FIBROSIS
Liver fibrosis is a wound-healing response to a range of chronic liver diseases of different etiology, and drives the progression of chronic hepatic diseases towards advanced liver cirrhosis and even hepatic carcinoma. Effective therapies are lacking besides diet control and physical exercise. Persisting parenchymal cell injury results in recruitment of immune cells, and activation and accumulation of fibrogenic cells. As the main source of liver fibrogenic cells, hepatic stellate cells (HSCs) lose cytoplasmic lipid droplets composed of retinyl esters to transdifferentiate from quiescent cells to activated myofibroblasts upon liver injury[1]. Myofibroblasts synthesize and secrete extracellular matrix (ECM) in an attempt to limit liver injury[2]. In addition, they also produce a wide range of matrix metalloproteinases (MMPs) that degrade ECM, and specific tissue inhibitors of metalloproteinase to inhibit activation of MMPs[3]. In brief, hepatocyte injury, immune cell recruitment, and fibrogenic cell activation contribute to the imbalance of ECM accumulation and degradation, which ultimately lead to fibrosis.
AUTOPHAGY
Autophagy is a catabolic intracellular pathway, targeting defective or excessive organelles to the lysosomes for degradation into amino acids, free fatty acids or other small molecules used for material recycling or energy harvest. Autophagy, usually stimulated by energy restriction, stress or inflammation, is regarded as a survival mechanism that plays a critical role in maintaining cellular homeostasis, which is involved in many human disorders including fibrotic disease. Three different kinds of autophagy are defined based on how the substrates are delivered to the lysosomes for degradation: macroautophagy, microautophagy, and chaperone-mediated autophagy, with macroautophagy being the major type. Although it is regarded as a cell-protective mechanism, excessive autophagy can cause cell death, known as type II programmed cell death[4]. However, it is unclear whether autophagy directly executes cell death or is a secondary effect of apoptosis. Autophagy can be considered a double-edged sword[5], and more investigations are needed to explore the complicated roles of autophagy.
AUTOPHAGY IN FIBROSIS: "HERO" OR "VILLAIN"?
Autophagy reduces fibrosis by hepatocyte injury attenuation
An increasing body of evidence supports the notion that autophagy participates in the pathophysiology of many human disorders including hepatic fibrosis. However, whether it is a hero or villain in hepatic fibrosis is still controversial.
Recent studies have demonstrated that autophagy impairment results in liver disease exacerbation due to reduction of degradation of defective organelles and unfolded proteins, which causes oxidative and endoplasmic reticulum stress[6-9] (Figure 1). Autophagy is increased in mice treated acutely with alcohol, in parallel with a marked reduction of serum inflammatory markers and tissue triglyceride level[10]. Autophagy may degrade activated caspase-8, a death receptor[11], thus exhibiting an antifibrotic effect by limiting liver injury. Furthermore, in α-1 antitrypsin (AT) deficiency, a disease in which the α-1 AT mutant Z protein results in protein aggregation and chronic liver injury, an autophagy-enhancing drug was demonstrated to reduce the hepatic load and reversed fibrosis[12]. Collectively, all the studies consistently supported that autophagy acted as a hero in hepatic fibrosis.
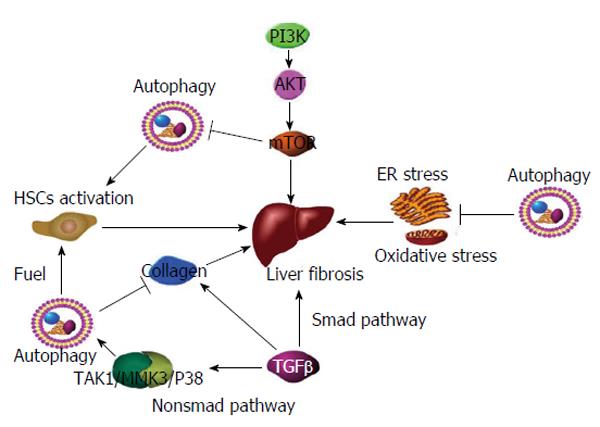
Figure 1 Mechanisms involved in autophagy and fibrosis.
(1) Phosphoinositide 3-kinase promotes phosphorylation of AKT, which subsequently leads to stimulation of mTOR and inhibition of autophagy. mTOR activation promotes hepatic fibrosis; (2) Autophagy fuels HSC activation, leading to hepatic fibrosis; (3) TGF-β promotes collagen synthesis and fibrosis via the Smad pathway. Furthermore, TGF-β stimulates autophagy via the non-Smad TAK1/MMK3/P38 pathway, leading to collagen degradation and fibrosis reduction; and (4) Autophagy attenuating ER stress and oxidative stress, and ultimately reduces fibrosis. mTOR: Mammalian target of rapamycin; HSC: Hepatic stellate cells; TGF-β: Transforming growth factor-β; TAK1/MMK3/P38: TGF-β-activated kinase 1-MAPK kinase 3-p38.
Controversial issues of autophagy and HSC activation
It had been unclear whether autophagy participates in HSC activation until the study of Zhu et al[13] in 1999, which demonstrated that rapamycin, a known immunosuppressive agent, inhibited HSC proliferation and limited fibrogenesis in mouse models treated with carbon tetrachloride (CCl4). They further demonstrated that rapamycin decreased HSC proliferation. As an immunosuppressant, rapamycin inhibited growth factor signaling in nonimmune as well as immune cells[14], which may largely explain its antifibrotic effect. The authors pointed out that mammalian target of rapamycin (mTOR) negatively regulated autophagy. The binding of rapamycin and mTOR appeared to block interleukin-2-dependent proliferation of T cells and even other cells[14]. Similar results were gained in another two studies[15,16]. However, it is unfortunate that no one has detected any change in autophagy during improvement of fibrosis, because rapamycin or its analogs stimulate autophagy by inhibiting mTOR. The antifibrotic effect of rapamycin depends on its antiproliferative effect on fibrotic cells or the indirect effect of autophagy stimulation remains unclear.
Fortunately, 10 years later, another study discovered that autophagic flux was increased during HSC activation and was inhibited by bafilomycin A1, an autophagy inhibitor. HSC activation was blocked by 3-methyladenine (MA) or chloroquine, suggesting that inhibition of HSC activation could be achieved by interruption of different phases of autophagy[17]. This evidence strongly indicates that autophagy is involved in HSC activation (Figure 1). Another discovery that merits further consideration is that platelet-derived growth factor BB, which activates HSCs, stimulates the location of microtubule associated protein light chain 3 II, an important biomarker protein of autophagy and lipid droplets, implying a potent relationship between HSC activation and lipid metabolism.
Hernández-Gea et al[18] have shown that autophagy releases lipid that promotes fibrogenesis by activating HSCs in mice and in human tissues. Inhibition of autophagy by pharmacological antagonism or Atg5 and Atg7 knockdown in mice also resulted in attenuation of fibrogenesis, as well as increased lipid content in stellate cells isolated from Atg7F/F mice[18]. Likewise, HSC-specific deletion of Atg7 in mice which were treated with either CCl4 or thioacetamide, also lead to obvious reduction of tissue fibrosis with preserved intracellular lipid droplets[19]. These results strongly support that autophagy induces tissue fibrogenesis by degradation of intracellular lipid droplets, which is known as lipophagy. Autophagy is a generalized feature of fibrotic cells, and a similar phenomenon is not only observed in the liver, but also in other organs such as the kidneys and lungs[19].
Autophagy and transforming growth factor-β1 related signaling pathways
Transforming growth factor (TGF)-β1 is a classical signaling pathway in liver fibrosis and induces autophagy[20], suggesting that autophagy participates in fibrosis via the TGF-β pathway. TGF-β1 may stimulate autophagy via the TGF-β-activated kinase (TAK)1-MAPK kinase (MKK)3-p38 and TAK1-AMP-activated protein kinase (AMPK) pathways, leading to profibrotic responses[21] (Figure 1). However, it is plausible that TGF-β acts as both an apoptosis promoter and suppressor, which may relate to its regulation of autophagy, and plays dual roles in apoptosis[21]. TGF-β protects glomerular mesangial cells from apoptosis during serum deprivation via autophagy induction[22]. Moreover, TGF-β is involved in ECM synthesis and degradation. One study showed that TGF-β induced autophagy in MMC via the TAK1-MKK3-p38 signaling pathway, and autophagy promoted intracellular degradation of collagen (Figure 1). The dual functions of TGF-β as both an inducer of collagen synthesis and an inducer of autophagy and collagen degradation underscore the multifunctional nature of TGF-β[23].
Autophagy and collagen degradation
Genetic and pharmacological inhibition of autophagy in mice resulted in increased levels of type I collagen in mouse kidneys and primary mesangial cells, suggesting that autophagy promotes intracellular degradation of type I collagen, which is a major component of ECM[23]. Autophagy attenuates endoplasmic reticulum stress by eliminating misfolded procollagen[24]. Furthermore, Beta (2)-adrenergic stimulation triggers autophagy in cardiac fibroblasts and promotes intracellular collagen degradation and inhibits cardiac fibrosis[25]. However, this effect has been demonstrated in other organs, and whether it exhibits the same effect in liver remains unclear.
The above studies marked a milestone in the exploration of the role of autophagy in hepatic fibrosis. Autophagy is mostly a cell survival mechanism that attenuates hepatic inflammatory injury and ultimately inhibits liver fibrosis. Given more insight into the role of autophagy in HSC activation, we have realized a new perspective that autophagy is responsible for activation of HSCs and other hepatic fibrogenic cells, by intracellular lipid degradation, leading to fibrosis. TGF-β induces autophagy, therefore, its role in liver fibrosis needs further investigation. We have to take into account that although autophagy may be a critical pathway in ameliorating hepatic injury in the short term, its long-term effect in fibrogenic cells may worsen chronic liver diseases, which could be regarded as a side effect in antifibrotic therapy[19].
We suggest that if autophagy could be selectively inhibited in HSCs and other fibrotic cells, autophagy special blocker would be an attractive candidate of antifibrotic strategies. Nonetheless, inhibition of cell-specific autophagy is exciting, yet more challenging in a tissue containing various types of cells. Further research is needed, targeting different receptors on the cell surface that may activate different effect of autophagy. It would also be useful to determine whether HSC activation is completely blocked by autophagy inhibition or just partly reversed to a quiescent phase, and the appropriate extent and time of autophagy should be seriously considered. Several genes participate in induction of autophagy. This raises the question of whether there is a link between autophagy and HSC phenotypic transformation.
Controversial issues of autophagy and mTOR
The data from Thoen et al[26] seem to contradict the HSC-activating yet autophagy-inhibiting effect of mTOR[27], because mTOR contributes to cell proliferation, including HSCs[13,28]. Likewise, it has been demonstrated that rapamycin, an mTOR target inhibitor and autophagy stimulator, reduces liver fibrosis[13,14,16], which is contradicted by later studies showing that autophagy induces HSC activation. Liu et al[29] have indicated that autophagy inhibitor 3-MA significantly inhibits proliferation and activation of HSCs by arresting the cells in G2 phase. Whether autophagy inhibits or promotes HSC proliferation is controversial. Whether the inhibitory effect on proliferation of fibrogenic cells depends on mTOR inhibition itself or an indirect action on autophagy remains unclear. In a recent study, TGF-β rapidly activated its canonical Smad signaling pathway, and recruited a noncanonical pathway involving mTOR kinase to induce matrix protein collagen I expression, thus inducing fibrosis[30]. Therefore, it is essential to investigate the relationship among mTOR, autophagy, and HSC proliferation. Few studies have focused on lipid metabolism and HSC activation, leaving the mechanism of intracellular lipid degradation poorly understood. More research, especially with selective knockdown of Atg in mice, or HSCs derived from Atg-deleted mice, will shed light on this[26].
Finally, we hypothesize that since fibrosis is the result of imbalances of ECM accumulation and degradation, could it be a new direction to focus on the translocation of ECM turning into cell from extracellular matrix. Then intracellular matrix could be enclosed by autophagosome and subsequently fuses with lysosome to be degraded. Since autophagy has been demonstrated to promote the degradation of type I collagen in kidney, some level of autophagy may help in treatment of hepatic fibrosis.