Published online Aug 18, 2015. doi: 10.4254/wjh.v7.i17.2119
Peer-review started: October 13, 2014
First decision: December 17, 2014
Revised: June 24, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: August 18, 2015
Processing time: 315 Days and 15.7 Hours
AIM: To compare the ability of model for end-stage liver disease (MELD)-Na and Maddrey discrimination function index (DFI) to predict mortality at 30 and 90 d in patients with alcoholic hepatitis (AH).
METHODS: We prospectively assessed 52 patients with AH. Demographic, clinical and laboratory parameters were obtained. MELD-Na and Maddrey DFI were calculated on admission. Short-term mortality was assessed at 30 and 90 d. Receiver operating characteristic curve analysis was performed.
RESULTS: Thirty-day and 90-d mortality was 44% and 58%, respectively. In the univariate analysis, sodium levels was associated with mortality at 30 and 90 d (P = 0.001 and P = 0.03). Child stage, encephalopathy, ascites, or types of treatment were not associated with mortality. MELD-Na was the only predictive factor for mortality at 90 d. For 30-d mortality area under the curve (AUC) was 0.763 (95%CI: 0.63-0.89) for Maddrey DFI and 0.784 for MELD-Na (95%CI: 0.65-0.91, P = 0.82). For 90-d mortality AUC was 0.685 (95%CI: 0.54-0.83) for Maddrey DFI and 0.8710 for MELD-Na (95%CI: 0.76-0.97, P = 0.041).
CONCLUSION: AH is associated with high short-term mortality. Our results show that MELD-Na is a more valuable model than DFI to predict short-term mortality.
Core tip: Alcoholic hepatitis (AH) is a severe condition associated with high mortality. The model for end-stage disease (MELD) score is widely used to predict mortality in end-stage liver disease, and the addition of sodium (MELD-Na) increase its utility. However, few studies have evaluated the utility of MELD-Na in AH. In this study, we found that MELD-Na is useful for predicting 90-d mortality in patients with AH and preserve prognostic advantage over Maddrey discrimination function index score. It represents a valuable tool to stratify patients by risk, however further studies are required to validate the prognostic utility of MELD-Na score in patients with AH.
- Citation: Amieva-Balmori M, Mejia-Loza SMI, Ramos-González R, Zamarripa-Dorsey F, García-Ruiz E, Pérez y López N, Juárez-Valdés EI, López-Luria A, Remes-Troche JM. Model for end-stage liver disease-Na score or Maddrey discrimination function index, which score is best? World J Hepatol 2015; 7(17): 2119-2126
- URL: https://www.wjgnet.com/1948-5182/full/v7/i17/2119.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i17.2119
It is estimated that 6% of the Mexican population is dependent on alcohol which equals to 4.9 million people[1]. Chronic alcohol consumption is the leading cause of liver failure in our country, and alcohol intake > 120 g/d is a factor associated with the development of alcoholic hepatitis (AH)[1-3]. AH was first described by Gordon Beckett in 1961 and clinical description of the syndrome is still valid after 50 years[4]. This entity is an acute form of alcohol induced liver injury that is seen in patients who consume large quantities of alcohol during a prolonged period of time. Its spectrum is wide and ranges from a silent disease to fulminant liver failure with a high mortality rate. Patients with severe AH have been reported to have 30-d mortality up to 50%[5,6].
Therefore, assessment of the disease severity becomes an important and practical issue for clinicians involved in the management of patients with AH[6]. There are several prognostic models to assess severity in patients with AH including the Maddrey’s discriminant function index (DFI)[7], the Glasgow AH score (GAHS)[8], the age- bilirubin- international normalized ratio (INR) - creatinine (ABIC) score[9], the Lille score[10] and the model for end-stage liver disease (MELD)[11]. Among the many scoring systems, the DFI is the most used. A score higher than 32 in the DFI is considered as a severe AH and mortality rates are close to 65% at 28 d[8,12]. Also, DFI allows identifying patients who may benefit from treatment with steroids[13]. However, some studies have shown that the cut-point of 32 of DFI could be inaccurate and higher cut-offs have been proposed (from 37 to 44)[12,14].
Although MELD score was designed for evaluation of patients awaiting liver transplantation[15], its use has been expanded and now, is used as a prognostic scale in various liver diseases such as AH[16,17], viral hepatitis[18], hepatocellular carcinoma[19] and autoimmune diseases[20]. As hyponatremia is associated with poor prognosis in cirrhosis, inclusion of serum sodium (Na) into the MELD (MELD-Na) was found to improve its predictive value in chronic liver diseases[21,22]. MELD-Na is more efficient than MELD to identify subjects with poor outcome and significantly increase the efficacy of the score to predict waitlist mortality[22].
Several studies have examined the use of MELD in assessing the severity of AH[12,16,17,23,24] and sensitivity and specificity in predicting 30-d mortality ranges from 75% to 86%. Few studies, have evaluated the usefulness of the MELD-Na in AH[24-26] and results are controversial.
As sodium abnormalities are close related to end stage liver disease conditions such as ascites and hepatorenal syndrome (HRS), we hypothesize that MELD-Na is better to predict short-term mortality in patients with AH compared to the Maddrey DFI (the most used score).
We prospectively identified 52 patients admitted to our Gastroenterology Service (Hospital Juarez de Mexico, Mexico City, Mexico) between March 2011 and March 2013, with a diagnosis of AH and history of long alcohol consumption. The patients were diagnosed with AH based on the following clinical and biochemical characteristics: excessive alcohol consumption (> 100 g/d) at least 2 mo prior to admission, serum total bilirubin level above 5 mg/dL, aspartate/alanine aminotransferase ratio above 2, aspartate aminotransferase level below 300 IU/mL, history of longstanding alcoholism, and finally the absence of a coexistent primary cause of liver disease, such as viral hepatitis, drug induce liver diseases, non-alcoholic hepatitis, autoimmune hepatitis and hepatocellular carcinoma. Only patients with laboratory values available within 24 h of admission were included.
The following data were obtained for all patients: age, sex, history of alcohol consumption, clinical complications at admission and during hospitalization [ascites, hepatic encephalopathy, renal failure (as defined as serum creatinine ≥ 1.5 mg/dL), HRS, bacterial infections and gastrointestinal bleeding]; length of hospital stay, treatment received and cause of death. The analytical parameters at admission or within 48 h of admission included serum glucose, cholesterol, triglycerides, sodium, albumin, aminotransferases, bilirubin and creatinine levels, blood urea nitrogen, INR, leukocyte count, neutrophil count, platelet count, and hematocrit.
Short-term mortality was assessed at 30 and 90 d. The Child-Turcotte-Pugh (CTP) score was calculated for all patients regardless the presence or absence of cirrhosis. Medical treatment was also assessed. Both, Maddrey DFI and MELD-Na scores were based on clinical and laboratory parameters collected at the time of diagnosis of AH. Maddrey DFI was calculated using the formula: DFI = 4.6 × (PTsec-control PTsec) + serum total bilirubin in mg/dL. MELD-Na score was calculated using the formula: 3.8 (log bilirubin mg/dL) + 11.2 (ln INR) + 9.6 (ln creatinine mg/dL) + 6.4 + 1.59 (135 - Na). Maddrey DFI and MELD-Na scores higher than 32 and 21, respectively, were considered as a more severe disease and associated with poor outcomes[6,8]. Patients received oral corticosteroids if they met the following criteria: a modified Maddrey’s DFI > 32 or hepatic encephalopathy at admission, recent onset of jaundice, and biochemical changes suggestive of AH. Prednisone was given orally (40 mg/d) for 4 wk followed by a taper of 2-3 wk. Contraindications for corticosteroid treatment were severe bacterial infections, renal dysfunction, diabetes mellitus with poor metabolic control, and the presence of acute gastrointestinal bleeding. For those patients, pentoxifylline was prescribed 400 mg thrice/d.
Continuous variables were expressed as means with standard deviation and range. Categorical variables were expressed with percentage. χ2 analysis was used to compare categorical variables, and continuous variables were analyzed using the Student t-test and Mann-Whitney. The primary end point was death from any cause at 30 and 90 d from hospital admission. With the significant prognostic variables obtained from the univariate analysis, multivariate logistic regression was carried out using forward selection model.
The accuracy of the MELD-Na score was compared with the Maddrey DFI score, through the analysis of their area under the receiver operating characteristic (AUROC) curve. Receiver operating characteristic (ROC) curves were generated to assess the prognostic utility of Maddrey DF and MELD score, evaluated by their ability to rank patients according to the risk of mortality at 30 and 90 d. An AUROC value of > 0.70 was considered clinically relevant. Comparison between AUROC curves was performed by the method of Hanley and McNeil[27] using MedCalc version 9.3.0.0. (Medisoftware, Mariakerke, Belgium). From ROC curves coordinates, cut-off points with best sensitivity and specificity of the different scores were determined. A P value less than 0.05 was considered statistically significant. Statistical interpretation of data was performed using statistical package for social sciences (SPSS) version 16.0 for Windows (SPSS, Inc., Chicago, Illinois, United States). The Institutional Review Board and the Ethics Committee approved this study.
Fifty two subjects met the inclusion criteria. Forty eight patients (92%) were males, and mean age was 42.8 ± 8.7 years. Mean alcohol consumption per day was 283 g and mean days of continuous alcohol consumption prior to admission was 24 d. Thirty eight patients (73%) developed ascites and 24 (46%) encephalopathy. A concomitant infection process was detected in 16 (31%) of the patients; 7 (44%) had a urinary tract infections and 5 (31%) spontaneous bacterial peritonitis, and 4 (25%) had both urinary tract infection and spontaneous bacterial peritonitis. At admission mean MELD score was 30.8 ± 3.3, MELD-Na was 27.5 ± 7.7 (range, 12 to 48) and Maddrey DFI values was 79.7 ± 54 (range, 13 to 321). Specific treatment for AH was used in 75% (n = 39) of patients: pentoxifylline was used in 48% (n = 25), prednisone alone was used in 17% (n = 9), and 10% (n = 5) received prednisone in combination with pentoxyfylline.
Mortality rate at 30 d was 44% (n = 23), and the attributable causes were: multiple organ failure in 44% (n = 10), renal insufficiency from HRS in 44% (n = 10) and gastrointestinal hemorrhage in 13% (n = 3). Mortality rate at 90 d was 57.6% (n = 30) and multiple organ failure occurred in 47% (n = 13), renal insufficiency from HRS in 40% (n = 12) and gastrointestinal hemorrhage in 13% (n = 5). The variables that were significantly associated with 30-d and 90-d mortality in the univariate analysis are presented in Tables 1 and 2.
| Variables | Survived (n = 29) | Deceased (n = 23) | P |
| Demographic | |||
| Age (yr) | 40 ± 9.6 | 44 ± 12 | 0.114 |
| Alcohol consumption per day (g/d) | 291 ± 140 | 302 ± 159 | 0.809 |
| Male, n (%) | 28 (97) | 20 (87) | 0.222 |
| Laboratory parameters at admission | |||
| White blood cell counts (103/μL) | 17362 ± 9807 | 21772 ± 10131 | 0.11 |
| Glucose (mg/dL) | 102 ± 49 | 102 ± 61 | 0.987 |
| Sodium (mmol/L) | 132 ± 6 | 128 ± 6 | 0.019a |
| Total bilirubin (mg/dL) | 17.3 ± 8.9 | 23.6 ± 9.4 | 0.018a |
| AST (IU/L) | 172 ± 111 | 189 ± 93 | 0.55 |
| ALT (IU/L) | 66.9 ± 40.5 | 71.5 ± 33 | 0.66 |
| γGT (IU/L) | 369 ± 254 | 291 ± 183 | 0.282 |
| Alkaline phosphatase (IU/L) | 254 ± 109 | 222 ± 112 | 0.344 |
| Creatinine (mg/dL) | 1.61 ± 1.5 | 3.5 ± 2.5 | 0.001a |
| INR | 2.05 ± 0.6 | 2.49 ± 1.48 | 0.079 |
| Prothrombin time (s) | 23.14 ± 8.1 | 27.2 ± 11.2 | 0.142 |
| Albumin (mg/dL) | 2.8 ± 0.5 | 2.5 ± 0.6 | 0.73 |
| Cholesterol (mg/dL) | 150.8 ± 68 | 116 ± 53 | 0.081 |
| Triglycerides (mg/dL) | 222 ± 122 | 230 ± 178 | 0.869 |
| Calcium (mg/dL) | 7.9 ± 0.7 | 7.5 ± 1.1 | 0.10 |
| Clinical manifestations at admission | |||
| Ascites, n (%) | 25 (86) | 22 (95) | 0.468 |
| Child status | 0.023a | ||
| Grade B, n (%) | 6 (20) | 0 | |
| Grade C, n (%) | 23 (80) | 23 (100) | |
| Encephalophaty, n (%) | 0.335 | ||
| None | 13 (45) | 7 (30) | |
| Stage I | 4 (14) | 5 (22) | |
| Stage II | 8 (28) | 10 (43) | |
| Stage III | 4 (14) | 1 (4) | |
| Hepatorenal syndrome, n (%) | 5 (17) | 14 (61) | 0.001a |
| Severity of liver disease at admission | |||
| MELD-Na score | 25.5 ± 8 | 31.9 ± 6 | 0.003a |
| Maddrey DFI | 69.4 ± 42 | 93 ± 53.8 | 0.08 |
| MELD | 32.1 ± 6.5 | 25.1 ± 2.9 | 0.79 |
| Variables | Survived (n = 22) | Deceased (n = 30) | P |
| Demographic | |||
| Age (yr) | 41 ± 9 | 44 ± 11 | 0.27 |
| Alcohol consumption per day (g/d) | 284 ± 148 | 303 ± 143 | 0.584 |
| Male, n (%) | 21 (95) | 27 (90) | 0.94 |
| Laboratory parameters at admission | |||
| White blood cell counts (103 /μL) | 284 ± 148 | 303 ± 143 | 0.584 |
| Glucose (mg/dL) | 108 ± 59 | 99 ± 49 | 0.55 |
| Sodium (mmol/L) | 133 ± 5 | 129 ± 6 | 0.03a |
| Total bilirubin (mg/dL) | 16 ± 8 | 22 ± 10 | 0.009a |
| AST (IU/L) | 192 ± 137 | 177 ± 84 | 0.61 |
| ALT (IU/L) | 103 ± 150 | 67 ± 39 | 0.24 |
| γGT (IU/L) | 577 ± 656 | 399 ± 480 | 0.29 |
| Alkaline phosphatase (IU/L) | 281 ± 91 | 211 ± 105 | 0.01a |
| Creatinine (mg/dL) | 2 ± 1.8 | 3 ± 2.11 | 0.01a |
| INR | 2 ± 0.4 | 3 ± 1.3 | 0.002a |
| Prothrombin time (s) | 19 ± 4 | 28 ± 12 | 0.0003a |
| Albumin (mg/dL) | 3 ± 0.5 | 3 ± 5 | 0.54 |
| Cholesterol (mg/dL) | 176 ± 90 | 119 ± 51 | 0.01a |
| Triglycerides (mg/dL) | 240 ± 163 | 226 ± 162 | 0.76 |
| Calcium (mg/dL) | 7.9 ± 0.8 | 7.3 ± 1.5 | 0.28 |
| Clinical manifestations at admission | |||
| Ascites, n (%) | 19 (86) | 28 (93) | 0.71 |
| Child status | 0.05a | ||
| Grade B, n (%) | 8 (37) | 3 (10) | |
| Grade C, n (%) | 14 (63) | 27 (90) | |
| Encephalophaty, n (%) | 0 (0) | ||
| None | 10 (45) | 0 (30) | 0.106 |
| Stage I | 8 (37) | 3 (10) | |
| Stage II | 4 (18) | 19(63) | |
| Stage III | 8 (26) | ||
| Hepatorrenal syndrome, n (%) | 5 (22) | 16 (53) | 0.05a |
| Severity of liver disease at admission | |||
| MELD-Na score | 24.95 ± 8 | 30.9 ± 7.79 | 0.01a |
| Maddrey DFI | 68.5 ± 42 | 88.3 ± 48.6 | 0.12 |
| MELD | 22.1 ± 7.5 | 23.1 ± 3.1 | 0.28 |
Lower sodium levels (P = 0.019), higher total bilirubin levels (P = 0.018), higher creatinine levels (P = 0.001), Child class C (P = 0.023), development of HRS (P = 0.001) and a higher MELD-Na (P = 0.003) were significant factors associated with 30-d mortality. Lower sodium levels (P = 0.03), higher total bilirubin levels (P = 0.009), higher creatinine levels (P = 0.01), higher INR (P = 0.002), higher prothrombin time (P = 0.0003), lower cholesterol levels (P = 0.01), Child class C (P = 0.05), development of HRS (P = 0.05) and a higher MELD-Na (P = 0.01) were significant factors associated with 90-d mortality. Treatment with specific medication, development of infections or gastrointestinal bleeding did not influence survival.
In the multivariate logistic regression, HRS was the strongest and independent predictor of mortality at 30-d (P = 0.001). MELD-Na was a predictor of mortality at 90-d (P = 0.036) (Table 3). No additional variables increased the predictive accuracy of MELD-Na (bilirubin, INR, and creatinine, as factors included in Maddrey DF and/or MELD score, were excluded from the analysis).
| Significance | Odds ratio | 95%CI | |
| 30-d mortality | |||
| MELD-Na | 0.11 | 1.25 | 0.78-1.7 |
| Maddrey DFI | 0.14 | 1.14 | 0.82-3.04 |
| Bilirubin | 0.45 | 0.7 | 0.47-3.6 |
| Creatinine | 0.38 | 0.31 | 0.74-1.98 |
| INR | 0.41 | 0.78 | 0.68-1.52 |
| Hepatorenal syndrome | 0.001 | 11.5 | 2.7-48.11 |
| 90-d mortality | |||
| MELD-Na | 0.036 | 1.19 | 1.06-1.232 |
| Maddrey DFI | 0.09 | 1.03 | 0.87-1.86 |
| Bilirubin | 0.23 | 0.67 | 0.65-3.56 |
| Creatinine | 0.35 | 0.37 | 0.8-4.2 |
| INR | 0.17 | 0.272 | 0.78-2.6 |
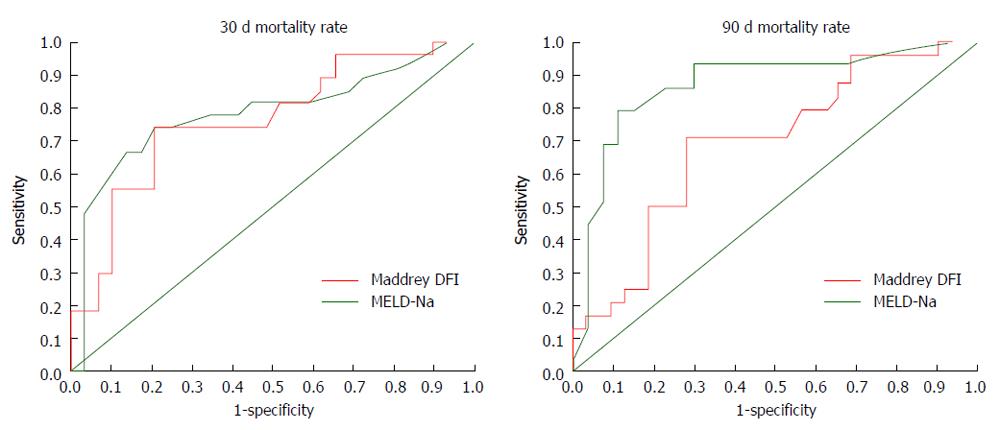
A clinical utility analysis was performed using the pre-established cut-off values for Maddrey DFI and MELD-NA (> 32 and > 21, respectively) and considering death at 30 and 90 d as the outcome. Sensitivity, specificity, positive predictive values, negative predictive values and accuracy are shown in Table 4. Receiver operating characteristic curves were created in order to estimate the predictive accuracy of the different scores to evaluate 30-d and 90-d mortality (Figure 1). For 30-d mortality the area under the curve (AUC) was 0.763 (95%CI: 0.63-0.89) for Maddrey DFI and 0.784 for MELD-Na (95%CI: 0.65-0.91, P = 0.82). For 90-d mortality the AUC was 0.685 (95%CI: 0.54-0.83) for Maddrey DFI and 0.8710 for MELD-Na (95%CI: 0.76-0.97, P = 0.041).
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
| Maddrey DFI > 32 | |||||
| Mortality at day 30 | 96 | 21 | 53 | 86 | 57 |
| Mortality at day 90 | 93 | 22.7 | 62.2 | 71.4 | 63.5 |
| MELD-Na > 21 | |||||
| Mortality at day 30 | 85 | 31 | 53 | 69 | 57.1 |
| Mortality at day 90 | 87 | 40 | 66 | 69 | 67.3 |
Excessive alcohol consumption is a social problem in Mexico and it has been estimated that alcohol related liver diseases (ALD) are responsible to approximately 9% of all diseases in Mexico[28]. A subset of patients with ALD will develop severe AH (AH), which has a substantially worse short-term prognosis[29]. The true prevalence is unknown, but histologic studies of patients with ALD suggest that AH may be present in as many as 10%-35% of hospitalized alcoholic patients[30,31].
Although a recent publication reported that the inpatient mortality rate in AH has decreased in the United States (from 10.07% in 2002 to 5.76% in 2010), in this cohort of Mexican patients with AH we found a high mortality rate, 44% at 30 d and 57.6% at 90 d[32]. Our results are similar to that reported in a recent multicentric study in Mexico in 175 patients with AH, where overall and 90-d mortality rate were 36% and 51%, respectively[3]. Similar to other cohorts, we found that most common causes of mortality were portal hypertension and HRS. This increased mortality rate could be explained by socioeconomic factors, quality of health services, higher amount of alcohol consumption in Mexican patients, as well as genetic factors[3,29]. For example, several studiesin Mexican-American and Mestizo populations have identified a virtual absence of some of the alcohol “protective” genes variations (ADH1B and ALDH2) and a high frequency of CPY2E c2polymorphic allele, which result in increased enzymatic activity,augmented acetaldehyde production, and more severe liver damage[33,34].
Many strategies have been used to predict morbidity and mortality in AH allowing a better medical support for those very ill patients. Such strategies include the search for single parameters (i.e., alkaline phosphatase) or the development of scoring systems like the Maddrey DFI, the GAHS, the ABIC, the Lille score and MELD[20-24]. According to our results we propose that MELD-Na is also a useful scoring system to predict severity in AH.
Although several studies have explored the clinical utility of severity scores in AH, results are variable. For example, Lafferty et al[35] in a cohort of 182 patients prospectively evaluated GAHS, MELD, ABIC and DFI scores anddid not found differences in the outcome among them. Other studies have focused in the specific use of MELD in evaluating the severity of AH. Dunn et al[11] in a study with 73 patients with AH found that a MELD score of 21 had the highest sensitivity and specificity to predict mortality at 30 and 90 d. In contrast, Monsanto et al[17] in a small sample size (n = 45) and retrospective study found that Maddrey DFI was a more valuable model to predict short-term mortality in patients with AH. Recently, a prospective study in 47 subjects with AH, found that both the MELD score and the Maddrey DFI score at admission were strong and equally good predictors of 28-d mortality in patients with AH[16]. However, in this study the optimal Maddrey DFI cut off point corresponding to the optimal MELD score was higher than the conventional one and the authors propose that MELD score may be used as an alternative to DFI score for predicting short-term mortality in AH[17].
Three previous studies have compared the ability of MELD-NA to predict mortality compared to other scores[24-26]. The first study, a small sample size study from the Mayo Clinic, showed that MELD-Na was a better predictor of 180-d mortality than MELD in patients with ascites[26]. In another study, Kasztelan-Szczerbinska et al[25] compared Maddrey DFI, CPT, GAHS, ABIC MELD and MELD-Na in 116 subjects with AH and no statistically significant differences in the models’ performances were found. Specifically for MELD-Na, the AUC was 0.83 to predict mortality at 90 d, similar to our findings. In a more recent study, nine scoring models were compared in 71 biopsy-proven patients with AH and all models showed excellent negative predictive values and MELD modifications incorporating sodium did not confer any prognostic advantage over classical MELD[24]. Interestingly, in this cohort the 30-d mortality and 90-d mortality rates were lower compared to other studies (14.1% and 19.7%, respectively). Also the authors did not report the incidence of ascites and HRS.
Hyponatremia is a common clinical problem in patients with end stage liver disease, and has a close relationship with portal hypertension, ascites and HRS. Low sodium levels are related to the impairment of renal solute-free water excretion most likely due to an increased vasopressin secretion, which results in increased sodium retention and reduced renal free water clearance, which predispose to life threatening conditions in the cirrhotic such as HRS and refractory ascites[36]. Also, hyponatremia represents an independent risk factor for brain edema, a fatal complication of acute liver failure[37,38]. Interestingly, we found that low sodium levels were associated with mortality at 30 and 90 d. Also, HRS was associated to mortality in the univariate and multivariate analysis. Thus, for us, was not surprisingly that MELD-Na had better clinical utility performance and ability to predict mortality at 90 d compared to Maddrey DFI.
We need to acknowledge that although we showed that MELD-Na is a useful tool to predict mortality, the treatment provided to our patients did not influence in their survival. Currently, corticosteroids or pentoxifylline are the main pharmacological treatment options; though the outcomes from the therapies are poor. Because of the limitations in the therapeutic options, it is no doubt that there is a critical need for the newer and more effective.
Other limitations that should be acknowledge include: a small sample size, some patients with suspected AH could not be included in the final analysis because they had incomplete laboratory parameters at admission, lack of comparison with other models that have been shown utility in Mexican population such as ABIC[3] and histological diagnosis of AH was not confirmed. However, several studies have shown that diagnosis of AH is confirmed in almost 80% of the suspected cases when high levels of recent alcohol consumption is confirmed and histological confirmation is not required[39,40]. Intriguingly, we did not find that encephalopathy, ascites and CPT were associated with mortality. However this finding is probably related with the power in our small sample size. Finally, although we found a better performance for MELD-Na to predict 90 d mortality, the clinical relevance of our findings should be assessed in future prospective, multicentric and larger sample size studies.
In conclusion, AH, is associated with high short-term mortality. We found that MELD-Na is useful for predicting 90-d mortality in patients with AH and preserve prognostic advantage over Maddrey DFI score. It represents a valuable tool to stratify patients by risk, however further studies are required to validate the prognostic utility of admission MELD-Na score in patients with AH.
Alcoholic hepatitis (AH) is a severe condition associated with high mortality. The model for end-stage disease (MELD) score is widely used to predict mortality in end-stage liver disease, and the addition of sodium (MELD-Na) increase its utility. However, few studies have evaluated the utility of MELD-Na in AH.
Few studies have compared the ability of MELD-NA to predict mortality compared to other scores.
In this study, the authors found that MELD-Na is useful for predicting 90-d mortality in patients with AH and preserve prognostic advantage over Maddrey discrimination function index (DFI) score.
MELD-Na may represent a valuable tool to stratify patients by risk and to predict in patients with AH.
AH: Alcoholic hepatitis; MELD: Model for end-stage disease; MELD-Na: MELD plus sodium; DFI: Discriminant function index; ALD: Alcoholic liver diseases.
This is a well written small study that recommends the use of MELD-Na in the prognostic scoring of patients with acute hepatitis. It warrants publication in its current form.
| 1. | Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Crocè L, Sasso F, Pozzato G, Cristianini G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845-850. [PubMed] |
| 2. | Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 535] [Article Influence: 17.8] [Reference Citation Analysis (3)] |
| 3. | Altamirano J, Higuera-de laTijera F, Duarte-Rojo A, Martínez-Vázquez MA, Abraldes JG, Herrera-Jiménez LE, Michelena J, Zapata L, Perez-Hernández J, Torre A. The amount of alcohol consumption negatively impacts short-term mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol. 2011;106:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Choi G, Runyon BA. Alcoholic hepatitis: a clinician’s guide. Clin Liver Dis. 2012;16:371-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin North Am. 2011;40:611-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 699] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 7. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] |
| 8. | Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, Singhal S, Brind A, Haydon G. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174-1179. [PubMed] |
| 9. | Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 381] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3779] [Article Influence: 151.2] [Reference Citation Analysis (2)] |
| 13. | Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56 Suppl 1:S39-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 14. | Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Kadian M, Kakkar R, Dhar M, Kaushik RM. Model for end-stage liver disease score versus Maddrey discriminant function score in assessing short-term outcome in alcoholic hepatitis. J Gastroenterol Hepatol. 2014;29:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Monsanto P, Almeida N, Lrias C, Pina JE, Sofia C. Evaluation of MELD score and Maddrey discriminant function for mortality prediction in patients with alcoholic hepatitis. Hepatogastroenterology. 2013;60:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Yang Y, Deng L, Li X, Shi Z, Chen D, Chen X, Li M, Ma L. Evaluation of the prognosis of fulminant viral hepatitis in late pregnancy by the MELD scoring system. Eur J Clin Microbiol Infect Dis. 2012;31:2673-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Huo TI, Lin HC, Hsia CY, Huang YH, Wu JC, Chiang JH, Chiou YY, Lui WY, Lee PC, Lee SD. The MELD-Na is an independent short- and long-term prognostic predictor for hepatocellular carcinoma: a prospective survey. Dig Liver Dis. 2008;40:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Khalaf H, Mourad W, El-Sheikh Y, Abdo A, Helmy A, Medhat Y, Al-Sofayan M, Al-Sagheir M, Al-Sebayel M. Liver transplantation for autoimmune hepatitis: a single-center experience. Transplant Proc. 2007;39:1166-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 573] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 22. | Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Papastergiou V, Tsochatzis EA, Pieri G, Thalassinos E, Dhar A, Bruno S, Karatapanis S, Luong TV, O’Beirne J, Patch D. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014;39:721-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Kasztelan-Szczerbinska B, Slomka M, Celinski K, Szczerbinski M. Alkaline phosphatase: the next independent predictor of the poor 90-day outcome in alcoholic hepatitis. Biomed Res Int. 2013;2013:614081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Vaa BE, Asrani SK, Dunn W, Kamath PS, Shah VH. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc. 2011;86:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5096] [Cited by in RCA: 5143] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 28. | Méndez-Sánchez N, Aguilar-Ramírez JR, Reyes A, Dehesa M, Juórez A, Castñeda B, Sánchez-Avila F, Poo JL, Guevara González L, Lizardi J. Etiology of liver cirrhosis in Mexico. Ann Hepatol. 2004;3:30-33. [PubMed] |
| 29. | Orrego H, Blake JE, Blendis LM, Medline A. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology. 1987;92:208-214. [PubMed] |
| 30. | Christoffersen P, Nielsen K. Histological changes in human liver biopsies from chronic alcoholics. Acta Pathol Microbiol Scand A. 1972;80:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Trabut JB, Plat A, Thepot V, Fontaine H, Vallet-Pichard A, Nalpas B, Pol S. Influence of liver biopsy on abstinence in alcohol-dependent patients. Alcohol Alcohol. 2008;43:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Jinjuvadia R, Liangpunsakul S. Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States. J Clin Gastroenterol. 2015;49:506-511. [PubMed] |
| 33. | Gordillo-Bastidas E, Panduro A, Gordillo-Bastidas D, Zepeda-Carrillo EA, García-Bañuelos JJ, Muñoz-Valle JF, Bastidas-Ramírez BE. Polymorphisms of alcohol metabolizing enzymes in indigenous Mexican population: unusual high frequency of CYP2E1*c2 allele. Alcohol Clin Exp Res. 2010;34:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Montano Loza AJ, Ramirez Iglesias MT, Perez Diaz I, Cruz Castellanos S, Garcia Andrade C, Medina Mora ME, Robles Díaz G, Kershenobich D, Gutierrez Reyes G. Association of alcohol-metabolizing genes with alcoholism in a Mexican Indian (Otomi) population. Alcohol. 2006;39:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Lafferty H, Stanley AJ, Forrest EH. The management of alcoholic hepatitis: a prospective comparison of scoring systems. Aliment Pharmacol Ther. 2013;38:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Larsen FS, Wendon J. Prevention and management of brain edema in patients with acute liver failure. Liver Transpl. 2008;14 Suppl 2:S90-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 261] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Spahr L, Rubbia-Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, Hadengue A. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
P- Reviewer: Bramhall S, Jin B S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/