransferases[108]. In addition, cyclosporine has been reported to increase the incidence of cholelithiasis[109]. Earlier studies evaluating cyclosporine use in post-renal transplant patients reported evidence of hepatotoxicity in 20%-82% of patients, characterized by transaminase elevations as well as elevations in bilirubin and alkaline phosphatase, which were dose responsive[110-112]. In IBD patients, the rate of liver tests abnormalities is closer to 1%-4% of patients, with a recent systematic review of the literature reporting only 11 cases of elevated liver tests in IBD patients treated with cyclosporine[113,114].
Though their benefits are not well defined in prospective randomized trials, antimicrobials have long been used as either adjunctive therapy for IBD complications such as CD related abscesses, or as the primary therapy in pouchitis, an IBD like condition occurring in surgically constructed continent jejunal or “J-pouch” reservoirs following total colectomy for UC. Since current IBD models suggest that even normal bowel bacteria may play a role in initiating and propagating the aberrant immune response typical of IBD, the appeal of antibiotics is straight forward; that decreasing bacterial concentrations may lessen this immune stimulation and thus decrease the inflammatory response. Though there is some evidence of benefit to this approach in fistulizing peri-anal and colonic CD, a clearer benefit in UC is lacking. Currently, antibiotic therapy for UC is only recommended for end-stage cases of toxic megacolon, when bacteremia is common.
The two most commonly used antibiotics are metronidazole and ciprofloxacin, either individually or in combination. Metronidazole is a synthetic nitroimidazole derivative used to treat both anaerobic bacteria and protozoa, generally dosed orally at 10 to 20 mg/kg per day. Ornidazole, another nitroimidazole derivative not currently available in the United States, has also been studied for the treatment of CD. Though metronidazole is often given intravenously when utilized for inpatients with CD related abscesses, most clinical experience in CD is with oral use. While dose adjustment is recommended in patients with severe liver impairment, primary hepatotoxicity is extremely rare. Cases of DILI have been reported, typically presenting with a hepatocelluar injury pattern several weeks after the initiation of therapy[115,116]. Small prospective trials of metronidazole and ornidazole in CD have reported elevations of liver enzymes, that did not significantly differ from the placebo group[117,118].
Ciprofloxacin is a member of the fluoroquinolone class of antibiotics whose mechanism involves inhibition of DNA gyrase within sensitive bacteria, preventing the relaxation of supercoiled DNA and subsequent breakage of double stranded DNA. Minor elevations of serum AST and ALT related to ciprofloxacin use is quite common, affecting approximately 1% of users. A recent large study of a Veterans Administration population of 7862 patients with fluoroquinolone exposure matched against 45512 controls, each group excluding underlying liver disease, showed an increased risk of hepatotoxicity in exposed compared to unexposed patients, odds ratio (OR) = 1.20; 95%CI: 1.04-1.38[119]. Much rarer cases of severe hepatocellular, cholestatic or a mixed pattern of injury have been reported with fluoroquinolones, which are among the group of antibiotics most commonly associated with this complication. Clinical trials of ciprofloxacin for the treatment of CD have not shown any significant rates of liver injury, but given the small size of these studies it is not possible to determine whether there is any disease specific associated liver risk[120,121].
Finally, though CD is not widely considered to be caused by a specific infection, there is a possible association of CD with the organism Mycobacterium avium paratuberculosis (MAP), which has been found in the serum and tissue of some CD patients. Though there has been modest success with antimicrobial treatment regimens directed at MAP, it is unclear whether this success is related to its effect on MAP or a general alteration of the bowel flora. While isoniazid (INH) has an anti-mycobacterial effect, and has been used as part of MAP directed therapy, the common usage of INH in individuals with IBD is not as a direct therapy, but as a co-therapy to prevent reactivation of mycobacterium tuberculosis (TB) in individuals with suspected latent infection treated with anti-TNF-α therapy[122,123]. In the United States population the risk of latent TB has been estimated at 4%, with significant variations depending upon the region and socioeconomic status[124]. Current prescribing guidelines recommend testing for latent TB prior to initiation of biologic therapy with anti-TNF-α agents[125]. In patients at risk for latent TB, at least one month of INH therapy is suggested prior to the initiation of biologic therapy[126].
INH inhibits the synthesis of mycolic acid, an essential component of the bacterial cell wall, and is commonly associated with DILI. Mild elevations of serum transaminases occur in up to 20% of INH treated patients, and usually requires close monitoring without discontinuation of therapy. Traditionally more severe, and potentially fatal INH hepatitis has been directly associated with increasing patient age, ranging from a rate of 0.3% in individuals 20 to 34 years of age, and as high as 4.6% in those over age 65 years manifesting as an acute hepatocellular pattern. Though, more recent studies have suggested that rates may be as low as 0.1%[127,128]. Fatality rates once hepatitis develops have been reported as high as 10%, and are worse with increasing age, though overall fatality rates in those treated and monitored following current American Thoracic Society guidelines appear to be very low[129,130]. Current guidelines recommend routine monitoring of patients treated with INH prophylaxis who are at higher risk of underlying liver disease such as those with chronic viral hepatitis, recent pregnancy, and regular alcohol consumption. However, monitoring may be considered for anyone greater than 35 years of age. Suggested intervals of AST, ALT, and bilirubin testing are at baseline, then as often as every 4 wk while on therapy. Discontinuation of therapy should be considered for an ALT > 5 × the upper limit of normal, or > 3 × the upper limit of normal with associated nausea, vomiting, abdominal pain, jaundice, or unexplained fatigue[131].
While both patients and physicians alike may look forward to a true “cure” for illness, the management of chronic medical conditions typically requires the use of medications, often for an indefinite length of time. This is certainly true for the management of IBD. The fact that IBD is itself an immune related disease, requiring immune suppressing or modifying therapies, for long periods of time, further increases the side effect potential of these treatments. IBD medications are prominent on the list of well described agents causing DILI, as well as other hepatobiliary manifestations, each with their own prevalence and management strategy (Table 2)[25]. While the data on newer therapies appears encouraging with regard to DILI specifically, further post marketing experience will be needed to determine the exact nature of this risk and appropriate prophylactic and management strategies.
| Medication | Prevalence of liver injury | Manifestation | Management1 |
| Sulfasalazine | 0.4%-2.9% | Mild liver test abnormalities | |
| Severe systemic hypersensitivity | |||
| Granulomatous hepatitis | |||
| DRESS syndrome | |||
| 5-ASA | Approximately 2.6% | Mild liver test abnormalities | |
| Drug induced autoimmune hepatitis | |||
| Systemic hypersensitivity reaction | |||
| AZA/6-MP | 1.4%-7.1% | Hypersensitivity reaction | Slight liver test elevation → |
| Cholestasis | Monitor | ||
| Peliosis hepatitis | |||
| Disse space fibrosis | Persistent elevation → | ||
| Veno-occlussive disease | Consider stopping medication/reducing dose | ||
| Nodular regenerative hyperplasia | Marked elevation → | ||
| Hepatosplenic T-cell lymphoma | Stop medication | ||
| Methotrexate | Approximately 10.2% | Elevated liver tests | |
| Fibrosis | |||
| Anti-TNF-α agents | 0.37%-6.7% | Hepatocellular injury | |
| Cholestasis | |||
| Hepatosplenic T-cell lymphoma | |||
| Autoimmune hepatitis | |||
| Anti-integrin therapy | < 5% | Hepatocellular injury | |
| Cholestasis | |||
| Autoimmune hepatitis | |||
| Liver failure | |||
| Ustekinumab | 1.4% | Elevated liver tests | |
| Cyclosporine | 1%-4% | Increased bile acids | |
| Cholestasis | |||
| Hepatocellular injury |
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3604] [Article Influence: 257.4] [Reference Citation Analysis (6)] |
| 2. | Mendes FD, Levy C, Enders FB, Loftus EV, Angulo P, Lindor KD. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am J Gastroenterol. 2007;102:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Svartz N. Salazyoprin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparation. Acta Med Scand. 1942;110:557-590. |
| 4. | Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 180] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Verspaget HW, Aparicio-Pagés MN, Verver S, Edelbroek PM, Hafkenscheid JC, Crama-Bohbouth GE, Peña AS, Weterman IT, Lamers CB. Influence of sulphasalazine and mesalazine on cellular and biochemical oxygen metabolite production. Effect of in vivo administration and an in vitro analysis. Scand J Gastroenterol. 1991;26:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Amos RS, Pullar T, Bax DE, Situnayake D, Capell HA, McConkey B. Sulphasalazine for rheumatoid arthritis: toxicity in 774 patients monitored for one to 11 years. Br Med J (Clin Res Ed). 1986;293:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 814] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | Boyer DL, Li BU, Fyda JN, Friedman RA. Sulfasalazine-induced hepatotoxicity in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1989;8:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Rafoth RJ. Systemic granulomatous reaction to salicylazosulfapyridine (Azulfidine) in a patient with Crohn’s disease. Am J Dig Dis. 1974;19:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Namias A, Bhalotra R, Donowitz M. Reversible sulfasalazine-induced granulomatous hepatitis. J Clin Gastroenterol. 1981;3:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Callen JP, Soderstrom RM. Granulomatous hepatitis associated with salicylazosulfapyridine therapy. South Med J. 1978;71:1159-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Haines JD. Hepatotoxicity after treatment with sulfasalazine. Postgrad Med. 1986;79:193-194, 197-198. [PubMed] |
| 14. | Marinos G, Riley J, Painter DM, McCaughan GW. Sulfasalazine-induced fulminant hepatic failure. J Clin Gastroenterol. 1992;14:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ribe J, Benkov KJ, Thung SN, Shen SC, LeLeiko NS. Fatal massive hepatic necrosis: a probable hypersensitivity reaction to sulfasalazine. Am J Gastroenterol. 1986;81:205-208. [PubMed] |
| 16. | Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 17. | Aquino RT, Vergueiro CS, Magliari ME, de Freitas TH. Sulfasalazine-induced DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms). Sao Paulo Med J. 2008;126:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Michel F, Navellou JC, Ferraud D, Toussirot E, Wendling D. DRESS syndrome in a patient on sulfasalazine for rheumatoid arthritis. Joint Bone Spine. 2005;72:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Piñana E, Lei SH, Merino R, Melgosa M, De La Vega R, Gonzales-Obeso E, Ramírez E, Borobia A, Carcas A. DRESS-syndrome on sulfasalazine and naproxen treatment for juvenile idiopathic arthritis and reactivation of human herpevirus 6 in an 11-year-old Caucasian boy. J Clin Pharm Ther. 2010;35:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Loftus EV, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Deltenre P, Berson A, Marcellin P, Degott C, Biour M, Pessayre D. Mesalazine (5-aminosalicylic acid) induced chronic hepatitis. Gut. 1999;44:886-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Hautekeete ML, Bourgeois N, Potvin P, Duville L, Reynaert H, Devis G, Adler M, Klöppel G. Hypersensitivity with hepatotoxicity to mesalazine after hypersensitivity to sulfasalazine. Gastroenterology. 1992;103:1925-1927. [PubMed] |
| 24. | Stoschus B, Meybehm M, Spengler U, Scheurlen C, Sauerbruch T. Cholestasis associated with mesalazine therapy in a patient with Crohn’s disease. J Hepatol. 1997;26:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966; quiz 967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 522] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 26. | Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn‘s disease in adults. Am J Gastroenterol. 2009;104:465-483; quiz 464, 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 596] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 27. | Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Gisbert JP, Luna M, González-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Maté J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106-1114. [PubMed] |
| 30. | Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Romagnuolo J, Sadowski DC, Lalor E, Jewell L, Thomson AB. Cholestatic hepatocellular injury with azathioprine: a case report and review of the mechanisms of hepatotoxicity. Can J Gastroenterol. 1998;12:479-483. [PubMed] |
| 32. | Haboubi NY, Ali HH, Whitwell HL, Ackrill P. Role of endothelial cell injury in the spectrum of azathioprine-induced liver disease after renal transplant: light microscopy and ultrastructural observations. Am J Gastroenterol. 1988;83:256-261. [PubMed] |
| 33. | Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651-662. [PubMed] |
| 34. | de Boer NK, Mulder CJ, van Bodegraven AA. Nodular regenerative hyperplasia and thiopurines: the case for level-dependent toxicity. Liver Transpl. 2005;11:1300-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Schaeffeler E, Fischer C, Brockmeier D, Wernet D, Moerike K, Eichelbaum M, Zanger UM, Schwab M. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Bastida G, Nos P, Aguas M, Beltrán B, Rubín A, Dasí F, Ponce J. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 519] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 38. | Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Prey S, Paul C. Effect of folic or folinic acid supplementation on methotrexate-associated safety and efficacy in inflammatory disease: a systematic review. Br J Dermatol. 2009;160:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | van Ede AE, Laan RF, Rood MJ, Huizinga TW, van de Laar MA, van Denderen CJ, Westgeest TA, Romme TC, de Rooij DJ, Jacobs MJ. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001;44:1515-1524. [PubMed] |
| 41. | Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A, Reed G, Strand V, Kremer JM. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010;69:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Fournier MR, Klein J, Minuk GY, Bernstein CN. Changes in liver biochemistry during methotrexate use for inflammatory bowel disease. Am J Gastroenterol. 2010;105:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Valentino PL, Church PC, Shah PS, Beyene J, Griffiths AM, Feldman BM, Kamath BM. Hepatotoxicity caused by methotrexate therapy in children with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60:824-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Themido R, Loureiro M, Pecegueiro M, Brandão M, Campos MC. Methotrexate hepatotoxicity in psoriatic patients submitted to long-term therapy. Acta Derm Venereol. 1992;72:361-364. [PubMed] |
| 46. | Bath RK, Brar NK, Forouhar FA, Wu GY. A review of methotrexate-associated hepatotoxicity. J Dig Dis. 2014;15:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 47. | MacDonald A, Burden AD. Noninvasive monitoring for methotrexate hepatotoxicity. Br J Dermatol. 2005;152:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Whiting-O’Keefe QE, Fye KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1032] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 50. | Kremer JM, Alarcón GS, Lightfoot RW, Willkens RF, Furst DE, Williams HJ, Dent PB, Weinblatt ME. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994;37:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 369] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 51. | Van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut. 1997;40:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 52. | van Hogezand RA, Verspaget HW. The future role of anti-tumour necrosis factor-alpha products in the treatment of Crohn’s disease. Drugs. 1998;56:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 54. | Khanna R, Feagan BG. Safety of infliximab for the treatment of inflammatory bowel disease: current understanding of the potential for serious adverse events. Expert Opin Drug Saf. 2015;14:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Sokolove J, Strand V, Greenberg JD, Curtis JR, Kavanaugh A, Kremer JM, Anofrei A, Reed G, Calabrese L, Hooper M. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1612-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Shelton E, Chaudrey K, Sauk J, Khalili H, Masia R, Nguyen DD, Yajnik V, Ananthakrishnan AN. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Möller S, Lohse AW. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 58. | Fujii K, Rokutanda R, Osugi Y, Koyama Y, Ota T. Adult-onset Still’s disease complicated by autoimmune hepatitis: successful treatment with infliximab. Intern Med. 2012;51:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Rajanayagam J, Lewindon PJ. Infliximab as rescue therapy in paediatric autoimmune hepatitis. J Hepatol. 2013;59:908-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 60. | Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, Serrano J, Rochon J, Fontana RJ, Bonacini M. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11:558-564.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Menghini VV, Arora AS. Infliximab-associated reversible cholestatic liver disease. Mayo Clin Proc. 2001;76:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Ierardi E, Valle ND, Nacchiero MC, De Francesco V, Stoppino G, Panella C. Onset of liver damage after a single administration of infliximab in a patient with refractory ulcerative colitis. Clin Drug Investig. 2006;26:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 64. | Lee YH, Bae SC, Song GG. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int J Rheum Dis. 2013;16:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011;17:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 66. | Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-219; quiz e16-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 466] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 67. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (3)] |
| 68. | Björnsson ES, Gunnarsson BI, Gröndal G, Jonasson JG, Einarsdottir R, Ludviksson BR, Gudbjörnsson B, Olafsson S. Risk of drug-induced liver injury from tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2015;13:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Adar T, Mizrahi M, Pappo O, Scheiman-Elazary A, Shibolet O. Adalimumab-induced autoimmune hepatitis. J Clin Gastroenterol. 2010;44:e20-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Grasland A, Sterpu R, Boussoukaya S, Mahe I. Autoimmune hepatitis induced by adalimumab with successful switch to abatacept. Eur J Clin Pharmacol. 2012;68:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Cravo M, Silva R, Serrano M. Autoimmune hepatitis induced by infliximab in a patient with Crohn’s disease with no relapse after switching to adalimumab. BioDrugs. 2010;24 Suppl 1:25-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Goldfeld DA, Verna EC, Lefkowitch J, Swaminath A. Infliximab-induced autoimmune hepatitis with successful switch to adalimumab in a patient with Crohn’s disease: the index case. Dig Dis Sci. 2011;56:3386-3388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Kim E, Bressler B, Schaeffer DF, Yoshida EM. Severe cholestasis due to adalimumab in a Crohn’s disease patient. World J Hepatol. 2013;5:592-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Matsumoto T, Marusawa H, Dogaki M, Suginoshita Y, Inokuma T. Adalimumab-induced lethal hepatitis B virus reactivation in an HBsAg-negative patient with clinically resolved hepatitis B virus infection. Liver Int. 2010;30:1241-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Verhelst X, Orlent H, Colle I, Geerts A, De Vos M, Van Vlierberghe H. Subfulminant hepatitis B during treatment with adalimumab in a patient with rheumatoid arthritis and chronic hepatitis B. Eur J Gastroenterol Hepatol. 2010;22:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis. 2010;4:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 621] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 78. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 674] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 79. | Gordon FH, Lai CW, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, Donoghue S, Greenlees C, Subhani J, Amlot PL. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 880] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 81. | Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 82. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 461] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 83. | Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn’s disease. Ann Pharmacother. 2005;39:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2672] [Cited by in RCA: 2441] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 85. | Bezabeh S, Flowers CM, Kortepeter C, Avigan M. Clinically significant liver injury in patients treated with natalizumab. Aliment Pharmacol Ther. 2010;31:1028-1035. [PubMed] |
| 86. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1969] [Article Influence: 151.5] [Reference Citation Analysis (1)] |
| 87. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1636] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 88. | Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D’Haens G, Ben-Horin S, Xu J, Rosario M. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 89. | Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I, Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 90. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 91. | Vermeire S, O’Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 92. | Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, Schreiber S, Mansfield JC, Williams M, Tang M. A randomised phase I study of etrolizumab (rhuMAb β7) in moderate to severe ulcerative colitis. Gut. 2013;62:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, Mascelli MA. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 94. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 894] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 95. | Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 96. | Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, Yeilding N, Li S, Szapary P, Gordon KB. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 97. | Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1104] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 98. | Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, Chan D, Hsu MC, Ho V, Ghislain PD. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 99. | Zeuzem S, Carreño V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antiviral Res. 2001;52:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236-3243. [PubMed] |
| 101. | Chiu HY, Chen CH, Wu MS, Cheng YP, Tsai TF. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 102. | Kornbluth A, Present DH, Lichtiger S, Hanauer S. Cyclosporin for severe ulcerative colitis: a user’s guide. Am J Gastroenterol. 1997;92:1424-1428. [PubMed] |
| 103. | Van Assche G, D’Haens G, Noman M, Vermeire S, Hiele M, Asnong K, Arts J, D’Hoore A, Penninckx F, Rutgeerts P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 274] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 104. | Rayner CK, McCormack G, Emmanuel AV, Kamm MA. Long-term results of low-dose intravenous ciclosporin for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2003;18:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1191] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 106. | Galán AI, Fernández E, Morán D, Muñoz ME, Jiménez R. Cyclosporine A hepatotoxicity: effect of prolonged treatment with cyclosporine on biliary lipid secretion in the rat. Clin Exp Pharmacol Physiol. 1995;22:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Stone BG, Udani M, Sanghvi A, Warty V, Plocki K, Bedetti CD, Van Thiel DH. Cyclosporin A-induced cholestasis. The mechanism in a rat model. Gastroenterology. 1987;93:344-351. [PubMed] |
| 108. | Kassianides C, Nussenblatt R, Palestine AG, Mellow SD, Hoofnagle JH. Liver injury from cyclosporine A. Dig Dis Sci. 1990;35:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | van Petersen AS, van der Pijl HW, Ringers J, Lemkes HH, de Fijter HW, Masclee AA. Gallstone formation after pancreas and/or kidney transplantation: an analysis of risk factors. Clin Transplant. 2007;21:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Kahan BD, Flechner SM, Lorber MI, Golden D, Conley S, Van Buren CT. Complications of cyclosporine-prednisone immunosuppression in 402 renal allograft recipients exclusively followed at a single center for from one to five years. Transplantation. 1987;43:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 111. | Lorber MI, Van Buren CT, Flechner SM, Williams C, Kahan BD. Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients. Transplantation. 1987;43:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 111] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Klintmalm GB, Iwatsuki S, Starzl TE. Cyclosporin A hepatotoxicity in 66 renal allograft recipients. Transplantation. 1981;32:488-489. [PubMed] |
| 113. | Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | García-López S, Gomollón-García F, Pérez-Gisbert J. Cyclosporine in the treatment of severe attack of ulcerative colitis: a systematic review. Gastroenterol Hepatol. 2005;28:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Kancherla D, Gajendran M, Vallabhaneni P, Vipperla K. Metronidazole induced liver injury: a rare immune mediated drug reaction. Case Rep Gastrointest Med. 2013;2013:568193. [PubMed] |
| 116. | Tabak F, Ozaras R, Erzin Y, Celik AF, Ozbay G, Senturk H. Ornidazole-induced liver damage: report of three cases and review of the literature. Liver Int. 2003;23:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Rutgeerts P, Van Assche G, Vermeire S, D’Haens G, Baert F, Noman M, Aerden I, De Hertogh G, Geboes K, Hiele M. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 118. | Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 495] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 119. | Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm. 2014;71:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Arnold GL, Beaves MR, Pryjdun VO, Mook WJ. Preliminary study of ciprofloxacin in active Crohn’s disease. Inflamm Bowel Dis. 2002;8:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 121. | Colombel JF, Lémann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, Notteghem B, Mary JY. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID). Am J Gastroenterol. 1999;94:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 156] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Swift GL, Srivastava ED, Stone R, Pullan RD, Newcombe RG, Rhodes J, Wilkinson S, Rhodes P, Roberts G, Lawrie BW. Controlled trial of anti-tuberculous chemotherapy for two years in Crohn’s disease. Gut. 1994;35:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2608] [Cited by in RCA: 2524] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 124. | Bennett DE, Courval JM, Onorato I, Agerton T, Gibson JD, Lambert L, McQuillan GM, Lewis B, Navin TR, Castro KG. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999-2000. Am J Respir Crit Care Med. 2008;177:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 125. | Centers for Disease Control and Prevention (CDC). Tuberculosis associated with blocking agents against tumor necrosis factor-alpha--California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2004;53:683-686. [PubMed] |
| 126. | Doherty SD, Van Voorhees A, Lebwohl MG, Korman NJ, Young MS, Hsu S. National Psoriasis Foundation consensus statement on screening for latent tuberculosis infection in patients with psoriasis treated with systemic and biologic agents. J Am Acad Dermatol. 2008;59:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | Kopanoff DE, Snider DE, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991-1001. [PubMed] |
| 128. | Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA. 1999;281:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 129. | Black M, Mitchell JR, Zimmerman HJ, Ishak KG, Epler GR. Isoniazid-associated hepatitis in 114 patients. Gastroenterology. 1975;69:289-302. [PubMed] |
| 130. | Salpeter SR. Fatal isoniazid-induced hepatitis. Its risk during chemoprophylaxis. West J Med. 1993;159:560-564. [PubMed] |
| 131. | Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 710] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
P- Reviewer: De Ponti F, Malnick SDH, Narciso-Schiavon JL, Triantafillidis JK, Xu R S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/