Published online Oct 28, 2015. doi: 10.4254/wjh.v7.i24.2559
Peer-review started: June 9, 2015
First decision: July 6, 2015
Revised: July 25, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: October 28, 2015
Processing time: 147 Days and 22.9 Hours
Glucosamine (GS) and chondroitin sulfate (CS) are common over-the-counter (OTC) supplements used in the treatment of osteoarthritis. These medications are seemingly safe, but there are increasing reports of hepatotoxicity with these supplements. We reported a unique case of drug-induced cholestasis caused by GS and CS in a combination tablet. The etiology of the jaundice was overlooked despite extensive investigations over a three-month period. Unlike drug-induced hepatocellular injury, drug-induced cholestatic jaundice with GS and CS has only been reported twice before. This case emphasizes the importance of a complete medication history, especially OTC supplements, in the assessment of cholestasis.
Core tip: Glucosamine and chondroitin sulfate are common over-the-counter medications available in North America and other countries in the treatment of osteoarthritis. We report a unique case of drug-induced cholestatic injury caused by this combination tablet. The etiology of this patient’s new jaundice went undiagnosed despite extensive investigations over three months. Only after careful questioning of his medication history and review of his liver biopsy was the correct diagnosis obtained. This case adds to the increasing reports of hepatoxicity related to this supplement. Furthermore, it highlights the importance of a complete medication history, especially over-the-counter medications, in the assessment of cholestatic jaundice.
- Citation: Ip S, Jeong R, Schaeffer DF, Yoshida EM. Unusual case of drug-induced cholestasis due to glucosamine and chondroitin sulfate. World J Hepatol 2015; 7(24): 2559-2562
- URL: https://www.wjgnet.com/1948-5182/full/v7/i24/2559.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i24.2559
In North America and other countries, glucosamine (GS) and chondroitin sulfate (CS) are common over-the-counter (OTC) supplements marketed for supporting the structure and function of joints, especially in treating osteoarthritis (OA). Although clinical trials have suggested that these medications have minimal to no significant clinical benefit in OA[1,2], they continue to be utilized, which may be attributed to their reputation as “natural products”, easy tolerability, and low side-effect profile[3]. A rare complication related to GS and/or CS use is hepatotoxicity especially classic hepatocellular drug-induced liver injury (DILI)[3-5]; however, purely cholestatic injury is less documented[4,6]. We report a unique case of drug-induced cholestasis caused by GS and CS. The etiology of the jaundice was missed despite exhaustive medical investigations over three months. This case adds to the growing literature of hepatotoxicity related to this supplement. Furthermore, it highlights the importance of a complete medication history, especially OTC supplements, in the assessment of cholestatic jaundice.
A 78-year-old man originally presented with a three-month history of jaundice of unknown etiology at his home hospital. He was subsequently transferred to a tertiary centre for further investigations. His past medical history was significant for a subarachnoid hemorrhage and OA. He was previously well until he reported a three-month history of pruritus, fatigue, nausea, vomiting, and a thirty-pound weight loss with no abdominal pain. He had no history of alcohol abuse or intravenous drug use, no recent travel history, no mushroom ingestion, and no family history of similar jaundice or any liver disease. According to records from his home hospital, he had not taken any other medications except acetaminophen intermittently and vitamin D, which he had been consuming for many years. On direct questioning, however, he disclosed that he had taken GS and CS approximately two months prior to the onset of jaundice. This was his first exposure to this supplement, and he took three tablets per day for his joint pain (maximum daily dose outlined on the bottle). When he started to become jaundiced three months ago, he discontinued the supplement.
On exam, he was clearly jaundiced with no asterixis. His body mass index was 28 kg/m2. He had no other stigmata of chronic liver disease. His abdominal exam was benign with no appreciable hepatosplenomegaly.
His bloodwork drawn at the tertiary centre revealed a total bilirubin of 470 mol/L (normal is < 18 mol/L), direct bilirubin of 383 mol/L (normal is < 5 mol/L), alkaline phosphate of 136 U/L (normal is 30-135 U/L), gamma-glutamyl transferase of 59 U/L (normal is 0-80 U/L), aspartate transferase of 46 U/L (normal is 10-80 U/L), and alanine transferase (ALT) of 52 U/L (normal is 10-80 U/L). This was virtually unchanged from his previous bloodwork at his home hospital (Table 1). His international normalized ratio and albumin levels were near normal. The remainder of his bloodwork was non-contributory. Liver enzymes and liver function tests were previously normal.
| Presentation at home hospital | Presentation at tertiary centre | |
| Total bilirubin (mol/L) | 476 | 440 |
| Normal: < 18 mol/L | ||
| AST (U/L) | 45 | 40 |
| Normal: 10-80 U/L | ||
| ALT (U/L) | 47 | 48 |
| Normal: 10-80 U/L | ||
| GGT (U/L) | 59 | 136 |
| Normal: 0-80 U/L | ||
| Alkaline phosphatase (U/L) | 136 | 136 |
| Normal: 30-135 U/L |
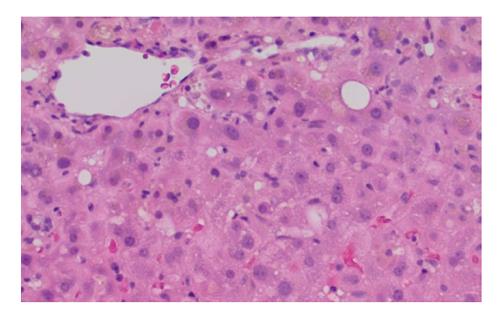
At his home hospital, he had had extensive investigations for his jaundice. Serology for hepatitis A, B and C were negative. Antinuclear antibody, antimitochondrial antibody, and antineutrophil cytoplasmic antibody were negative. Ceruloplasmin and alpha-antitrypsin levels were normal, and anti-tissue transglutaminase was negative with a normal IgA level. A skin biopsy was negative for vasculitis. He had had multiple abdominal ultrasounds that showed cholelithiasis, a somewhat inhomogeneous liver with no discrete lesions, but specifically no evidence of intra or extrahepatic bile duct dilatation. He had two endoscopic retrograde cholangiopancreatographies (ERCPs), which revealed no biliary tract abnormalities. A sphincterotomy and stent insertion were completed in hopes of relieving any kind of obstruction with no success. A subsequent magnetic resonance cholangiopancreatography (MRCP) showed no evidence of duct dilatation. Two liver biopsies showed non-specific signs of acute cholestasis (Figure 1). Upon further review with a pathologist at the tertiary centre, these biopsies were most consistent with drug-related cholestasis. Temporally, GS and CS fit as the likely culprit.
GS is an aminosaccharide that is important for proteoglycan formation, which helps preserve cartilage integrity of joints[7] while CS is an essential part of aggrecan, a component of the cartilage structure[8]. Both supplements are frequently taken together in the treatment of OA. Previous systemic reviews have shown that these supplements are safe with no significant side effects[8,9]; however, reports of hepatotoxicity have been documented[3-5]. We report a unique case of drug-related cholestasis that adds to the expanding literature of this mechanism of liver injury associated with GS and CS.
There have been two other similar reports regarding cholestatic DILI with GS and/or CS. Ossendza et al[6] describe a case of hepatitis with significant ALT and bilirubin elevation, approximately 6- and 10-fold respectively, in a patient taking therapeutic doses of GS four months prior to presenting with pruritus and jaundice. This patient recovered with discontinuation of the supplement. Smith et al[4] describe a case of elevated cholestastic liver enzymes and normal bilirubin that return to normal after stopping GS and CS. Our case is unique in that the patient presented with purely hyperbilirubinemia with mild liver enzyme derangement. An exhaustive search for a cause was undertaken including multiple investigations (e.g., ERCPs, MRCP, etc.), highlighting the importance of a complete medication history in the evaluation of new onset jaundice.
The mechanism by which GS and/or CS causes hepatotoxicity is unclear. An allergic mechanism has been proposed given the presence of rash and/or eosinophilia in previous case reports[3]. Furthermore, GS is derived from the exoskeleton of shellfish, which would theoretically worsen in those with known seafood allergies. No cases of hepatotoxicity, however, have been recorded in patients with known shellfish allergy[10]. The purity of the supplement may be another factor in causing hepatotoxicity. In Europe, GS and/or CS is classified as a medication, but in North America and other countries, they are available as an OTC supplements, which is not under regulative scrutiny regarding purity or efficacy. In our case, our patient appeared only to be taking therapeutic doses suggested by the manufacturer.
In conclusion, we report a rare case of cholestatic injury related to GS and CS, adding to increasing reports of hepatotoxicity of this supplement. Although this supplement may be thought to be harmless, clinicians need to consider this supplement in the evaluation of liver enzyme derangement and/or jaundice.
A 78-year-old man presents with 3 mo of painless jaundice.
The patient clearly had scleral icterus but no other stigmata of chronic liver disease. His abdominal exam revealed no masses.
Medication (e.g., over-the-counter), malignant (e.g., pancreatic cancer), benign obstruction (e.g., gallstones), autoimmune hepatitis.
His bloodwork revealed significant hyperbilirubinemia of 470 mol/L (normal is < 18 mol/L) with relatively preserved liver enzymes and liver function tests.
He has multiple normal abdominal ultrasounds, endoscopic retrograde cholangiopancreatographies and a magnetic resonance cholangiopancreatography.
The liver biopsy was consisted with drug-related cholestasis.
His glucosamine and chondroitin sulfate supplements were stopped.
There have been only two reports of drug-related cholestasis with glucosamine and/or chondroitin sulfate.
Glucosamine and chondroitin sulfate are commonly available over-the-counter supplements in the treatment of osteoarthritis.
A complete drug history, especially over-the-counter medications, are important in the evaluation of liver enzyme derangement and/or jaundice.
This is a good instructive case which will benefit readers.
| 1. | Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, Bradley JD, Bingham CO, Weisman MH, Jackson CG. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 778] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, Bradley JD, Silver D, Jackson CG, Lane NE. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Cerda C, Bruguera M, Parés A. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J Gastroenterol. 2013;19:5381-5384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Smith A, Dillon J. Acute liver injury associated with the use of herbal preparations containing glucosamine: three case studies. BMJ Case Rep. 2009;2009:bcr02.2009.1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy. 2010;30:750, 258e-262e. [PubMed] |
| 6. | Ossendza RA, Grandval P, Chinoune F, Rocher F, Chapel F, Bernardini D. [Acute cholestatic hepatitis due to glucosamine forte]. Gastroenterol Clin Biol. 2007;31:449-450. [PubMed] |
| 7. | Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, Jan S, March L, Edmonds J, Norton R, Woodward M, Day R; LEGS study collaborative group. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, Hochberg MC, Wells G. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;CD002946. [PubMed] |
| 9. | Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, Reichenbach S, Trelle S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. [PubMed] |
| 10. | Gray HC, Hutcheson PS, Slavin RG. Is glucosamine safe in patients with seafood allergy? J Allergy Clin Immunol. 2004;114:459-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
P- Reviewer: Chetty R, Loomis T, Morales-Gonzalez J S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/