Published online Sep 18, 2015. doi: 10.4254/wjh.v7.i20.2237
Peer-review started: May 14, 2015
First decision: June 24, 2015
Revised: August 2, 2015
Accepted: September 1, 2015
Article in press: September 2, 2015
Published online: September 18, 2015
Processing time: 124 Days and 16.7 Hours
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related deaths worldwide. Various treatment modalities have been applied to HCC depending on the tumor load, functional capacity of the liver and the general condition of the patient. According to Barcelona Clinic Liver Cancer staging strategy and The American Association for the Study of Liver Disease guidelines, surgical resection is not advocated in the tretment of multinodular HCC. Despite this, many recent clinical studies show that, resection can achieve good results in patients with multinodular HCC and 5-year survival rate around 40% can be reached. If resection or transplantation is not performed, these patients are usually managed with palliative procedures such as transarterial chemoembolization, radioembolization and cytotoxic chemotherapy and 5-year survival of this group of patients will be extremely low. Although survival rates are lower and complications may be increased in this group of patients, liver resection can safely be performed in selected patients in experienced centers for the management of multinodular HCC.
Core tip: Liver resection is underutilized in the management of multinodular hepatocellular carcinoma. The presence of multiple nodules should not be considered as a contraindication for surgical resection. Acceptable 5-year survival rates can be achieved in selected patients.
- Citation: Abbasoglu O. Role of liver resection in the management of multinodular hepatocellular carcinoma. World J Hepatol 2015; 7(20): 2237-2240
- URL: https://www.wjgnet.com/1948-5182/full/v7/i20/2237.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i20.2237
Hepatocellular carcinoma (HCC) is a major health problem and is the sixth most common malignancy in the world[1]. HCC usually develops in the presence of underlying chronic liver disease, most commonly due to chronic hepatitis B in underdeveloped countries and due to hepatitis C in developed countries. Although resection remains the cornerstone treatment for HCC, the role of resection in multinodular HCC is controversial. The early literature suggests that transplantation is the only treatment of choice that can offer long-term survival in patients with multinodular disease[2,3].
Liver transplantation eliminates not only the tumor but also the underlying liver disease and excellent outcomes with 5-year survival rates exceeding 60% can be achieved but, many patients are not candidates for transplantation because of tumor size, advanced age, high costs, and finally organ shortage[4]. Clearly, in patients with advanced underlying liver disease and portal hypertension, transplantation is the only option with a chance of cure. Tumors meeting Milan criteria defined as a single tumor smaller than 5 cm or up to three nodules smaller than 3 cm each are good candidates for liver transplantation.
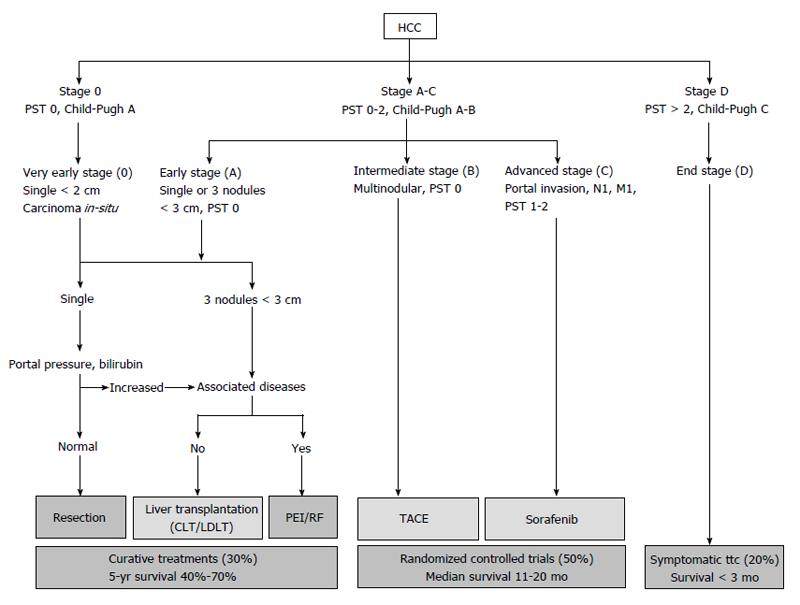
Many staging systems and management algorithms have been proposed for HCC and the Barcelona Clinic Liver Cancer (BCLC) staging and treatment strategy is the most commonly used system. In the BCLC treatment algorithm, surgical resection is limited to patients with early stage single tumors. Patients with up to three nodules may be offered liver transplantation. Patients with more than three nodules are considered for palliative treatments including chemoembolization. BCLC algorithm was first introduced by Llovet et al[5] in 1999 in Seminars in liver disease. Since this original publication, some modifications has been emerged, but the suggestions in the management of multinodular HCC has remained unchanged[6] (Figure 1). The American Association for the Study of Liver Disease guidelines also advocates liver resection only for solitary HCC smaller than 3 cm with well-preserved liver function[7].
On the other hand, the resectability for HCC mainly depends on the volume and functional capacity of the remnant liver, but not to the resected tumor amount. The severity of underlying liver dysfunction is critical in the evaluation of patients preoperatively. During daily practice, surgeons, gastroenterogists and oncologists may see many HCC patients with good liver reserve, good general condition but with multinodular HCC. Multinodular HCC has generally been regarded as a contraindication to resection, but recently there is evolving evidence for the resection of these tumors[8-14].
Anatomic vs nonanatomic resection in patients with HCC is an ongoing discussion[15]. The surgeons performing resection in the treatment of multinodular HCC have to face the difficulty of achieving a curative intervention and at the same time preventing postoperative liver failure as a result of removal of too much liver. Proponents of anatomic resection claim that, eradication of intrahepatic metastasis along the portal venous system can only be achieved by systematic removal based on segmental liver anatomy[16]. On the contrary, some surgeons prefer non-anatomic resections, aiming to preserve enough tumor free margin to maximize the volume of the remnant liver. In the presence of multiple HCC nodules, anatomical resections may be difficult to perform as postoperative liver failure may be increased due to insufficient remnant liver. In an analysis of 434 patients from Japan, anatomic resections could be performed in only 36% of patients with multinodular HCC while 71% of patients had undergone anatomical resections in the presence of a single tumor[7]. Although the risk of intrahepatic disseminations is considered trivial in small tumors under 2 cm, it may be challenging to justify resection in the treatment of multinodular HCC for the anatomic resection advocates[17].
Nathan et al[18] analyzed the factors predictive of receipt of surgical treatment for early HCC that is, those patients with non-metastatic tumors 5 cm or smaller and without evidence of lymph node metastasis, extra-hepatic tumor growth, or major vascular invasion. Of the 1745 patients meeting the selection criteria, a total of 820 patients (47%) did not receive any type of surgical intervention. Seventy-six percent of those (n = 622) had found to have no documentation of any treatment modality in their medical records. The authors examined the factors associated with receipt of surgical therapy in a bivariate analysis. With respect to tumor characteristics, patients who receive surgical therapy generally had solitary tumors (68% vs 60%, P < 0.001) and less often had bilobar disease (14% vs 22%, P < 0.001). Surprisingly surgical treatment was not offered to many patients without any apparent reason. This Johns Hopkins University data suggest that surgical treatment options are not offered to many patients with HCC, and their opportunity of achieving better survival may be hindered.
There is not enough data about the maximum number of nodules that can be resected safely. In a recent study of 399 patients, Nojiri et al[19] showed that even if patients have four or more nodules without portal vein invasion and with well-preserved liver function, resection for HCC may be the treatment of choice. The 3- and 5-year overall survival rates of patients with multinodular HCC were 62% and 38% respectively. Ishizawa et al[8] reported 5-year survival of 58% in 126 patients with multinodular HCC and in this series 22 patients (17%) had four or more HCC nodules. In this group of patients, existence of multiple tumors was not found to be a predictor of overall survival but independently increased the risk of recurrence (relative risk 1.64)[8]. With the development of radiofrequency ablation, the combination of this modality with resection may increase the resectability rates and surgical treatment can be performed in more advanced multinodular HCC[20].
In a recent systematic review of 50 studies involving 14808 patients Zhong et al[21] showed that resection can safely be performed in both large and multinodular HCC. In this systematic review, hospital mortality rates were found to be 2.7% to 7.3% depending on the ethnicity and type of HCC and these rates were similar to the mortality rates of early HCC surgery. The median rate of postoperative complications in this study (26.6% to 32.3%) is also comparable to early HCC series. Overall 5-year survival and 5-year disease free survival rates in these large and/or multinodular HCC patients were 42% and 26% respectively. These numbers are definitely lower than the corresponding 5-year survival of 67% and 5-year disease free survival of 37% for patients with early HCC, but still acceptable, suggesting that resection can be considered a reasonable approach in carefully selected patients.
Despite the fact that many patients with multinodular HCC may not be amenable to surgical treatment, these patients need careful evaluation for possible surgical therapy to provide that all therapeutic opportunities are being applied (Table 1).
If resection or transplantation is not offered to patients with multinodular HCC, these patients are usually managed with palliative procedures such as transarterial chemoembolization (TACE) and cytotoxic chemotherapy (sorafenib, etc.). Although the survival of patients after TACE is improved compared to conservative management, 5-year survival of this group of patients is still extremely low[22].
In conclusion, there is increasing evidence showing that resection can be safely extended to selected patients with multinodular HCC to achieve acceptable survival rates. Advances in the surgical treatment of HCC over the recent years have broadened the available surgical options for these patients. Although success of resection decreases in multinodular HCC, the overall 5-year survival rates approaching 40% to 50% can be achieved. With the improvements in surgical technique and perioperative management, liver resection can be considered as a safe treatment with an acceptable mortality and morbidity rates in the treatment of multinodular HCC in experienced centers.
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11886] [Article Influence: 792.4] [Reference Citation Analysis (6)] |
| 2. | Ochiai T, Sonoyama T, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ueda Y, Otsuji E, Itoi H, Hagiwara A. Poor prognostic factors of hepatectomy in patients with resectable small hepatocellular carcinoma and cirrhosis. J Cancer Res Clin Oncol. 2004;130:197-202. [PubMed] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5388] [Article Influence: 179.6] [Reference Citation Analysis (7)] |
| 4. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2915] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1332] [Article Influence: 74.0] [Reference Citation Analysis (1)] |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 8. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 9. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 875] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 10. | Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D’Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (3)] |
| 13. | Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005;92:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 326] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, Shen F, Pinna AD. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 17. | Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Nathan H, Hyder O, Mayo SC, Hirose K, Wolfgang CL, Choti MA, Pawlik TM. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Ann Surg. 2013;258:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Nojiri K, Tanaka K, Takeda K, Ueda M, Matsuyama R, Taniguchi K, Kumamoto T, Mori R, Endo I. The efficacy of liver resection for multinodular hepatocellular carcinoma. Anticancer Res. 2014;34:2421-2426. [PubMed] |
| 20. | Wong TC, Lo CM. Resection strategies for hepatocellular carcinoma. Semin Liver Dis. 2013;33:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Zhong JH, Rodríguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore). 2015;94:e396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503-509. [PubMed] |
P- Reviewer: Kapoor S, Wang GY S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/